Abstract
The aetiology of both term and preterm labour remains incompletely understood. Maternal infectious diseases as well as intra-uterine infections were shown to be a well established cause of uncontrollable preterm delivery, indicating that inflammatory reactions, regulated by maternal immunecompetent cells, are implicated in labour-promoting mechanisms. To investigate the possibility that the activation of the fetal immune system may be involved in labour induction, we examined cytokine production patterns of different cord blood cell populations obtained from neonates after spontaneous onset of normal term labour and vaginal delivery (n = 25), vaginal delivery but induced term labour (n = 17), and preterm delivery because of uncontrollable labour (n = 27, 20 patients received corticoid treatment for fetal lung maturation), in comparison with cells obtained from neonates after elective term caesarean delivery in the absence of labour (n = 15). Our results demonstrate that spontaneous term labour, but not induced term labour, was associated with significantly increased IL-6 production by myelomonocytic cell populations. Preterm delivery due to uncontrollable labour with resistance to tocolysis was not associated with increased IL-6 production by fetal myelomonocytic cells. Two-colour flow cytometry combined with intracellular cytokine staining was used to identify fetal monocytes as sources of labour-associated IL-6 release at term. We did not find any activation of cord blood T cells in association with spontaneous term or uncontrollable preterm labour. Therefore, fetal T cell responses may not cause monocyte activation. Our results suggest that increased release of IL-6 from fetal monocytes is involved in mechanisms promoting normal term, but not preterm labour, and that mechanisms inducing term and preterm labour are completely different.
Keywords: fetal immune system, monocyte activation, labour, inflammatory cytokines
INTRODUCTION
Recent progress in the study of the aetiology of labour has shown that increased intra-uterine concentrations of inflammatory cytokines (IL-1β, IL-6, IL-8 and tumour necrosis factor-alpha (TNF-α)) are a main cause of infection-associated uncontrollable preterm labour and subsequent premature delivery [1–4]. These cytokines, thought to have an important role in the intra-uterine response to infection, have currently been reported to be also elevated in the amniotic fluid and cervical secretions during the normal course of term labour [5, 6] and an increased production of inflammatory cytokines was demonstrated for placental cell cultures obtained from women with spontaneous term labour in comparison with women with an elective caesarean delivery at term in the absence of labour [7]. With regard to premature deliveries, uncontrollable preterm labour concomitant with high cytokine concentrations in the amniotic fluid was observed much more often than the appearance of pathogens in the amniotic fluid or the occurrence of histologically confirmed chorioamnionitis [8, 9]. Moreover, we were able to demonstrate an elevated release of inflammatory cytokines from placental tissue cultures, obtained from women with uncontrollable preterm labour in the absence of intra-uterine infections, while placental cells obtained from women with confirmed infection produced only small amounts of these cytokines [10]. Therefore, inflammatory regulated processes unrelated to intra-uterine infection may be involved in the production of cytokines in association with normal term and also preterm uterine activation.
Published results concerning immunohistologic detection of cytokine-producing cell types within placental tissues are very contradictory. Expression was found in the fetal vessels, macrophages, cytotrophoblast, syncytiotrophoblast and also in fetal blood cells [11–13]. Currently, we were able to demonstrate labour-associated production of inflammatory cytokines by placental cell types, using magnetic cell sorting (MACS) and immunohistochemical techniques. Thereby it was shown that term as well as preterm labour was accompanied by increased production of IL-1β and IL-6 by fetal endothelial cells, while TNF-α was predominantly released from placental macrophages [14]. These results suggest that the activation of fetal immunocompetent cells in combination with the activation of the placental vascular system provokes inflammatory processes, characterized by increased local cytokine production within the placental compartment. Participation of endothelial cell reactions in labour-promoting mechanisms are also supported by morphological findings demonstrating an association between preterm labour and placental vasculopathy. Also uteroplacental hypoperfusion associated with placental ischaemia were shown to be the most common process implicated in the aetiology of preterm delivery [15]. These vascular alterations in the placenta may be induced by in utero activation of the fetal immune system, and probably occur in both term and also non-infection-induced preterm labour. Thereby, rejection processes due to irregular cell traffic between the fetal and the maternal circulation may provoke extensive immunological responses. To evaluate this hypothesis, we examined the cytokine release of different cell populations of the fetal immune system in association with normal term and uncontrollable preterm labour.
