Abstract
CD40 and its ligand CD40L are key players in T cell–B cell interaction and T cell–antigen-presenting cell (APC) interaction. Inhibition of CD40–CD40L interaction leads to severe humoral and cellular immunodeficiency. In this study we examined the presence of soluble CD40 (sCD40) in the serum of haemodialysis (HD) patients, CAPD patients, chronic renal failure (CRF) patients and healthy donors in order to evaluate the possible involvement of CD40 in uraemic immunodeficiency. Soluble CD40 was detected in the serum of healthy donors (n = 41) with a mean of 0.14 ± 0.12 ng/ml and in the urine of healthy donors with a mean of 1.80 ± 0.74 ng/ml. Soluble CD40 was highly elevated in all patients with impaired renal function. HD patients (n = 22) had up to 100-fold elevated sCD40 levels with a mean concentration of 8.32 ± 4.11 ng/ml, whereas CAPD patients (n = 10) had considerably lower levels of sCD40 with a mean of 3.58 ± 2.40 ng/ml. A strong correlation between sCD40 and serum creatinine levels was noted in CRF patients (n = 66). The highly elevated levels of sCD40 may point to the involvement of CD40 and its ligand CD40L in the clinical manifestation of uraemic immunodeficiency.
Keywords: CD40, soluble receptor, uraemia, dialysis
INTRODUCTION
Patients with end-stage renal failure are often severely immunocompromised [1]. They show a high rate of infections [2, 3], low response to hepatitis B vaccine [4, 5], cutaneous anergy [6] and possibly higher rates of malignant tumours [7] and lower rates of allograft rejection [8] than healthy persons. Some features of this cellular immunodeficiency are thought to result from a suppression of T helper cell type 1 (Th1) cytokines [9] and a defective costimulation of T cells via the B7–CD28 pathway [10]. Elevated circulating levels of cytokines and their corresponding receptors have been reported in the setting of chronic renal failure (CRF) [11–17], indicating that dysregulations in the cytokine network may be in part responsible for the development of uraemic immunodeficiency. In this study we investigated the in vivo presence of soluble CD40 and its possible changes in haemodialysis patients, CAPD patients and patients with CRF.
The 50-kD transmembrane protein CD40 is primarily expressed on B cells [18], but has also been detected on thymic epithelium [19] dendritic cells [20], monocytes [21], basal epithelium [22], kidney epithelium [23], vascular endothelium [24, 25], and some carcinomas including bladder and renal cell carcinoma [18, 22, 26, 27]. The corresponding ligand CD40L is transiently expressed on activated T cells [28–31], natural killer (NK) cells [32], mast cells, basophils [33] and eosinophils [34]. CD40 and CD40L are members of the tumour necrosis factor (TNF) receptor ligand family [35, 36] and key players in T cell–B cell and T cell–antigen-presenting cell (APC) interaction [37, 38]. Activation of CD40 triggers important B cell functions such as isotype switching and proliferation and rescues germinal centre B cells from apoptotic cell death [38]. CD40 activation on APC induces IL-12 secretion and thus promotes the switch from Th0 to Th1 cells [39]. Furthermore, CD40–CD40L interaction provides important costimulatory signals for T cells which seem to be mediated partially by the up-regulation of B7..1 (CD80) and B7.2 (CD86) on APC, which in turn stimulate T cells via CD28 [40, 41]. The X-linked hyper-IgM syndrome represents a naturally occurring condition in which CD40–CD40L interaction is impaired due to mutations in the CD40L gene and leads to severe defects in humoral and cellular immunity [42]. The blockade of CD40–CD40L interaction by anti-CD40L antibodies is immunosuppressive and has been successfully applied to suppress autoimmune disease [43, 44] and allograft rejection [45, 46].
Soluble CD40 might exert immunosuppressive effects in a similar fashion by interfering with CD40–CD40L interaction. Soluble CD40 has been demonstrated in vitro in the supernatants of B cell lines and bound to CD40L, which was thought to regulate CD40–CD40L interaction in a negative fashion [47, 48]. A dimeric CD40 fusion protein effectively suppressed IL-12 production by monocytes in vitro [49]. Thus, sCD40 is a candidate molecule to explain the development of immunological disturbances seen in end-stage renal failure.
