Abstract
T helper (Th) responses are mediated in part by immunoregulatory cytokines and the signals delivered by the costimulatory CD28–B7 pathway. In this study, we have investigated the relationship between the regulation of B7 isoform expression on antigen-presenting cells from HIV+ individuals and the production of Th cytokines. The level of expression of both B7.1 and B7.2 isoforms as measured by mean channel fluorescence was significantly decreased on freshly isolated monocytes from HIV+ individuals compared with HIV− controls. However, the levels of expression of B7.1 and B7.2 on both B cells and monocytes increased significantly following culture in HIV+ individuals compared with HIV− controls. B7 expression is subject to regulation by immunoregulatory cytokines. Therefore, we analysed the regulation of B7 expression by cytokines, namely IL-10 and tumour necrosis factor-alpha (TNF-α), the production of which is enhanced in HIV infection and have similar inhibitory effects on B7 expression. Two groups of HIV+ individuals were distinguished on the basis of the inhibitory effect of IL-10 and TNF-α on monocyte B7.2 expression. IL-10 inhibited B7.2 expression on monocytes from some HIV+ individuals (termed responders) like the HIV− controls. However, in a subset of HIV+ individuals (non-responders) this inhibitory effect was lost. Loss of inhibition of B7.2 expression by IL-10 was associated with significantly reduced IL-2 production by phytohaemagglutinin (PHA)- stimulated peripheral blood mononuclear cells (PBMC). These observations showing an association of B7 dysregulation on monocytes and B cells with altered production of IL-2 may have implications in HIV immunopathogenesis.
Keywords: HIV-1, B7, cytokines
INTRODUCTION
Infection of CD4+ T cells and monocytes with HIV initiates a series of complex events within the cellular immune system which ultimately result in depletion of CD4+ T cells, dysregulation of T helper (Th) cytokines and impaired immune function [1]. Even before depletion of CD4+ T cells is detected, immune alterations evidenced by loss of proliferative responses to specific recall antigens have been observed [2, 3]. This deficiency in proliferative responses has been attributed at least in part to the loss of IL-2 production by T cells [2, 3]. It is now well established that T cells require two distinct signals to become activated. The first signal is delivered by recognition of antigen–MHC complex by the T cell receptor. The second signal, the interaction of CD28 with B7, provides a costimulatory signal for T cell activation and proliferation that results in IL-2 mRNA stabilization and accumulation [4, 5]. The loss of T cell proliferation in response to antigen/mitogens may be attributed at least in part to the imbalance in the signals delivered to T cells via the CD28–B7 pathway.
In HIV infection, the findings of altered expression of CD28 on CD8+ T cells [6] and the inability of CD28+CD8+ T cells to produce IL-2 provide evidence for impaired signals delivered to the T cells via the CD28–B7 pathway [6, 7]. Dysregulation of B7 receptors on antigen-presenting cells (APC) may also result in altered immune responses. B7 exists in at least three different forms, namely B7.1, B7.2 and B7.3, the first two of which have been extensively studied [5, 8]. It has been suggested that B7 receptors play a vital role in the development of Th responses [9–11]. While both B7.1 and B7.2 can provide costimulatory signals, whether or not B7.1 and B7.2 provide distinct signals is controversial. While several studies have failed to find functional differences between B7.1 and B7.2, other studies support the notion that B7.1 and B7.2 may provide qualitatively different signals [5, 9–11]. Differential roles of B7.1 and B7.2 in T cell development have also been suggested by in vivo studies [9–11]. In experimental allergic encephalomyelitis, a condition believed to depend on exaggerated Th1-type immunity, blockade of B7.1 by anti-B7.1 antibodies results in the generation of Th2 cells and reduction in disease severity. In contrast, treatment with anti-B7.2 antibodies results in the generation of Th1 responses and increased disease severity [10]. In another study of a non-obese diabetic mouse model for insulin-dependent diabetes mellitus, a condition also involving Th1-type immunity, anti-B7.2 antibodies prevented the onset of disease whereas anti-B7.1 antibodies resulted in more rapid onset and severe disease [9]. The reason for contradictory results in these studies are not understood, but clearly indicate that skewing the balance of B7 isoform signalling towards B7.1 or B7.2 may modulate Th responses.
Alterations in B7 expression on APC induced by cell activation or by immunoregulatory cytokines present in the microenvironment may influence B7-dependent biological activity. We have shown that cytokines which induce the development of Th2 responses (IL-4, IL-10) down-regulate B7.2 and moderately enhance B7.1 expression on monocytes. In contrast, interferon-gamma (IFN-γ), a cytokine that induces the development of Th1 responses, enhances the expression of both B7.1 and B7.2 isoforms on monocytes [12]. Imbalance in the regulation of Th1 and Th2 cytokines may be associated with alterations in B7 expression and the costimulatory signals that they deliver. In HIV infection, several reports have suggested up-regulation of Th2 cytokines [13, 14], though there have been reports contrary to this hypothesis [15–19]. Constitutive production of IL-10 has been shown to be enhanced especially in patients with < 400 CD4+ T cells/μl [15]. Altered production of IL-10 in HIV infection may be of significance, since the immunoregulatory effects of this cytokine may be mediated through B7 [20] and MHC class II molecules [21]. The expression of B7 isoforms on various APC such as monocytes and B cells, and the role of these isoforms in HIV immunopathogenesis, is not known. In this study, we analysed the modulation of B7 expression on monocytes and B cells of HIV+ individuals by cytokines, namely IL-10 and TNF-α, the production of which is also enhanced in HIV infection and have similar inhibitory effects on B7 expression. The effects of IL-10 and TNF-α on the expression of B7.1 and B7.2 on monocytes and B cells of HIV+ individuals were correlated with alterations in Th cytokine production.