PATIENTS AND METHODS
Patient populations
Human umbilical cord blood was collected immediately after delivery, from 84 women with a singleton gestation who had attended our delivery rooms between February 1997 and May 1998. According to status of labour and gestational age, the study population was divided into four different groups of patients. In the first group 27 women had undergone preterm parturition because of uncontrollable preterm labour (25–36 weeks gestation) with resistance to tocolysis. In this group, 18 women had a caesarean delivery and nine patients delivered spontaneously. Rupture of fetal membranes was diagnosed in 14 cases and 14 women had rising indicators of infection such as increasing numbers of leucocytes (> 10 000/l) and high levels of C-reactive protein (CRP; > 1 mg/dl). Intra-amniotic infection, confirmed by histological examination of the fetal membranes, umbilical cord and chorionic plate was diagnosed in 16 cases. In this group 22 patients received tocolytic treatment. Tocolytics were administered intravenously and consisted of fenoterolhydrobromide and/or magnesium sulphate according to standardized protocols. Five patients did not receive tocolytics because of advanced cervical dilatation. For induction of lung maturity, a formulation of betamethason-hydrogenphosphate (8 mg) and betamethasone-acetate (6 mg) was used at a dose of 2 × 14 mg intramuscularly given 24 h apart. Seven patients did not receive corticosteroid therapy because the gestational age of these women was more than 34 weeks. Oral antibiotics were given in case of rising infectious indices (CRP, ascending leucocytes), as vaginal cream in case of positive vaginal smears, prophylactically in case of premature rupture of membranes, and as i.v. therapy in case of ruptured membranes associated with rising infectious indices. The second group comprised 15 women near term (38–40 weeks gestation) who had not undergone spontaneous labour. The patients in that group had a primary or repeated caesarean delivery before the onset of labour because of breech presentation or cephalopelvic disproportion. The third group consisted of 17 women near term (38–42 weeks gestation) who had delivered vaginally after labour induction with labour-promoting drugs like prostaglandin E2 gel because of medical indications (gestational diabetes, preterm rupture of fetal membranes, prolonged pregnancy or fetal heart distress). Dinoproston (0.5 mg or 1 mg) was administered as intracervical or vaginal gel every 6 h at most two times a day. In the fourth group 25 women delivered vaginally after spontaneous onset of normal term labour (38–42 weeks gestation).
Isolation of myelomonocytic cells
Human umbilical cord blood (10 ml) was collected into tubes containing EDTA immediately after delivery. Peripheral blood leucocytes were prepared by lysis of whole blood using FACS Lysing Solution (Becton Dickinson, Heidelberg, Germany). Myelomonocytic cell fractions consisting of monocytes, granulocytes and natural killer (NK) cells were isolated using MACS techniques. About 1 × 107 leucocytes were centrifuged at 300 g and resuspended in 80 μl PBS supplemented with 0.5% bovine serum albumin (BSA) and 5 mm EDTA. The cells were labelled with 20 μl super-paramagnetic Micro Beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) conjugated to rat anti-human CD11b-IgG2b MoAbs for 15 min at 4°C. Cells were separated using columns of type RS + (Miltenyi Biotec GmbH), which were placed in the magnetic field of the Variomacs separator (Miltenyi Biotec GmbH). The columns were washed with buffer solution before use and the labelled cells were applied on top of the column. The magnetically labelled CD11b+ cells were retained in the column, while the unlabeled CD11b− cells passed through. After washing the columns with 2 ml buffer solution, the columns were removed from the magnetic field of the Variomacs separator, and the retained CD11b+ cells were flushed out with 2 ml buffer solution. The CD11b+ and the CD11b− cell populations were reanalysed to determine their purity after sorting, which was expressed as a percentage of total viable cells. For that, 5 × 105 cells of both fractions were labelled with 20 μl fluorescein-conjugated mouse anti-human CD11b MoAb (CAMON, Wiesbaden, Germany) in 80 μl PBS for 30 min at 4°C. After washing, cells were analysed using a flow cytometer (Becton Dickinson, San Jose, CA). Both fractions of CD11b+ and CD11b− cells were washed twice with PBS, and counted after trypan blue staining. Aliquots of 5 × 105 cells were resuspended in 1 ml of RPMI medium (Gibco BRL, Eggenstein, Germany), supplemented with 10% fetal calf serum (FCS), 1% glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, and kept in suspension culture. Aliquots of culture supernatant for cytokine detection were taken after 24 h of incubation at 37°C and 5% CO2 atmosphere. The supernatants were frozen at 80°C until assayed. Concentrations of IL-6, IL-1β and TNF-α were determined, using ELISA kits (BIOSOURCE Europe, S.A., Ratingen, Germany) according to the manufacturer's instructions. Medium controls, incubated under the same conditions, contained no detectable amounts of cytokines. The immunoassay's interassay and intra-assay coefficients of variations were < 9%. The sensitivity of the IL-1β and TNF-α assay was 1 pg/ml and of the IL-6 assay was 2 pg/ml.