In this study we investigated the in vivo presence of sCD40 in the serum of healthy donors and different patient groups with renal failure in order to determine whether sCD40 may be involved in the pathogenesis of immunodeficiency in uraemia. High levels of sCD40 could be detected in all sera of patients with renal insufficiency, and may suggest an immunosuppressive role of sCD40 in CRF.
PATIENTS AND METHODS
Patients
Twenty-two patients were receiving intermittent haemodialysis. Of the 22 patients receiving haemodialysis, six had diabetic nephropathy, four hypertensive nephropathy, two polycystic kidneys, two chronic glomerulonephritis, one c-ANCA+ vasculitis as underlying disease, the remaining seven patients were diagnosed clinically as having chronic glomerulonephritis. All patients were long-term haemodialysis patients and were either anuric or had a residual renal function of < 100 ml/day. Intermittent haemodialysis was performed three times a week for 4–5 h using a biocompatible membrane (F60, hollow fibre dialyser, high-flux polysulphone, 1.25 m2; Fresenius, Bad Homburg, Germany). Ten patients were receiving CAPD consisting of four to six exchanges during the day using 2 l of glucose-based solution (Fresenius). The sera of 66 patients with CRF due to different renal diseases were collected when the patients visited our out-patient department. Patients suffering from autoimmune diseases with known B cell activation (e.g. systemic lupus erythematosus (SLE)) were not included in this study. Control groups included 20 patients with metastatic bronchial, colorectal carcinoma or leukaemia, 23 patients with chronic inflammatory bowel diseases (CIBD) and 41 healthy volunteers. Each serum sample was additionally measured for creatinine, blood urea nitrogen and C-reactive protein (CRP) by the routine laboratory.
Antibodies
The G28-5 hybridoma was obtained from American Type Culture Collection (ATCC, Rockville, MD). The hybridoma supernatant was purified over a protein G Sepharose Fast Flow column (Pharmacia, Freiburg, Germany). For the production of polyclonal anti-CD40 antibodies, sCD40 was purified from supernatants of Chinese hamster ovary (CHO) cells transfected with the extracellular domain of CD40 as previously described [50]. A rabbit was immunized with 10 μg of the soluble extracellular domain of CD40 four times and boosted twice before bleeding. Anti-CD40 MoAb Ro1 was purified from hybridoma supernatants with a protein G column as described previously [50]. Peroxidase-conjugated goat anti-rabbit IgG (F(ab)2) and FITC-labelled goat anti-mouse IgG were obtained from Dianova (Hilgen, Germany).
ELISA for detection of soluble CD40
Ninety-six-well plates (Nunc, Wiesbaden, Germany) were coated for 2 h with MoAb G28-5 at a concentration of 1 μg/ml for 2 h and blocked for 1 h with PBS containing 1% bovine serum albumin (PBS–BSA). Samples of human serum or urine were diluted in PBS containing 1% BSA and 0.3 m NaCl. These samples were added for 12 h at 4°C. After washing, the plates were incubated with anti-CD40 rabbit serum at a dilution of 1:500 for 6 h at 4°C. After three further washing steps a peroxidase-linked goat anti-rabbit antibody was added at a concentration of 1 μg/ml for 1 h. The plates were developed with ABTS (Sigma, Deisenhofen, Germany) in citrate buffer pH 3.1 and read after 20 min at 405 nm. Concentrations were calculated by comparison with purified CD40Fc (kindly provided by Dr L. Kurrle and Dr R. Laufer; Behring Werke, Marburg, Germany). Each serum sample was measured twice and the mean concentration was calculated. The detection limit of the ELISA was about 50 pg/ml, the intra-assay variation and the interassay variation were 10% and 15%, respectively. Since MoAb G28-5 has been shown to bind closely to the site of CD40–CD40L interaction [50], this ELISA did not detect CD40–CD40L complexes.