PATIENTS AND METHODS
Isolation and culture of peripheral blood mononuclear cells, culture medium and reagents
Blood was obtained for isolation of peripheral blood mononuclear cells (PBMC) from healthy adult volunteers and HIV+ individuals with CD4+ T cell counts ranging from 100 to 600 cells/μl following approval of the protocol by the Ethics Review Committee of the Ottawa General Hospital (University of Ottawa, Ottawa, Ontario, Canada). Blood was collected in tubes containing acid citrate dextrose (ACD) as anticoagulant. All patients were Epstein–Barr virus (EBV)-seropositive but had no clinical evidence of infectious mononucleosis or EBV-related malignancies. All patients were receiving reverse transcriptase inhibitors, and none had clinical evidence of acute infection at the time of specimen collection. PBMC were isolated by density gradient centrifugation over Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) as previously described [15, 22]. The cell layer consisting mainly of mononuclear cells was collected and washed three times in PBS. PBMC were resuspended at a concentration of 2 × 106 cells/ml in Iscove's modified Dulbecco's medium (IMDM; Sigma Chemical Co., St Louis, MO) supplemented with 10% fetal bovine serum (FBS; Gibco Labs, Grand Island, NY), 100 U/ml penicillin, 100 μg/ml gentamycin, 10 mm HEPES and 2 mm glutamine. IL-10 and TNF-α were purchased from R&D Systems (Minneapolis, MN).
Cell stimulation and collection of culture supernatants
PBMC (2 × 106 cells/ml) were stimulated with either anti-CD3 antibodies (anti-CD3 antibody-secreting hybridoma, OKT 3, obtained from American Type Culture Collection (ATCC, Rockville, MD), 1 : 200 final dilution of the culture supernatant) or phytohaemagglutinin (PHA; 1 : 50 final dilution) in the presence or absence of IL-10 or TNF-α (1 ng/ml) in 24-well tissue culture plates (Falcon, Becton Dickinson, Lincoln Park, NJ). The supernatants were harvested after 24 h, 48 h and 72 h and frozen at −70°C. Initial kinetics experiments demonstrated that peak levels of IL-10 were produced after 48 h of cell culture (data not shown) and therefore in subsequent experiments cytokine production was assayed after 48 h of culture. Supernatants were thawed at the time of analysis for measurement of cytokine production by ELISA.
Measurement of cytokines in the culture supernatants
IL-2, IL-4, IL-10, IL-12 and IFN-γ were measured in the culture supernatant by ELISA using two different MoAbs which recognize distinct epitopes, as described previously [15, 22]. Briefly, the plates (Nunc Immunomodules, Roskilde, Denmark) were coated overnight at 4°C with the primary antibody at a concentration of 3–5 μg/ml in coating buffer (0.1 m NaHCO3, pH 8.2) as described below. The plates were washed with PBS–Tween 20 and blocked with PBS–10% FBS. The cytokines were detected by employing a second biotinylated MoAb in PBS–10% FBS at a final concentration as listed below. Streptavidin-peroxidase was used at a final dilution of 1:1000 (Jackson ImmunoResearch, West Grove, PA). The colour reaction was developed by OPD (Sigma) and hydrogen peroxide and read at 450 nm. Recombinant cytokines were used as standards. The concentrations of the primary antibodies and the secondary biotinylated antibodies used are listed in Table 1.
Table 1.
Primary and secondary monoclonal antibodies used for cytokine analysis
Flow cytometric analysis
Unstimulated PBMC (3 × 106/ml) were cultured in the presence or absence of either IL-10 or TNF-α (1 ng/ml). The B cells and monocytes were analysed for B7.1 and B7.2 expression after 24 h and 48 h of stimulation as described [12]. Briefly, for flow cytometric analysis, cells were washed at the time of harvesting once with PBS–0.05% sodium azide and distributed into flow cytometry tubes (Sarstedt, Numbrecht, Germany). Cells were stained with 5 μl of either PE-labelled anti-B7.1 (Becton Dickinson, Lincoln Park, NJ) or PE-labelled anti-B7.2 (PharMingen, Missisauga, Ontario, Canada) MoAbs and either 2.5 μl of FITC-labelled anti-CD14 MoAbs (Becton Dickinson) for monocytes or 5 μl of Tri-labelled anti-CD19 antibody (Sigma) for B cells. Autofluorescence and isotype controls (IgG1 for B7.1 and IgG2b for B7.2 (Becton Dickinson)) were included (data not shown). For PBMC, two distinct populations (lymphocytes and monocytes) could be visualized. For analysis of B7 expression on monocytes and B cells in PBMC, cells were gated in monocytic and lymphocytic gates, respectively. The gates were set in accordance with the gates obtained with the isotype-matched control antibodies. The gates obtained for the cultured monocytes and the resting monocytes in the whole blood were different because of varying amounts of autofluorescence. B7.1- and B7.2-expressing CD14+ monocytes were observed in distinct clusters. Mean channel fluorescence (MCF) was obtained from the B7.1/B7.2-expressing clusters of CD14+ monocytes. B7.1+ and B7.2+ monocytes were calculated as a percentage of B7.1- or B7.2-expressing monocytes, respectively, in the total number of CD14+ monocytes. Data were acquired on a Coulter EPICS XL Flow Cytometer (Coulter Corp., Hialeah, FL) by counting 5000 events for B cells and monocytes. Data were stored as list mode files for later analysis. Validity of comparisons in the expression levels of B7.1 and B7.2 isoforms between different patient populations was ensured through the use of Standard Brite Flow Cytometric Fluorescence Intensity Standardization beads (Coulter). Dot plot diagrams were analysed using the Epics Elite Flow Cytometric Workstation software (Coulter).