Isolation of T cells
Human umbilical CB (10 ml) was collected into tubes containing EDTA immediately after delivery. Mononuclear cells were separated by Ficoll density gradient centrifugation. T cells were isolated after labelling cells with MicroBeads (Miltenyi Biotec GmbH) conjugated to rat mouse–human CD3 antibodies and application of the MACS technique described above. Purity of T cells after sorting was analysed using fluorescein-conjugated mouse anti-human CD3 MoAb (Coulter-Immunotech Diagnostics, Hamburg, Germany). Cells (5 × 105) were cultured in RPMI medium and aliquots of culture supernatant for cytokine detection were taken after 24 h of incubation at 37°C and 5% CO2 atmosphere. The supernatants were frozen at −80°C until assayed. Concentrations of interferon-gamma (IFN-γ), IL-2 and IL-6 were determined, using ELISA kits (BIOSOURCE, Europe, S.A.) according to the manufacturer's instructions. The immunoassay's interassay and intra-assay coefficients of variations were < 9%. The sensitivity of the IFN-γ was 4 pg/ml and of the IL-2 assay was 9 pg/ml.
Intracellular cytokine staining and phenotype analysis
Human umbilical cord blood (10 ml) was collected into tubes containing EDTA from term neonates after spontaneous onset of labour immediately after vaginal delivery. Mononuclear cells (MNC) were separated by Ficoll density gradient centrifugation and cytofluorometric studies were performed using FITC- and PE-conjugated murine anti-human MoAbs. FITC–CD11b, FITC–CD14, FITC–CD15 and FITC–CD56 were purchased from Coulter-Immunotech Diagnostics. PE–IL-6 and PE–IgG1 (isotype control) were purchased from PharMingen (Hamburg, Germany). Determination of IL-6-producing cell types was done using two-colour flow cytometry. The surface phenotype of MNC was estimated by staining 1 × 106 cells with 0.5 μg of FITC-conjugated MoAb specific for cell surface antigens such as CD11b, CD14, CD15 and CD56 for 30 min at 4°C. Cells were washed twice with PBS supplemented with 0.5% FCS/5 mm EDTA and centrifuged. Intracellular staining for IL-6 was done using Cyto Stain Kits purchased from PharMingen according to the manufacturer's instructions. Briefly, cells were fixed and permeabilized by incubating cells in 250 μl Cytofix/Cytoperm solution for 20 min at 4°C and resuspended in 50 μl Perm/Wash solution containing 0.1 μg of rat anti-human IL-6 IgG1 MoAb. Cells were incubated at 4°C for 30 min. Negative staining controls were done using an IgG1 isotype control of irrelevant specificity in the same concentration of anti-IL-6 MoAb. Staining was analysed by flow cytometry (FACScan; Becton Dickinson).
Statistical analysis
The statistical significance of the differences between groups was evaluated by the non-parametric H-test of Kruskal–Wallis, which is used for simultaneous comparison of more than two sample populations. Each H-test was followed by a Dunn test. P < 0.05 was considered significant.
RESULTS
Spontaneous term labour but not preterm labour or induced labour is associated with activation of fetal myelomonocytic cells
In this study subpopulations of cord blood cells, obtained from neonates after spontaneous term labour, elective caesarean delivery in the absence of labour, induced labour, and uncontrollable preterm labour, were fractionated on the basis of CD11b and CD3 expression, using MACS technology. CD11b+ cell fractions consisting of monocytes, granulocytes and NK cells, as well as CD3+ T cells, were cultured and analysed for release of IL-1β, TNF-α, IL-6 and IFN-γ, IL-2, IL-6, respectively. Thereby, 64% (32–87%) of unfractionated cord blood cells were identified as CD11b+ myelomonocytic cells and 33% (7–52%) were identified as CD3+ T cells. Purity after sorting was 99% (98–99%) for myelomonocytic cells and 81% (55–94%) for T cells. The lesser purity of T cell preparations may be due to the small number of CD3+ T cells within a high number of CD3− cells in whole blood samples which contain mainly granulocytes.