Binding of sCD40 to CD40L
CD40L-transfected baby hamster kidney cells (1 × 106; BHKCD40L) [51] were incubated with 200 μl serum containing 10 mm EDTA from haemodialysis (HD) patients for 2 h at 4°C. The cells were washed with PBS containing 0.05% Tween and were incubated for 2 h with anti-CD40 MoAb Ro1 at 5 μg/ml, which detects a CD40 epitope that is not involved in CD40–CD40L interaction [50]. After washing, the cells were treated with FITC-conjugated goat anti-mouse immunoglobulin for 1 h. After three further washing steps the cells were resuspended in PBS containing 3% paraformaldehyde (PFA) and 5000 cells were analysed by flow cytometry on a FACScan (Becton Dickinson, Heidelberg, Germany). CD40Fc was used as positive control in a concentration of 5 μg/ml. Additionally, BHKCD40L cells were incubated with CD40Fc at 5 ng/ml to evaluate a threshold of sCD40 detection by flow cytometric analysis. BHKwt cells were used as negative control.
Statistical analysis
Statistical analysis was performed using SPSS 5.1 software (SPSS, Munich, Germany) on a Windows-based computer. Correlations and P levels were determined by Pearson correlation. Differences between sCD40 of the different patient groups were calculated by Mann–Whitney U-test, P levels ≤ 0.05 were considered significant.
RESULTS
Soluble CD40 in healthy individuals
Constitutively low levels of sCD40 were detected in the serum of healthy donors with an average concentration of 0.14 ± 0.12 ng/ml as measured by ELISA (Table 1). None of these donors had a history of impaired renal function or ongoing infections. Compared with serum levels, the sCD40 concentrations in the urine of these donors were about 10-fold higher with a mean concentration of 1.80 ± 0.74 ng/ml, indicating that sCD40 may possibly be eliminated via the kidneys.
Table 1.
Serum levels of sCD40 in healthy individuals and different patient groups
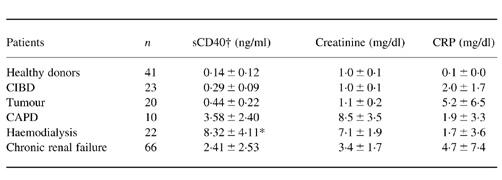
Dialysis patients and chronic renal failure (CRF) patients had highly elevated levels of sCD40, whereas tumour and chronic inflammatory bowel disease (CIBD) patients showed only slightly increased levels of sCD40, although their C-reactive protein (CRP) was markedly elevated. Compared with haemodialysis (HD) patients, CAPD patients had more than 50% lower levels of sCD40, although their creatinine and CRP were higher. The differences in sCD40 levels between all groups were significant according to Mann–Whitney U-test.
*Levels before haemodialysis. After haemodialysis sCD40 levels were 7.97 ± 4.66 ng/ml.
†The differences in sCD40 levels between each patient group and the respective control group were significant according to the Mann–Whitney U-test (P < 0.001).
Soluble CD40 in patients with neoplasia or inflammatory diseases
Patients with neoplastic disease had slightly, but significantly increased levels of sCD40, with a mean of 0.44 ± 0.22 ng/ml. These patients had normal kidney function with creatinine and blood urea nitrogen (BUN) in the upper normal range and a highly elevated CRP (Table 1). CIBD patients had also slight elevations of their serum sCD40, with a mean concentration of 0.29 ± 0.09 ng/ml and elevated levels of CRP (Table 1). It is conceivable that these weak elevations of sCD40 were caused by an activation of the immune system in these patients. There were no significant correlations between sCD40 and creatinine, BUN and CRP in tumour patients.