Statistical analysis
Means were compared by the two-tailed Student's t-test. The results are expressed as mean ± s.d.
RESULTS
Expression of B7.1 and B7.2 on monocytes and B cells from HIV− and HIV+ individuals
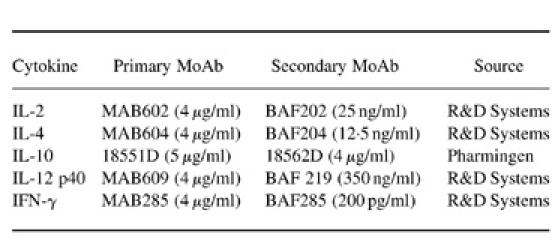
To study the expression of B7 isoforms on monocytes and B cells, whole blood obtained from six HIV− and 20 HIV+ individuals (CD4 counts 100–600/μl) was analysed by two-colour flow cytometry. Most of the monocytes from HIV-infected and uninfected individuals expressed B7.2 isoform (> 95%). The level of B7.1 expression on monocytes of both HIV-infected and uninfected individuals was very low. The number of monocytes expressing B7.1 remained < 10% in both groups of individuals. The number of B cells expressing either B7.1 or B7.2 was also comparable in HIV+ and HIV− individuals. Expression of B7.1 and B7.2 was detected on 13.4 ± 1.9% and 22.6 ± 4.9% B cells in HIV− individuals, and 15.8 ± 2.1% and 22.2 ± 4.4% B cells of HIV+ individuals, respectively. The level of expression of B7.1 and B7.2 receptors on resting monocytes and B cells in whole blood from HIV+ and HIV− individuals was also compared by measuring MCF with concurrent use of Standard Brite Flow Cytometric Fluorescence Intensity Standardization beads (Coulter). The level of B7.1 expression on monocytes and B cells of both HIV+ and HIV− individuals was very low. Interestingly, the expression of both B7.1 (2.2 ± 0.57 versus 7.6 ± 0.58; P < 0.005) and B7.2 (11.6 ± 1.3 versus 24.9 ± 3.1; P < 0.005) was significantly reduced on resting monocytes of HIV+ individuals compared with HIV− controls (Fig. 1a,b). However, the level of expression of both B7.1 (3.3 ± 0.2 versus 4.1 ± 0.2) and B7.2 (4.4 ± 0.3 versus 3.9 ± 0.3) on CD19+ B cells was comparable in HIV+ and HIV− individuals (Fig. 1a,b).
Fig. 1.
Analysis of B7.1 (a) and B7.2 (b) expression on freshly isolated (resting) B cells and monocytes of HIV+ (□) and HIV− individuals (▪) by flow cytometry. Peripheral blood mononuclear cells (PBMC) from six HIV+ and five HIV− individuals were stained with either PE-labelled anti-B7.1 or PE-labelled anti-B7.2 antibodies and counterstained with either FITC-labelled anti-CD14 antibodies for monocytes or Tri-labelled anti-CD19 antibodies for B cells as described in Patients and Methods. The cells in the lymphocytic and monocytic gate were analysed for the expression of B7.1 and B7.2 on B cells and monocytes, respectively. The expression of B7.1 and B7.2 is represented as the mean ± s.d. of mean channel fluorescence (MCF). The comparability in the expression levels of B7.1 and B7.2 isoforms between different patient populations was ensured through the use of Standard Brite Flow Cytometric Fluorescence Intensity Standardization beads (Coulter).
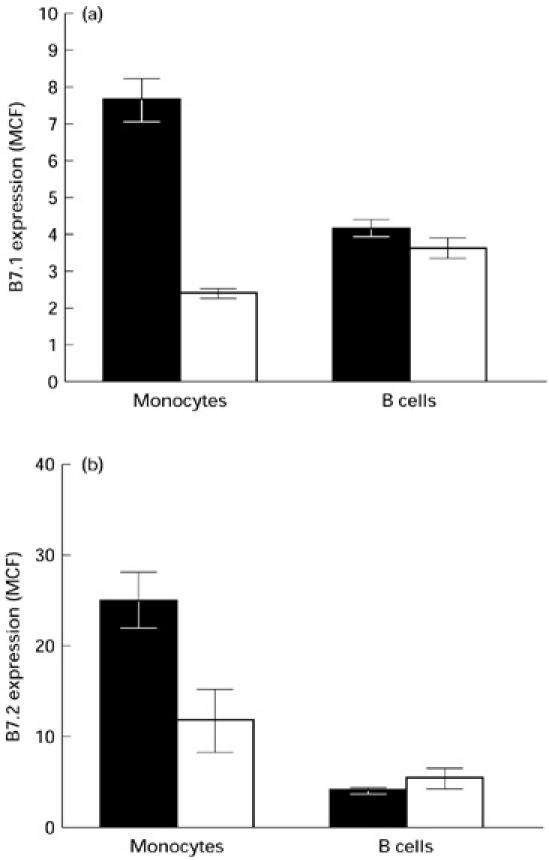
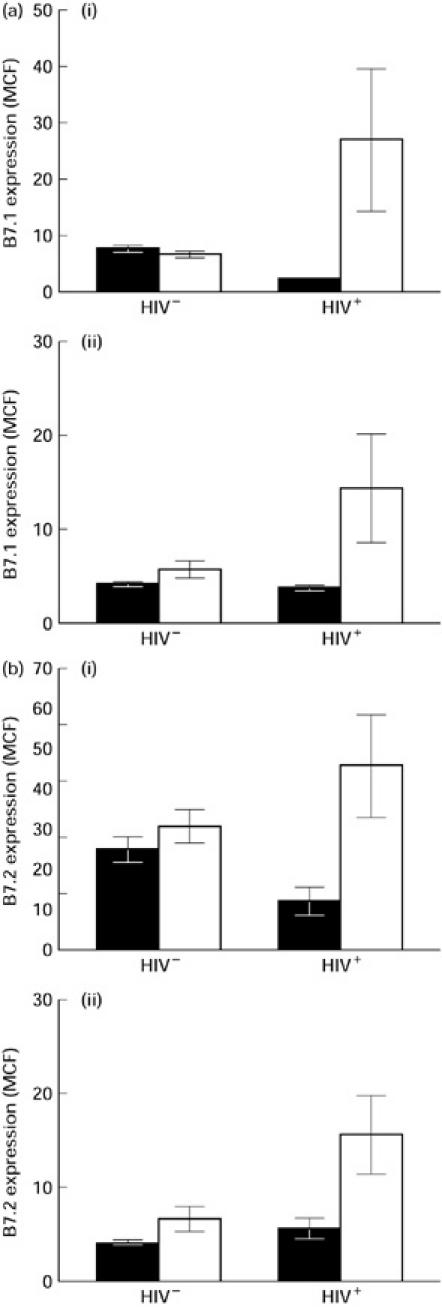