Cell cultures of fetal myelomonocytic cord blood cells obtained from neonates in all four groups produced only small amounts of IL-1β and TNF-α, and with regard to the status of labour, there were no significant differences in cytokine release between these groups. Surprisingly, myelomonocytic cells obtained from neonates after spontaneous onset of normal term labour and vaginal delivery released strongly elevated amounts of IL-6 (median 320 pg/ml, range 80–1659 pg/ml) in comparison with myelomonocytic cells obtained after preterm delivery due to uncontrollable labour (median 7 pg/ml, range 0–430 pg/ml; P < 0.001) and term delivery after caesarean section in the absence of labour (median 15 pg/ml, range 0–218 pg/ml; P < 0.001) (Fig. 1).
Fig. 1.
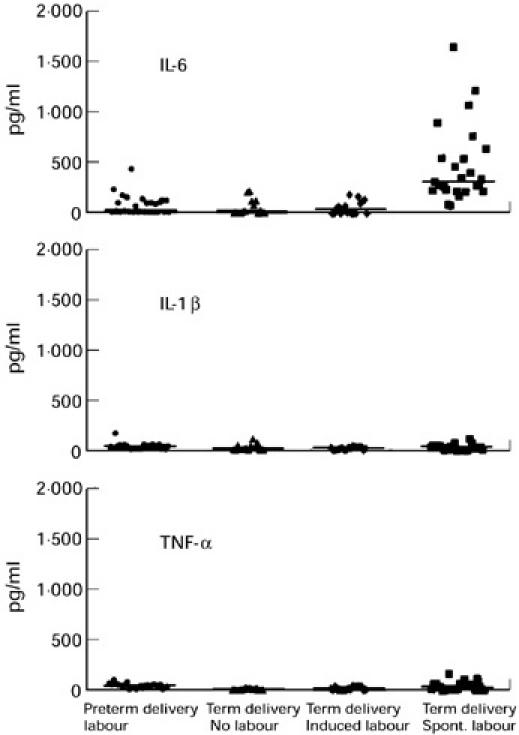
Myelomonocytic cell populations were isolated from cord blood by magnetic cell-sorting (MACS) technique on the basis of CD11b expression. CD11b+ cell fractions were cultured for 24 h and concentrations of IL-6, IL-1β and tumour necrosis factor-alpha (TNF-α) were estimated in the culture supernatants. Cord blood specimens were obtained from 27 neonates after preterm delivery (26–36 weeks gestation) with resistance to tocolysis, 15 neonates had an elective caesarean delivery at term without any labour (30–40 weeks gestation), 17 neonates delivered vaginally at term after induction of labour (38–42 weeks gestation) and 25 neonates delivered vaginally at term after the onset of normal spontaneous term labour (38–42 weeks gestation).
Cell cultures of myelomonocytic cord blood cells obtained from neonates after vaginal delivery and spontaneous onset of normal term labour also produced significantly higher amounts of IL-6 in comparison with cord blood cells obtained from neonates after induced labour and vaginal delivery (median 46 pg/ml, range 0–189 pg/ml; P < 0.001). There were no significant differences in IL-6 production of cord blood cells obtained from term neonates after elective caesarean delivery and term neonates delivered vaginally after labour induction (P = 0.982). Also, no significant differences in IL-6 production of these cells were detectable between neonates delivered preterm because of uncontrollable labour with resistance to tocolysis and term neonates delivered after caesarean section (P = 0.908) or term neonates delivered vaginally after labour induction (P = 0.783) (Fig. 1).
Isolated cord blood T cells of these four patient groups did not release detectable amounts of IFN-γ or IL-2, and IL-6 production of these cells was also below the detection limit of the assays. For positive control, cytokine release of cord blood T cells was estimated after stimulation with medium containing 1 μg/ml phytohaemagglutinin (PHA) for 72 h.
Spontaneous term delivery is associated with fetal monocyte activation
Two-colour flow cytometry was used to identify IL-6-producing cord blood cells obtained from 12 neonates delivered after spontaneous onset of normal term labour. Thereby, flow cytometric analysis was performed on cells isolated by Ficoll density gradient centrifugation immediately after delivery. To include contaminating granulocytes, the gate was set for both forward and side scatter. For these cells 45.2% were positive for CD11b antigen and 4.4% were positive for both CD11b antigen and intracellular IL-6 expression. CD11b antigen is present in large quantities on monocytes, at intermediate levels on granulocytes and at low levels on NK cells. Therefore, we investigated intracellular IL-6 expression of CD14+ cells (monocytes), CD15+ cells (granulocytes) and CD56+ cells (NK cells). Our results indicate that intracellular IL-6 production was exclusively detectable in CD14+ monocytes, while granulocytes and NK cells were shown to be negative for intracellular IL-6 expression. Thereby, 18.2% of the gated cells expressed CD14 antigen and 3.8% were positive for both CD14 antigen and intracellular IL-6 expression (Table 1,Fig. 2).