Soluble CD40 in haemodialysis and CAPD patients
Patients receiving haemodialysis had the highest sCD40 levels in this study, with a mean concentration of 8.32 ± 4.11 ng/ml before the onset of dialysis (Fig. 1). The mean concentration of sCD40 after the dialysis session was only slightly lower with 7.97 ± 4.66 ng/ml. Yet, in some patients an increase of their serum sCD40 level was observed after the haemodialysis session. Interestingly, CAPD patients had lower levels of sCD40, with a mean of 3.58 ± 2.40 ng/ml sCD40, although their creatinine was higher than in HD patients (8.5 mg/dl versus 7.1 mg/dl). Also, their sCD40 levels correlated well with creatinine, whereas in HD patients no correlation between these parameters was seen (Fig. 2). CRP levels were elevated in both groups, but did not correlate significantly with sCD40 levels. Since most patients were anuric or had a urine production of < 100 ml/day a correlation between residual renal function and sCD40 could not be calculated.
Fig. 1.
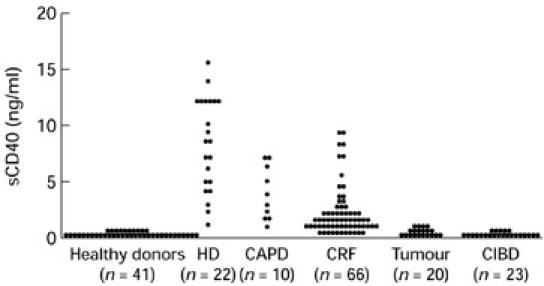
Distribution of serum levels of sCD40 in healthy donors, haemodialysis (HD), CAPD, chronic renal failure (CRF), tumour and chronic inflammatory bowel disease (CIBD) patients. The sCD40 serum levels of healthy donors were just above the detection limit, whereas sCD40 serum levels were highly elevated in all uraemic patients. Tumour and CIBD patients showed only weak elevations of sCD40.
Fig. 2.
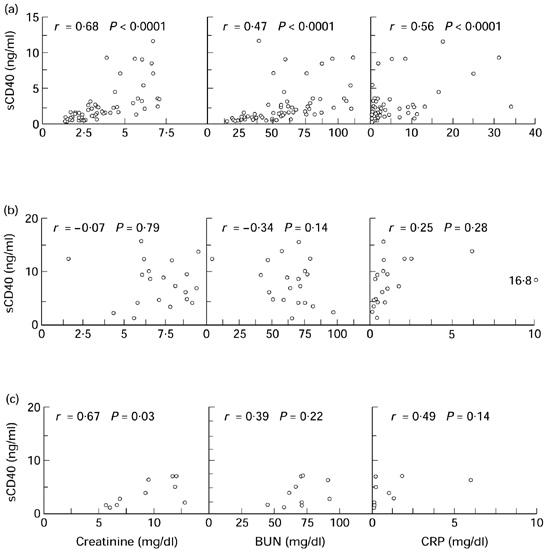
Correlations between sCD40 and creatinine, blood urea nitrogen (BUN) and C-reactive protein (CRP). (a) In chronic renal failure (CRF) patients high correlations were seen between sCD40 and creatinine and betweem sCD40 and BUN. (b) In haemodialysis (HD) patients, sCD40 did not correlate with any of the measured parameters. (c) In CAPD patients, sCD40 correlated with significantly with creatinine.
Soluble CD40 in patients with CRF
Patients with CRF had significantly higher sCD40 serum levels than healthy individuals, with a mean of 2.41 ± 2.53 ng/ml. Serum sCD40 levels correlated closely with serum creatinine, whereas the correlation with BUN and CRP was weaker (Fig. 2). None of the patients with elevated creatinine levels had normal levels of sCD40.
Binding of serum sCD40 to CD40L-expressing cells
In order to avoid possible modification of sCD40 through purification processes, binding of sCD40 to CD40L was studied in untreated serum using transfected baby hamster kidney cells. Binding of serum sCD40 to BHKCD40L could not be detected by flow cytometric analysis (Fig. 3a). Since binding of serum sCD40 to CD40L might have escaped detection simply through the low concentration of sCD40 in serum, we tested the binding of CD40Fc to BHKCD40L at an equally low concentration. At 5 ng/ml no binding of CD40Fc to BHKCD40L could be detected, either (Fig. 3b).
Fig. 3.