Monocytes become activated following culture in the absence of any exogenous stimulant, and this activation may affect the expression of B7 isoforms. To determine the expression of B7 isoforms on B cells and monocytes following culture, PBMC were isolated from five HIV+ (CD4 counts 100–600/μl) and five HIV− controls and cultured for 24 h in medium alone. In comparing freshly isolated PBMC with PBMC subjected to 24 h of culture, the expression of both B7.1 and B7.2 isoforms was significantly up-regulated following culture on both monocytes and B cells from HIV+ individuals (Fig. 2a,b). MCF of B7.2 expression was 11.7 ± 3.4 on resting monocytes versus 45.5 ± 12.7 on cultured monocytes and 5.4 ± 1.1 on resting B cells versus 15.3 ± 4.2 following culture. MCF of B7.1 expression was 2.4 ± 0.1 on resting monocytes versus 27.1 ± 12.6 on cultured monocytes and 3.6 ± 0.2 on resting B cells versus 14.3 ± 5.7 following culture. However, the level of expression of both B7.1 and B7.2 remained unchanged in resting and cultured monocytes as well as B cells of HIV− individuals (Fig. 2a,b). Thus, although expression of B7.1 and B7.2 is decreased on resting monocytes of HIV+ individuals compared with HIV− controls (Fig. 1), activation of monocytes by overnight culture in the absence of any stimulant enhances the expression of both B7 isoforms on both monocytes and B cells of HIV+ individuals. As this phenomenon was not seen in HIV− controls, these findings support dysregulation of B7 receptors on both monocytes and B cells of HIV+ individuals. Dot plot diagrams depicting the expression of B7.1 and B7.2 on resting and cultured monocytes from representative HIV− and HIV+ individuals are shown in Fig. 3a and 3b, respectively.
Fig. 2.
Analysis of B7.1 (a) and B7.2 (b) expression on monocytes (i) and B cells (ii) from HIV+ and HIV− individuals following activation by overnight culture, as measured by flow cytometric analysis. Peripheral blood mononuclear cells (PBMC) from six HIV+ and five HIV− individuals were cultured overnight in the absence of any stimulus. B cells and monocytes were stained for B7.1 and B7.2 expression as described in the legend to Fig. 1 and in Patients and Methods. The expression of B7.1 and B7.2 is represented as the mean ± s.d. of mean channel fluorescence (MCF). ▪, Resting; □, culture.
Fig. 3.
Dot plot diagrams of B7.1 (a) and B7.2 (b) expression on resting and cultured (activated) monocytes. Peripheral blood mononuclear cells (PBMC) were stained for the expression of B7.1 and B7.2 as described in the legend to Fig. 1. Dot plot diagrams from one representative HIV− and HIV+ individual are shown.
Effects of IL-10 and TNF-α on B7.1 and B7.2 expression on monocytes and B cells of HIV− and HIV+ individuals
Dysregulation of B7 expression on monocytes and B cells of HIV+ individuals was further investigated by studying the effects of immunoregulatory cytokines which are known to influence the expression of B7 isoforms. Several studies have shown that monocyte B7.2 expression is down-regulated by IL-10 and TNF-α in HIV− individuals [12, 23]. We and others have also demonstrated that IL-10 is produced constitutively by PBMC of HIV+ individuals [15]. Furthermore, TNF-α production by PBMC of HIV+ individuals is also increased compared with that of HIV− controls [24]. Excessive production of IL-10 and TNF-α in the microenvironment may influence B7 expression and may thus alter B7-mediated biological activity. To study the effect of IL-10 and TNF-α on B7 isoform expression on monocytes of HIV+ and HIV− individuals, PBMC were cultured in the presence or absence of either IL-10 or TNF-α for 24 h and cells were subsequently analysed by two-colour flow cytometry.