Table 1.
Flow cytometric analysis of intracellular IL-6 expression in myelomonocytic cell populations of cord blood cells obtained from neonates (n = 12) after spontaneous onset of normal term labour
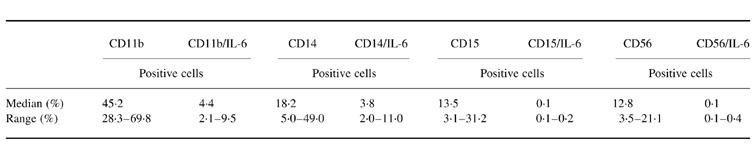
Fig. 2.
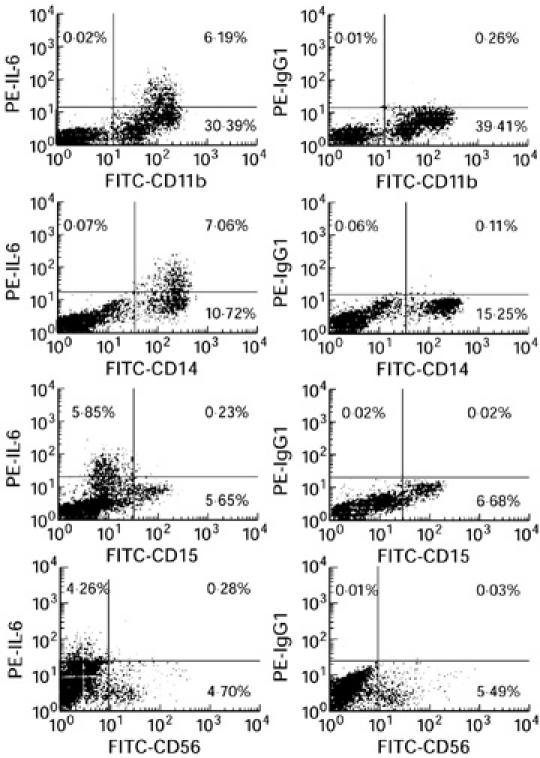
Flow cytometric analysis of IL-6 expression and cell surface phenotype of cord blood cells obtained from neonates after spontaneous term labour and vaginal delivery. Intracellular IL-6 expression was exclusively detectable in CD14+ monocytes, while CD15+ granulocytes and CD56+ natural killer (NK) cells were negative for IL-6 expression. Negative staining controls were done using an IgG1 isotype control of irrelevant specificity at the same concentration of the IL-6 MoAb, and are shown on the right.
DISCUSSION
In the present study we have documented that normal spontaneous term labour is associated with increased production of IL-6 by fetal myelomonocytic cell populations, and two-colour flow cytometry was used to identify fetal monocytes as sources of elevated cytokine release. These findings suggest that extensive activation of the fetal macrophage/monocyte system is involved in labour-promoting mechanisms, and that increased IL-6 levels in the fetal circulation contributes to labour induction at term.
Normally, the primary stimulus for antigen-presenting cell (APC) activation and subsequent release of proinflammatory cytokines from these cells is recognition and processing of bacterially derived lipopolysaccharide (LPS) antigens. These conditions may not be realized in all these cases of normal spontaneous term deliveries. Therefore, immunologically mediated mechanisms, based on allograft rejection processes, are probably the cause of fetal monocyte activation. Thereby activation of monocytes is mediated through T cell response reactions, due to differences in HLA antigens of recipient and donor cells. The consequences are extensive release of Th1-type cytokines like IFN-γ and IL-2 from CD4+ T-helper cells and TNF-α from CD8+ cytotoxic T cells, which are able to induce subsequent monocyte activation. However, we were not able to detect any T cell activation in association with spontaneous term labour. It seems likely that fetal T cell responses are not involved in labour-associated monocyte activation, particularly because there are many reports demonstrating a reduced cell-mediated immunity in neonates due to diminished IFN-γ production by cord blood MNC [16, 17]. The underlying factors responsible for defects in NK and LAK cytotoxicity are still unknown. Investigations examining IL-12 and IL-15 expression in LPS-stimulated cord blood and adult blood MNC propose the reduced ability to produce these cytokines as a cause for the decrease in IFN-γ and TNF-α release and cellular immunity [18, 19], but there are also indications that the presence of prostaglandin E2 plays an important role in inhibiting IFN-γ and IL-2 production by naive cord blood T cells and facilitating the development of a Th2-type cytokine production profile [20].