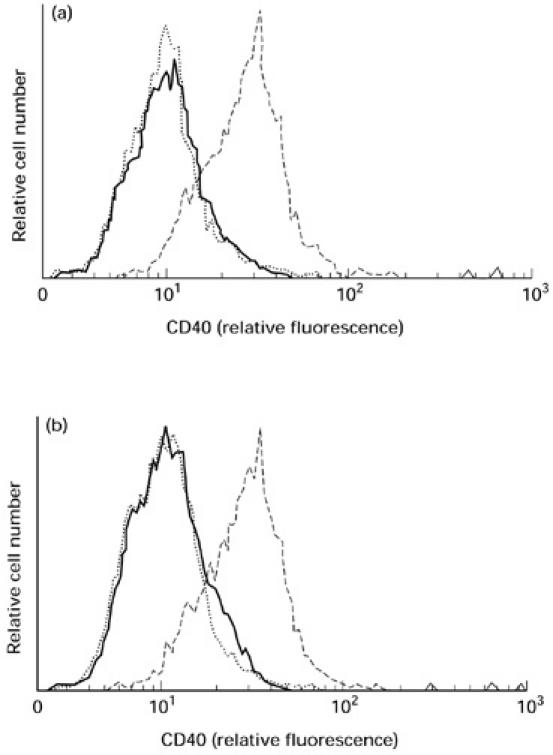
Binding of soluble CD40 from the serum of haemodialysis (HD) patients to CD40L. (a) BHKCD40L incubated in pooled serum from 10 HD patients (—) showed no flow cytometric staining for CD40. BHKwt cells (·····) incubated in pooled serum from HD patients were used as negative control, BHKCD40L incubated with 5 μg/ml CD40Fc served as positive control (–––––). (b) Neither BHKCD40L (—) nor BHKwt (·····) stained for CD40Fc at 5 ng/ml, whereas BHKCD40L incubated in CD40Fc at 5 μg/ml were strongly positive for CD40 (–––––). BHKwt did not stain for CD40FC at 5 μg/ml (not shown).
DISCUSSION
In this study the sera of healthy individuals and uraemic patients were examined for the presence of soluble forms of the B cell receptor CD40 to evaluate the possible role of CD40 in the immune system in end-stage renal failure. Compared with the constitutively low levels of sCD40 in the sera of healthy donors, the levels of sCD40 were highly increased in sera of uraemic patients. Among these the highest levels of sCD40 were found in HD patients. Although sCD40 correlated well with creatinine levels, factors associated with HD seemed to modulate sCD40 serum levels, since CAPD patients had much lower sCD40 levels than HD patients despite higher serum creatinine. Inflammation seemed to influence sCD40 levels, as demonstrated by the correlation of sCD40 and CRP in all patient groups and the elevation of sCD40 in control groups with metastatic tumour disease or CIBD. B cells represent the most likely source of serum sCD40, since they express high levels of CD40 and have been shown to shed sCD40 in vitro [47].
Urinary sCD40 concentrations were about 10-fold higher than sCD40 serum levels in healthy donors. Urinary sCD40 may either be the product of CD40 shedding in the kidney by CD40-expressing renal cells or it may stem from the same source as serum sCD40 being concentrated into the urine. Recently, CD40 was detected in the proximal tubulus when human kidneys were stained with a CD40 MoAb [27]. Since no CD40 mRNA was detected in these cells it was concluded that this staining possibly represented sCD40 in the tubulus. Altogether, a renal excretion of serum sCD40 seems more likely than shedding of sCD40 by renal parenchymal cells.
The high serum levels of sCD40 in CRF patients may be the result of an increased expression of CD40, an increased generation of sCD40 or a decreased elimination of this soluble receptor. Taking into account that sCD40 correlated significantly with creatinine levels and was found in high concentrations in normal human urine, passive accumulation of sCD40 due to a decreased elimination is the most likely mechanism. The activation of B cells in the uraemic patient [17] may further contribute to the increased levels of sCD40 by enhanced expression and shedding of this receptor. The latter hypothesis is supported by the correlation between sCD40 and CRP in CRF patients and the elevated sCD40 levels in patients with high CRP levels and normal kidney function such as tumour patients and CIBD patients.