For HIV− control subjects, IL-10 and TNF-α down-regulated the expression of B7.2 on monocytes, as reported previously [12] (Fig. 4a,b). Two groups of HIV+ individuals were distinguished on the basis of the inhibitory effect of IL-10 and TNF-α on monocyte B7.2 expression. IL-10 inhibited B7.2 expression on monocytes from some HIV+ individuals (termed responders) but not from others (termed non-responders). Similar results were obtained for TNF-α (Fig. 4b). Differences in the expression of B7 isoforms were not observed on resting monocytes and B cells and following culture between responders and non-responders (data not shown). The inhibitory effect of IL-10 correlated with circulating CD4+ T cell numbers. The number of CD4+ T cells in the responder group was significantly higher than the numbers in the non-responder group (346 ± 220 in the responders (n = 15) versus 140 ± 67 in the non-responders (n = 15); P < 0.003). These results provide further evidence for dysregulation of B7.2 receptor expression on monocytes of HIV+ individuals. Dot plot diagrams showing B7.2 expression in the presence and absence of IL-10 on monocytes from one representative HIV− individual, HIV+ IL-10 responder and IL-10 non-responder are shown (Fig. 5). Neither IL-10 nor TNF-α affected B7.2 expression on B cells of HIV− and HIV+ individuals (data not shown). IL-10 and TNF-α did not influence B7.1 expression on monocytes or B cells as well (data not shown).
Fig. 4.
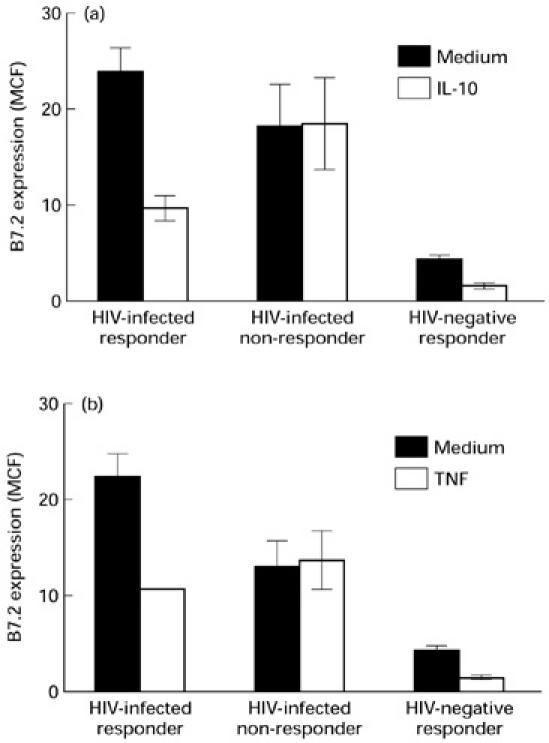
Effect of IL-10 (a) and tumour necrosis factor-alpha (TNF-α) (b) on B7.2 expression on monocytes of HIV+ and HIV− individuals. Peripheral blood mononuclear cells (PBMC) from 10 HIV+ and five HIV− individuals were cultured in the presence or absence of either IL-10 or TNF-α for 24 h. The CD14+ monocytes were analysed for the expression of B7.2 by flow cytometry as described in the legend to Fig. 1 and in Patients and Methods. Monocytes from HIV+ individuals showing inhibition of B7.2 expression (responders) and those showing resistance to the inhibitory effects of IL-10 and TNF-α (non-responders) are shown. The expression of B7.2 is represented as the mean ± s.d. of mean channel fluorescence (MCF).
Fig. 5.
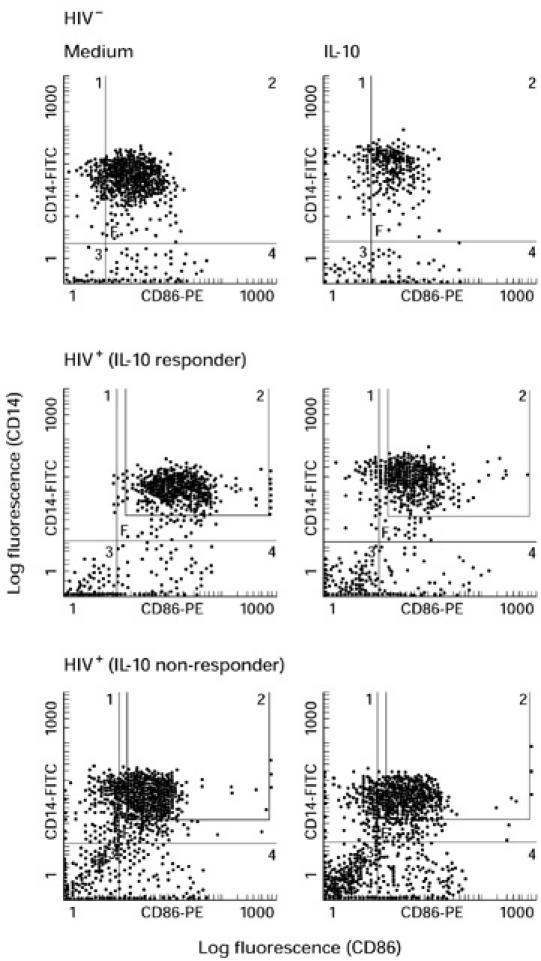
Dot plot diagrams showing the effect of IL-10 B7.2 expression on monocytes. Peripheral blood mononuclear cells (PBMC) from HIV+ and HIV− individuals were cultured in the presence or absence of IL-10 for 24 h and stained for B7.2 expression on monocytes as described in the legends to Figs 1 and 4. Dot plot diagrams of B7.2 expression on monocytes showing inhibition of B7.2 expression (responders) and those showing resistance to the inhibitory effects of IL-10 (non-responders) from representative HIV−, HIV+ responder and HIV+ non-responder individuals are shown.