The deficiency of fetal cellular immune functions suggests that the activation of the fetal phagocyte system may be caused by alloantigen recognition processes of maternal T cells infiltrating the fetal circulation in association with spontaneous term labour. Such cell traffic across the placenta may occur at the end of pregnancy due to insufficient function of the placental barrier and may provoke extensive activation of fetal monocytes. These conclusions may also be drawn with regard to reports demonstrating the contamination of cord blood with maternal lymphocytes, which lead to graft-versus-host disease (GVHD) disease in case of utilization of cord blood in bone marrow transplantations, even if the intended donor's neonatal lymphocytes are unresponsive. Thereby, it was shown that transplantable cord blood units carry a high probability of having maternal genetic materials in detectable amounts [21].
Another mechanism inducing IL-6 release from fetal monocytes without participation of T cell responses may be mediated by binding of IgG to Fc receptors on monocyte accessory cells. It was shown that incubation of monocytes purified from peripheral blood mononuclear cells (PBMC) with aggregated immunoglobulin or Fc fragments of immunoglobulin induced IL-6 activity in the culture supernatants of these cells [22]. Cross-linking of Fc receptors on monocytes seems to play an important role in triggering IL-6 production, because in contrast to solid-phase bound mouse IgG, soluble forms of antibody failed to induce IL-6 secretion [23]. Therefore it seems possible that fetal cells infiltrating the maternal circulation at the end of pregnancy may provoke secretion of HLA-specific immunoglobulin from maternal B cells. These antibodies may be bound by Fc receptors on monocytes in the fetal circulation and recognition of fetal immunocompetent cells by Fc receptor-bound immunoglobulin may induce subsequent IL-6 release from fetal monocytes.
Further reports, demonstrating that labour-associated leucocytosis is selective for neutrophils, monocytes and NK cells [24], and reported findings demonstrating elevated levels of LPS binding protein and soluble CD14 in cord blood and amniotic fluid of women in term labour [25], also indicate participation of fetal monocyte reactions in association with spontaneous term labour. Furthermore, in vitro experiments investigating GVH potential of cord blood cells after stimulation with allogeneic cells demonstrated a 5–27-fold reduction of IFN-γ release and a 50% determination of IL-2 production; however, an increase in IL-6 release by cord blood cells was observed in comparison with the controls [26].
Our results propose extensive fetal monocyte activation and IL-6 release in association with spontaneous term labour, but not in the case of elective caesarean delivery in the absence of labour or induced term labour. In agreement with our findings are reports demonstrating significantly increased plasma levels of IL-6 in cord blood of babies born by vaginal delivery in comparison with those born by elective caesarean section [27]. We did not find any fetal monocyte activation in association with uncontrollable preterm labour, although there are reports suggesting that premature parturition is characterized by in utero activation of the fetal immune system. It was shown that fetuses who were delivered prematurely because of uncontrollable preterm labour had an increase in populations of cells bearing markers associated with activation of the monocyte-neutrophil system [28]. Most of our preterm delivering patients received glucocorticoids to induce maturation of the fetal lungs. Glucocorticoids normally act as immunosuppressive drugs, mainly via their effects on APC. They are known to influence production of cytokines as well as expression of surface molecules necessary for transmission of costimulatory signals. Seven patients, who did not receive glucocorticoid treatment, also did not show any increased cytokine release in association with preterm labour. Therefore, we conclude that low cytokine release by monocytes isolated from preterm delivering fetuses may not be caused by suppressive effects of glucocorticoids given to the mothers. Concerning causes of uncontrollable preterm labour, it seems much more likely that inflammatory reactions of the maternal immune system are responsible for labour onset, rather than participation of fetal immune responses. This condition may be realized in the case of maternal infections, but inadequate maternal immune recognition of fetal alloantigens may also be able to provoke extensive inflammatory responses of maternal immunocompetent cells. Normal pregnancy is characterized by a lack of maternal cell-mediated anti-fetal immunity and a dominant humoral immune response [29]. Thereby, Th1-type responses are systemically suppressed during the normal course of pregnancy, while Th2-type responses are obviously occurring within fetal tissues, and there seems to be a shift in the balance of cytokine profiles away from Th1-type reactivity to a Th2-type reactivity [30, 31]. This mechanism may offer an explanation for the fact that pregnancy exerts suppressive effects on a number of chronic inflammatory conditions such as rheumatoid arthritis. Remission of symptoms was shown to be due to a depression of polymorphonuclear leucocyte functions such as respiratory burst activity, intracellular H2O2 production and NADPH oxidase activity [32]. In contrast, activation of maternal leucocytes was demonstrated in normal third-trimester pregnancy and in preeclampsia. Granulocytes and monocytes from normal pregnant women and women with preeclampsia showed significantly higher surface expression of CD11b, CD14 and CD64, and also increased intracellular reactive oxygen species in comparison with non-pregnant women [33].