The contact with dialysis membranes may lead to contact-dependent activation of the immune system. Although in our study the mean level of sCD40 was decreased after the HD session, we frequently found increased sCD40 after haemodialysis. These findings were inconsistent and were not attributable always to the same patients. Therefore sCD40 levels did not correlate with kt/v-values or with serum creatinine in HD patients, indicating that multiple factors may influence the shedding of this receptor.
Although the release of sCD40 has been demonstrated in vitro in cultured B cell lines, the functions that sCD40 exerts in vivo are not known. In vitro, sCD40 has been shown to bind to CD40L [47]. This may be interpreted as a mechanism that controls CD40–CD40L interaction in a negative fashion. The application of a dimeric CD40 fusion protein strongly suppressed IL-12 production of monocytes in vitro [49]. Supposing that sCD40 has similar effects on CD40–CD40L interaction in vivo, it might profoundly influence the immune system. An impaired CD40–CD40L interaction leads to humoral and cellular immunodeficiencies, as seen in patients with mutated CD40L. Likewise, the application of antibodies that block CD40–CD40L interaction is immunosuppressive and may improve autoimmune disease [44], suppress IL-12 production [43] and prolong allograft survival [45, 46]. Similar clinical features are noted in patients with end-stage renal failure. In our study no significant binding of serum sCD40 to CD40L could detected by flow cytometric analysis. This result may be explained by the low serum concentration of sCD40 and does not exclude binding of serum sCD40 in vivo, since CD40Fc was not detected on BHKCD40L when applied at a similar concentration. In view of the finding that sCD40 from cultured B cells bound to CD40L [47], it is not unlikely that sCD40 may act as a negative regulator of CD40–CD40L interaction in vivo. A supposed blockade of CD40–CD40L interaction might be held responsible for the decreased inducibility of Th1 cytokines [9] and the defective costimulation via CD28 [10] in uraemic patients [10], since CD40 is a potent up-regulator of IL-12 synthesis [43] and CD28 expression [41]. Since the concentrations of sCD40 in human serum were too low to show significant binding in our assays, a profound inhibition of CD40–CD40L in vivo seems unlikely. Yet, the constant presence of low amounts of sCD40 in uraemic patients may alter immune responses and contribute to the immune dysregulations in these patients.
In conclusion, this study demonstrates highly elevated levels of sCD40 in uraemic patients. Although binding of serum sCD40 to CD40L could not be demonstrated in vitro, an involvement of CD40 in the uraemic immunodeficiency cannot be excluded and requires further study.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgesellschaft (Gerhard Hess Programm and SFB217, projects A3 and A9). Dr Gert Riethmüller is thanked for encouragement and advice, Dr Peter Sauer is thanked for help with the statistical analysis. Christine Federle is thanked for excellent technical assistance.
REFERENCES
- 1.Descamps LB, Chatenoud L. T cells and B cells in chronic renal failure. Semin Nephrol. 1996;16:183–91. [PubMed] [Google Scholar]
- 2.Tolkoff-Rubin N, Rubin R. Uremia and host defenses. N Engl J Med. 1990;322:770–2. doi: 10.1056/NEJM199003153221109. [DOI] [PubMed] [Google Scholar]
- 3.Khan IH, Catto GR. Long-term complications of dialysis: infection. Kidney Int Suppl. 1993;41:S143–8. [PubMed] [Google Scholar]
- 4.Benhamou E, Courouce AM, Laplanche A. Long-term results of hepatitis B vaccination in patients on dialysis. N Engl J Med. 1986;314:1710–1. doi: 10.1056/NEJM198606263142613. [DOI] [PubMed] [Google Scholar]
- 5.Jungers P, Devillerk P, Salomon H. Randomised placebo-controlled trial of recombinant interleukin-2 in chronic uraemic patients who are nonresponders to heptitis B vaccine. Lancet. 1994;344:856–7. doi: 10.1016/s0140-6736(94)92829-0. [DOI] [PubMed] [Google Scholar]