Loss of inhibitory effect of IL-10 on B7.2 expression on monocytes is associated with the loss of IL-2 production by PHA-stimulated PBMC of HIV+ individuals
Recent evidence suggests that B7 receptors may play a vital role in the development of Th immune responses [9–11]. Our findings of dysregulated B7 expression on monocytes of HIV+ individuals raised the possibility of associated alterations in the production of the immunoregulatory cytokines IL-2, IL-4, IL-10, IL-12 and IFN-γ. Therefore, supernatants from PHA-stimulated PBMC of HIV+ and HIV− individuals were analysed for the production of these cytokines by ELISA. Simultaneously, PBMC from the same individuals were cultured in the presence or absence of IL-10 and analysed for expression of B7.2 on monocytes. PBMC from HIV+ individuals were identified as responders and non- responders based on their susceptibility to the inhibitory effects of IL-10 on B7.2 expression on monocytes. PBMC from HIV+ non-responders (n = 8) produced significantly lower levels of IL-2 than did PBMC from HIV+ responders (n = 7; P < 0.05) and HIV− individuals (n = 9; P < 0.05; Fig. 6). Furthermore, PBMC from HIV+ IL-10 non-responders produced relatively lower levels of IL-4, IL-10, and IFN-γ (Fig. 6), but the differences did not reach statistical significance. IL-10 produced by the non-responders was significantly lower compared with that produced by HIV− individuals (P < 0.05). However, there was no difference in the levels of IL-10 produced by the HIV+ responders and the HIV− individuals. PBMC from both groups of HIV+ individuals produced very low levels of IL-12 and were not significantly different. However, IL-12 produced by both groups of HIV+ individuals was significantly lower than that observed in HIV− individuals (P < 0.005).
Fig. 6.
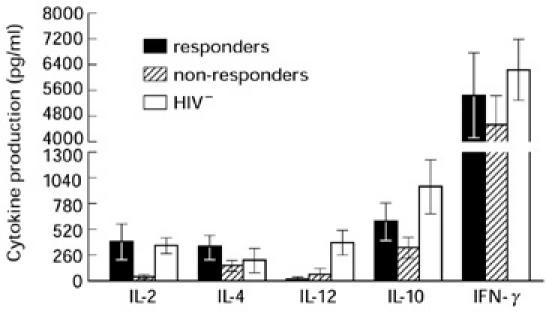
Analysis of Th1 and Th2 cytokines production by peripheral blood mononuclear cells (PBMC) from responder and non-responder HIV+ individuals. PBMC from eight HIV+ individuals showing inhibition of B7.2 expression on monocytes (responders) and those showing resistance to the inhibitory effects of IL-10 (non-responders) were stimulated with phytohaemagglutinin (PHA). The supernatants were harvested after 48 h of culture and were analysed for production of Th1 (IL-2, IL-12 and IFN-γ) and Th2 (IL-4, IL-10) cytokines. The results are reported as pg/ml (mean ± s.d.).
DISCUSSION
In this study we provide evidence for dysregulated B7 isoform expression on monocytes and B cells from HIV+ individuals which is correlated with alterations in IL-2 production. This is supported by a number of findings. The level of expression of both B7 isoforms as measured by MCF was significantly decreased on freshly isolated monocytes from HIV+ individuals compared with HIV− controls. However, the levels of expression of B7.1 and B7.2 on both B cells and monocytes increased significantly following culture in HIV+ individuals compared with HIV− controls. Furthermore, we show that in a subset of HIV+ individuals, IL-10 and TNF-α failed to inhibit B7.2 expression on monocytes, and that this loss of inhibition was correlated with loss of IL-2 production by PHA-stimulated PBMC. These observations suggest that dysregulated expression of B7 isoforms on monocytes and B cells may have implications in HIV immunopathogenesis.
There is evidence to suggest that B7 isoforms play a vital role in the pathogenesis of autoimmune and infectious diseases. Expression of both B7.1 and B7.2 is up-regulated following stimulation with bacterial lipopolysaccharides, whereas Neisserial porins up-regulate B7.2 expression alone [25]. In contrast, infection of monocytes with Mycobacterium tuberculosis and Leishmania donovani inhibits B7.1 expression [26, 27]. B7.1 expression is up-regulated on T cells and APC in experimental allergic encephalitis and blockade of B7.1 with anti-B7.1 antibodies can ameliorate disease severity [9, 10]. Several reports have also shown increased expression of B7.1 on peripheral blood T cells from patients suffering from chronic liver disease and autoimmune diseases [28]. In HIV infection, B7.1 and B7.2 expression on T cells and CD4+ T cell lines was found to be up-regulated [29, 30]. B7.1 expression was also found to be significantly higher on monocytes of HIV+ individuals, particularly those receiving anti-retroviral therapy [31]. In the present study we show that, compared with HIV− controls, freshly isolated monocytes and B cells from HIV+ individuals express lower levels of B7.1 and B7.2 (Fig. 1). Activation of monocytes and B cells by overnight culture in the absence of any stimulant resulted in up-regulation of B7.1 and B7.2 in HIV+ individuals, but not in HIV− controls. Our findings of up-regulation of B7 isoforms on monocytes and B cells from HIV+ individuals by mild stimuli such as overnight culture indicate dysregulation of B7 receptor expression on APC.
The pathophysiological consequences of altered expression of B7 molecules on APC are not clear. Increased expression of B7 on monocytes has been correlated with enhanced APC function [32], and high levels of B7.1 expression on tumour cells transfected with B7.1 were associated with increased susceptibility to natural killer (NK)-mediated lysis [33]. Similarly, interaction of CD4+ T cells with gp120 has been shown to decrease B7.1 expression, resulting in the loss of T cell signals and cell proliferation in response to anti-CD3 antibodies [34]. It has been suggested that over-expression of B7 molecules may break self tolerance and induce an autoimmune state [35, 36]. The question of the immunological consequences of altered B7 expression on APC was addressed using a transgenic mouse model in which B7 receptors were constitutively expressed on B cells [35]. Transgenic mice exhibited loss of immature B cells and loss of CD28 expression on T cells relative to their wild-type littermates, but their antigen-specific cellular responses were not affected [35]. Whether over-expression of B7 on activated monocytes and B cells induces loss of CD28 expression on T cells and development of predominantly CD28− memory T cells in HIV infection [6, 7] remains to be investigated.