Participation of maternal immune reactions in labour-promoting mechanisms has not been established. Possibly uncontrollable preterm labour is mediated by the induction of an inappropriately occurring Th1-type reaction of the maternal immune system. It seems that fetal host defence mechanisms play an important role in modulating the onset of spontaneous term but not preterm labour. Further investigations of both fetal and maternal immune response mechanisms may be necessary to understand the complex reactions responsible for the aetiology of uncontrollable preterm labour.
REFERENCES
- 1.Santhanam U, Avila C, Romero R, Vinguet H, Ida N, Sakurai S, Sehgal PB. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–63. doi: 10.1016/1043-4666(91)90037-e. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Copeland D, Cotton DB. Interleukin-1α and interleukin-1β in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–23. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor-necrosis-factor-α in term and preterm labour. Am J Obstet Gynecol. 1992;166:1576–87. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 4.Cherouny PH, Pankuch GA, Romero R, Botli JJ, Kuhn DC, Demers LM, Appelbaum PC. Neutrophil attractant, activating peptide-1, interleukin–8: association with histologic chorioamnionitis, preterm delivery and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 5.Opsjon SL, Wathen NC, Tingulstad S, Wiedswang G, Sundan A, Waage A, Austgulen R. Tumor-necrosis-factor, interleukin-1 and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 6.Steinborn A, Kühnert M, Halberstadt E. Immunmodulating cytokines induce term and preterm parturition. J Perinatal Med. 1996;24:381–90. doi: 10.1515/jpme.1996.24.4.381. [DOI] [PubMed] [Google Scholar]
- 7.Steinborn A, Günes H, Halberstadt E. Signal for term parturition is of trophoblast and therefore of fetal origin. Prostaglandins. 1995;50:237–52. doi: 10.1016/0090-6980(95)00138-7. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis and chorioamnion infection. Obstet Gynecol. 1993;81:941–8. [PubMed] [Google Scholar]
- 9.Hitti J, Krohn A, Patton DL, Tarczy-Hornoch P, Hillier SL, Cussen EM, Eschenbach DA. Amniotic fluid tumor-necrosis-factor-α and the risk of respiratory distress syndrome among preterm infants. Am J Obstet Gynecol. 1997;177:50–56. doi: 10.1016/s0002-9378(97)70437-x. [DOI] [PubMed] [Google Scholar]
- 10.Steinborn A, Günes H, Röddiger S, Halberstadt E. Elevated placental cytokine release, a process associated with preterm labour in the absence of intrauterine infection. Obstet Gynecol. 1996;88:534–9. doi: 10.1016/0029-7844(96)00224-4. [DOI] [PubMed] [Google Scholar]
- 11.Baergen R, Benirschke K, Ulich TR. Cytokine expression in the placenta. The role of interleukin-1 receptor antagonist expression in chorioamnionitis and parturition. Arch Pathol Lab Med. 1994;118:52–55. [PubMed] [Google Scholar]
- 12.Simon C, Frances A, Piquette G, Hendrickson M, Milki A, Polan ML. Interleukin-1 system in the materno-trophoblast unit in human implantation: immunohistochemical evidence for autocrine/paracrine function. J Clin Endocrinol Metab. 1994;78:847–54. doi: 10.1210/jcem.78.4.8157710. [DOI] [PubMed] [Google Scholar]
- 13.Stallmach T, Hebisch G, Joller-Jemelka HI, Orban P, Schwaller J, Engelmann M. Cytokine production and visualized effects in the feto-maternal unit. Quantitative and topographic data on cytokines during intrauterine disease. Lab Invest. 1995;73:384–92. [PubMed] [Google Scholar]
- 14.Steinborn A, von Gall C, Hildenbrand R, Stutte HJ, Kaufmann M. Identification of placental cytokine-producing cells in term and preterm labour. Obstet Gynecol. 1998;91:329–35. doi: 10.1016/s0029-7844(97)00680-7. [DOI] [PubMed] [Google Scholar]