- 6.Modai D, Sagi DB, Maor J. Effect of levamisole on skin reactions to DNCB in chronically dialyzed patients. Nephron. 1980;25:280–2. doi: 10.1159/000181858. [DOI] [PubMed] [Google Scholar]
- 7.Soubrane C, Jacobs C, Jacquillat C, et al. Influence of the uremic state on the development of malignancy. An experimental study in the rat. Am J Nephrol. 1986;6:363–8. doi: 10.1159/000167192. [DOI] [PubMed] [Google Scholar]
- 8.Rashid A, Sengar DP, Couture RA, Posen GA, Harris JE. Beneficial effect of hemodialysis on renal allograft survival. Nephron. 1975;14:413–20. doi: 10.1159/000180475. [DOI] [PubMed] [Google Scholar]
- 9.Gerez L, Madar L, Shkolnik T, et al. Regulation of interleukin-2 and interferon-gamma gene expression in renal failure. Kidney Int. 1991;40:266–72. doi: 10.1038/ki.1991.209. [DOI] [PubMed] [Google Scholar]
- 10.Girndt M, Kohler H, Schiedhelm-Weick E, Meyer-zum Buschenfelde KH, Fleischer B. T cell activation defect in hemodialysis patients: evidence for a role of the B7/CD28 pathway. Kidney Int. 1993;44:359–65. doi: 10.1038/ki.1993.252. [DOI] [PubMed] [Google Scholar]
- 11.Kurz P, Kohler H, Meuer S, Hutteroth T, Meyer zum Buschenfelde KH. Impaired cellular immune responses in chronic renal failure: evidence for a T cell defect. Kidney Int. 1986;29:1209–14. doi: 10.1038/ki.1986.129. [DOI] [PubMed] [Google Scholar]
- 12.Beaurain G, Naret C, Marcon L, et al. In vivo T cell preactivation in chronic uremic hemodailyzed and non-hemodialyzed patients. Kidney Int. 1989;36:636–44. doi: 10.1038/ki.1989.240. [DOI] [PubMed] [Google Scholar]
- 13.Herbelin A, Nguyen AT, Zingraff J, Urena P, Descamps LB. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int. 1990;37:116–25. doi: 10.1038/ki.1990.16. [DOI] [PubMed] [Google Scholar]
- 14.Brockhaus M, Bar KY, Gurwicz S, Frensdorff A, Haran N. Plasma tumor necrosis factor soluble receptors in chronic renal failure. Kidney Int. 1992;42:663–7. doi: 10.1038/ki.1992.332. [DOI] [PubMed] [Google Scholar]
- 15.Pereira BJ, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994;45:890–6. doi: 10.1038/ki.1994.117. [DOI] [PubMed] [Google Scholar]
- 16.Descamps LB, Herbelin A, Nguyen AT, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–92. [PubMed] [Google Scholar]
- 17.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Soluble CD23 as an effector of immune dysregulation in chronic uremia and dialysis. Kidney Int. 1993;43:878–84. doi: 10.1038/ki.1993.123. [DOI] [PubMed] [Google Scholar]
- 18.Paulie S, Ehlin HB, Mellstedt H, Koho H, Ben AH, Perlmann P. A p50 surface antigen restricted to human urinary bladder carcinomas and B lymphocytes. Cancer Immunol Immunother. 1985;20:23–28. doi: 10.1007/BF00199769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galy AH, Spits H. CD40 is functionally expressed on human thymic epithelial cells. J Immunol. 1992;149:775–82. [PubMed] [Google Scholar]
- 20.Hart DNJ, McKenzie JL. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988;168:157–70. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young LS, Dawson CW, Brown KW, Rickinson AB. Identification of a human epithelial cell surface protein sharing an epitope with the C3d/Epstein–Barr virus receptor molecule of B lymphocytes. Int J Cancer. 1989;43:786–94. doi: 10.1002/ijc.2910430508. [DOI] [PubMed] [Google Scholar]
- 23.Hess S, Rensing EA, Schwabe R, Bufler P, Engelmann H. CD40 function in nonhematopoietic cells. Nuclear factor kappa B mobilization and induction of IL-6 production. J Immunol. 1995;155:4588–95. [PubMed] [Google Scholar]
- 24.Hollenbaugh D, Mischel PN, Edwards CP, Simon JC, Denfeld RW, Kiener PA, Aruffo A. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995;182:33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc Natl Acad Sci USA. 1995;92:4342–6. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamenkovic I, Clark EA, Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989;8:1403–10. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluth B, Hess S, Engelmann H, Schnitzel S, Riethmüller G, Feucht HE. Endothelial expression of CD40 in renal cell carcinoma. Cancer Res. 1997;57:891–9. [PubMed] [Google Scholar]