B7 expression on APC is influenced by immunoregulatory cytokines. We have previously demonstrated that IL-10 and TNF-α decreased the expression of B7.2 on monocytes of HIV− individuals [12]. IL-10 is produced by Th2, Th0, CD8+ T cells and monocytes, and functions to inhibit cellular immune responses by inhibiting cytokines produced by Th1 cells, T cell proliferation and monocyte functions [21, 37]. It has been shown to induce immune unresponsiveness [38]. The production of IL-10 and TNF-α is significantly enhanced in monocytes of HIV+ individuals and in normal monocytes infected in vitro with HIV [24]. Enhanced production of these cytokines may exert a negative regulatory effect on B7 expression and induce immune unresponsiveness. Here we show that inhibition of B7.2 expression on monocytes by IL-10 and TNF-α was lost in a subgroup of HIV+ individuals. The molecular mechanism underlying the loss of B7 inhibition by IL-10 has not been studied, but may be attributed to the desensitization of monocytes caused by excessive production of IL-10 and TNF-α following HIV infection. Alternatively, HIV regulatory antigens such as tat, nef or rev may specifically modulate IL-10-mediated inhibitory effects on B7 expression. Inhibition of B7.2 expression by either IL-10 or TNF-α was not observed in B cells of either HIV+ or HIV− individuals, a finding which is in agreement with a previous report suggesting that IL-10 inhibits the APC function of monocytes but not of B cells [21].
B7 receptors have also been suggested to play a vital role in the induction of Th cytokines [5, 10, 39]. The physiological significance of the loss of inhibition of B7.2 expression was investigated by comparing the production of Th cytokines in PHA-stimulated PBMC between the responder and non-responder groups of HIV+ individuals. We show here correlation of dysregulation of B7 receptor expression on monocytes with the loss of IL-2 production following PHA stimulation of PBMC. This is in agreement with the proposed role of B7 in inducing the expression of IL-2 [40–42]. We also show that the loss of B7 inhibition was associated with relatively lower levels of IL-4 and IL-10 in the non-responder group. Lower levels of IL-10 produced by non-responders are in agreement with our previous observations [15, 19]. Furthermore, IL-12 production by both the groups was significantly decreased compared with the HIV− individuals, as previously reported [43]. Although we have not observed significant differences in the production of IL-4, IL-10, IL-12 and IFN-γ in the two subgroups, it is likely that the production of some of these cytokines may be impaired in specific cell types, e.g. IL-10 production by T cells or IL-12 production by monocytes. Studies are in progress to determine the expression of the above mentioned cytokines in specific cell types by intracellular staining. The trend towards lower IL-10 and IL-4 production in HIV+ non-responders may indicate the loss of immune responsiveness in these individuals. The current observation of a correlation between IL-2 production and loss of IL-10-induced B7.2 inhibition of expression on monocytes supports an important role for the B7–CD28 pathway in HIV immunopathogenesis.
The status of the patients showing loss of regulation of B7 receptors with respect to the clinical state of HIV infection is not clear. The non-responder group showed lower levels of CD4+ T cells compared with the responder group, indicating that HIV+ non-responders may be further in their HIV disease progression despite the absence of observed differences in measured clinical characteristics. Dysregulation of B7 receptors, loss of IL-2 production and the relative decreased production of other cytokines in the non-responder group would further suggest to be in the advanced disease stage. The present cross-sectional study of asymptomatic HIV+ individuals does not specifically address the effect of virus load on dysregulation of B7 receptor expression over time. HIV+ individuals receiving protease inhibitors, which dramatically reduce virus load, may be the ideal subjects to study this issue. We have previously demonstrated up-regulation of CD28 expression on CD8+ T cells, enhanced production of IL-2 and IL-12, and increased proliferative responses to recall antigens and mitogens in HIV+ patients receiving anti-retroviral therapy [44]. Further studies are necessary to understand the molecular mechanisms responsible for the observed dysregulation of B7 receptors.
Acknowledgments
This work was supported by grants from the Ministry of Health, Ontario, Canada, the Research Institute, Children's Hospital of Eastern Ontario and the Canadian Foundation for AIDS Research (A.K.).