- 15.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labour and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 16.Scott ME, Kubin M, Kohl S. High levels of interleukin-12 production, but diminished interferon-γ production by cord blood mononuclear cells. Pediatric Research. 1997;41:547–53. doi: 10.1203/00006450-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Sautois B, Fillet G, Beguin Y. Comparative cytokine production by in vitro stimulated mononucleated cells from cord blood and adult blood. Exp Immunol. 1997;25:103–8. [PubMed] [Google Scholar]
- 18.Quian JX, Lee SM, Suen Y, Knoppel E, van de Ven C, Cairo MS. Decreased interleukin-15 from activated cord versus adult peripheral blood mononuclear cells and the effect of interleukin-15 in upregulating antitumor immune activity and cytokine production in cord blood. Blood. 1997;90:3106–17. [PubMed] [Google Scholar]
- 19.Lee SM, Suen Y, Chang L, et al. Decreased interleukin-12 from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-γ, natural killer and lymphokine-activated killer activity by interleukin-12 in cord blood mononuclear cells. Blood. 1996;88:945–54. [PubMed] [Google Scholar]
- 20.Katamura K, Shintaku N, Yamauchi Y, Fukui T, Ohshima Y, Mayumi M, Furusho K. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-γ and IL-2 but not IL-4 and IL-5. J Immunol. 1995;155:4604–12. [PubMed] [Google Scholar]
- 21.Scaradavou A, Carrier C, Mollen N, Stevens C, Rubinstein P. Detection of maternal DNA in placental/umbilical cord blood by locus-specific amplification of noninherited maternal HLA gene. Blood. 1996;88:1494–500. [PubMed] [Google Scholar]
- 22.Ling ZD, Ziltener HJ, Webb BT, Matheson DS. Aggregated imunoglobulin and Fc fragment of IgG induce IL-6 release from human monocytes. Cell Immunol. 1990;129:95–103. doi: 10.1016/0008-8749(90)90189-x. [DOI] [PubMed] [Google Scholar]
- 23.Krutmann J, Kirnbauer R, Kock A, Schwarz T, Schopf E, May LT, Sehgal PB, Luger TA. Cross-linking Fc-receptors on monocytes triggers IL-6 production. Role in anti-CD3-induced T cell activation. J Immunol. 1990;145:1337–42. [PubMed] [Google Scholar]
- 24.Thilaganathan B, Meher-Homji N, Nicolaides KH. Labour: an immunologically beneficial process for the neonate. Am J Obstet Gynecol. 1994;171:1271–2. doi: 10.1016/0002-9378(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 25.Roos T, Martin TR, Ruzinski JT, Leturcq DJ, Hillier SL, Paton DL, Eschenbach DA. Lipopolysaccharide binding protein and soluble CD14 receptor protein in amniotic fluid and cord blood in patients at term. Am J Obstet Gynecol. 1997;177:1230–7. doi: 10.1016/s0002-9378(97)70044-9. [DOI] [PubMed] [Google Scholar]
- 26.Milosevits J, Pocsik E, Schmidt B, et al. Immunophenotypic and functional characteristics of haemapoietic cells from human cord blood. Scand J Immunol. 1995;42:493–500. doi: 10.1111/j.1365-3083.1995.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 27.Buonocore G, De Filippo M, Gioia D, Picciolini E, Luzzi E, Bocci V, Bracci R. Maternal and neonatal plasma cytokine levels in relation to mode of delivery. Biology of the Neonate. 1995;68:104–10. doi: 10.1159/000244225. [DOI] [PubMed] [Google Scholar]
- 28.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, Bianchi DW. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173:1315–20. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 29.Wegmann TG, Linn H, Guilert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 31.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–82. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 32.Crouch SPM, Crocker IP, Fletcher J. The effect of pregnancy on polymorphonuclear leukocyte function. J Immunol. 1995;155:5436–43. [Google Scholar]
- 33.Sacks GP, Studena K, Sargent IL, Redman WG. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]