- 28.Armitage RJ, Sato TA, Macduff BM, Clifford KN, Alpert AR, Smith CA, Fanslow WC. Identification of a source of biologically active CD40 ligand. Eur J Immunol. 1992;22:2071–6. doi: 10.1002/eji.1830220817. [DOI] [PubMed] [Google Scholar]
- 29.Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992;13:431–3. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- 30.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 31.Lederman S, Yellin MJ, Inghirami G, Lee JJ, Knowles DM, Chess L. Molecular interactions mediating T–B lymphocyte collaboration in human lymphoid follicles. Roles of T cell–B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J Immunol. 1992;149:3817–26. [PubMed] [Google Scholar]
- 32.Carbone E, Ruggiero G, Terrazzano G, et al. A new mechanism of NK cell cytotoxicity activation: the CD40–CD40 ligand interaction. J Exp Med. 1997;185:2053–60. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 34.Gauchat JF, Henchoz S, Fattah D, et al. CD40 ligand is functionally expressed on human eosinophils. Eur J Immunol. 1995;25:863–5. doi: 10.1002/eji.1830250335. [DOI] [PubMed] [Google Scholar]
- 35.Beutler B, van Huffel C. Unraveling function in the TNF ligand and receptor families. Science. 1994;264:667–8. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- 36.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–62. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 37.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–8. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 38.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 39.Kato T, Hakamada R, Yamane H, Nariuchi H. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40–CD40 ligand interaction. J Immunol. 1996;156:3932–8. [PubMed] [Google Scholar]
- 40.Caux C, Massacrier C, Vanbervliet B, Van Dubois BKC, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation. Requirement of B7–CD28 signaling through CD40. Science. 1996;273:1862–4. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 42.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–64. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 43.Stuber E, Strober W, Neurath M. Blocking the CD40L–CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–8. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desai MA, Lu L, Ramsey GR, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996;97:2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker DC, Greiner DL, Phillips NE, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci USA. 1995;92:9560–4. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 47.van Kooten C, Gaillard C, Galizzi JP, Hermann P, Fossiez F, Banchereau J, Blanchard D. B cells regulate expression of CD40 ligand on activated T cells by lowering the mRNA level and through the release of soluble CD40. Eur J Immunol. 1994;24:787–92. doi: 10.1002/eji.1830240402. [DOI] [PubMed] [Google Scholar]
- 48.Bjorck P, Braesch AS, Paulie S. Antibodies to distinct epitopes on the CD40 molecule co-operate in stimulation and can be used for the detection of soluble CD40. Immunology. 1994;83:430–7. [PMC free article] [PubMed] [Google Scholar]
- 49.Shu U, Kiniwa M, Wu CY, et al. Activated T cells induce interleukin-12 production by monocytes via CD40–CD40 ligand interaction. Eur J Immunol. 1995;25:1125–8. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 50.Schwabe RF, Hess S, Johnson JP, Engelmann H. Modulation of soluble CD40 ligand bioactivity with anti-CD40 antibodies. Hybridoma. 1997;16:217–26. doi: 10.1089/hyb.1997.16.217. [DOI] [PubMed] [Google Scholar]
- 51.Hess S, Kurrle R, Lauffer L, Riethmuller G, Engelmann H. A cytotoxic CD40/p55 tumor necrosis factor receptor hybrid detects CD40 ligand on herpesvirus saimiri-transformed T cells. Eur J Immunol. 1995;25:80–86. doi: 10.1002/eji.1830250116. [DOI] [PubMed] [Google Scholar]