REFERENCES
- 1.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–34. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 2.Clerici M, Hakim FT, Venzon DJ, et al. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:759–65. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerici M, Stocks NI, Zajac RA, et al. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.June CH, Bluestone JA, Nadler LM, et al. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 5.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 6.Brinchmann JE, Dobloug JH, Heger BH, et al. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–8. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 7.Zanussi S, Simonelli C, D'Andrea M, et al. CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin Exp Immunol. 1996;105:220–4. doi: 10.1046/j.1365-2249.1996.d01-746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boussiotis VA, Freeman GJ, Gribben JG, et al. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci USA. 1993;90:11059–63. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenschow DJ, Ho SC, Sattar H, et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7–2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 12.Creery WD, Diaz-Mitoma F, Filion L, et al. Differential modulation of B7-1 and B7-2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273–7. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 13.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 14.Clerici M, Wynn TA, Berzofsky JA, et al. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–75. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Mitoma F, Kumar A, Karimi S, et al. Expression of IL-10, IL-4 and interferon-gamma in unstimulated and mitogen-stimulated peripheral blood lymphocytes from HIV-seropositive patients. Clin Exp Immunol. 1995;102:31–39. doi: 10.1111/j.1365-2249.1995.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyaard L, Hovenkamp E, Keet IP, et al. Single cell analysis of IL-4 and IFN-gamma production by T cells from HIV-infected individuals: decreased IFN-gamma in the presence of preserved IL-4 production. J Immunol. 1996;157:2712–8. [PubMed] [Google Scholar]
- 17.Emilie D, Fior R, Llorente L, et al. Cytokines from lymphoid organs of HIV-infected patients: production and role in the immune disequilibrium of the disease and in the development of B lymphomas. Immunol Rev. 1994;140:5–34. doi: 10.1111/j.1600-065x.1994.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 18.Maggi E, Mazzetti M, Ravina A, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265:244–8. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Angel J, Daftarian MP, et al. Differential production of IL-10 by T cells and monocytes of HIV-infected individuals: association of IL-10 production with CD28-mediated immune responsiveness. Clin Exp Immunol. 1998;114:78–86. doi: 10.1046/j.1365-2249.1998.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, Linsley PS, Huang LY, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 21.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daftarian PM, Kumar A, Kryworuchko M, et al. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- 23.Buelens C, Willems F, Delvaux A, et al. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–72. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 24.Vyakarnam A, Matear P, Meager A, et al. Altered production of tumour necrosis factors alpha and beta and interferon gamma by HIV-infected individuals. Clin Exp Immunol. 1991;84:109–15. doi: 10.1111/j.1365-2249.1991.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetzler LW, Ho Y, Reiser H. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J Exp Med. 1996;183:1151–9. doi: 10.1084/jem.183.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye PM, Rogers NJ, Curry AJ, et al. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol. 1994;24:2850–4. doi: 10.1002/eji.1830241140. [DOI] [PubMed] [Google Scholar]
- 27.Saha B, Das G, Vohra H, et al. Macrophage–T cell interaction in experimental mycobacterial infection. Selective regulation of co- stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol. 1994;24:2618–24. doi: 10.1002/eji.1830241108. [DOI] [PubMed] [Google Scholar]
- 28.Wyss-Coray T, Mauri-Hellweg D, Baumann K, et al. The B7 adhesion molecule is expressed on activated human T cells: functional involvement in T–T cell interactions. Eur J Immunol. 1993;23:2175–80. doi: 10.1002/eji.1830230919. [DOI] [PubMed] [Google Scholar]
- 29.Wolthers KC, Otto SA, Lens SM, et al. Increased expression of CD80, CD86 and CD70 on T cells from HIV-infected individuals upon activation in vitro: regulation by CD4+ T cells. Eur J Immunol. 1996;26:1700–6. doi: 10.1002/eji.1830260806. [DOI] [PubMed] [Google Scholar]
- 30.Haffar OK, Smithgall MD, Bradshaw J, et al. Costimulation of T-cell activation and virus production by B7 antigen on activated CD4+ T cells from human immunodeficiency virus type 1-infected donors. Proc Natl Acad Sci USA. 1993;90:11094–8. doi: 10.1073/pnas.90.23.11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Low P, Weber C, Harrer E, et al. CD80 expression on monocytes in HIV-infected patients. J Acquir Immune Defic Syndr Hum Retrovir. 1997;15:264–8. doi: 10.1097/00042560-199708010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Larsen CP, Ritchie SC, Pearson TC, et al. Functional expression of the costimulatory molecule B7/BB1, on murine dendritic cell populations. J Exp Med. 1992;176:1215–20. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu TC, Huang AYC, Jaffee EM, et al. A reassessment of the role of B7-1 expression in tumor rejection. J Exp Med. 1995;182:1415–21. doi: 10.1084/jem.182.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chirmule N, McCloskey TW, Hu R, et al. HIV gp120 inhibits T cell activation by interfering with expression of costimulatory molecules CD40 ligand and CD80 (B71) J Immunol. 1995;155:917–24. [PubMed] [Google Scholar]
- 35.Van Parijs L, Sethna MP, Schweitzer AN, et al. Functional consequences of dysregulated B7-1 (CD80) and B7-2 (CD86) expression in B or T lymphocytes of transgenic mice. J Immunol. 1997;159:5336–44. [PubMed] [Google Scholar]
- 36.von Herrath MG, Guerder S, Lewicki H, et al. Coexpression of B7-1 and viral (‘self’) transgenes in pancreatic beta cells can break peripheral ignorance and lead to spontaneous autoimmune diabetes. Immunity. 1995;3:727–38. doi: 10.1016/1074-7613(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 37.Del Prete G, De Carli M, Almerigogna F, et al. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 38.Groux H, Bigler M, de Vries JE, et al. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JA, Titus RG, Nabavi N, et al. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J Infect Dis. 1996;174:1303–8. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- 40.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman GJ, Borriello F, Hodes RJ, et al. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993;178:2185–92. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiser H, Freeman GJ, Razi-Wolf Z, et al. Murine B7 antigen provides an efficient costimulatory signal for activation of murine T lymphocytes via the T-cell receptor/CD3 complex. Proc Natl Acad Sci USA. 1992;89:271–5. doi: 10.1073/pnas.89.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chehimi J, Starr SE, Frank I, et al. Impaired interleukin 12 production in human immunodeficiency virus- infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angel JB, Kumar A, Parato K, et al. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J Infect Dis. 1998;177:898–904. doi: 10.1086/515244. [DOI] [PubMed] [Google Scholar]


