Abstract
A human thymoma is a neoplasm derived from the thymic epithelial cell, and is well known for its association with autoimmune diseases, especially myasthenia gravis. The neoplastic epithelial cells of thymoma clearly retain thymic epithelial functions, but the development of T cells in thymoma is somewhat impaired. In this study, we quantified by flow cytometry the in vitro expression of MHC molecules on neoplastic epithelial cells precultured with IFN-γ. While MHC class I expression was comparable with that on normal thymic epithelial cells, the level of MHC class II molecules on neoplastic epithelial cells was lower than in controls, and also varied greatly from case to case. Additionally, there was a significant positive correlation between the expression level of MHC class II and the proportion of mature CD3+ cells in the CD4+CD8− subset. Thus, accumulation of CD3−CD4+CD8− cells in thymoma may result from impaired expression of the MHC class II molecules, suggesting that the function of the neoplastic epithelial cells might determine the maturation and the positively selected repertoire of T cells in thymomas.
Keywords: thymoma, thymic epithelial cell, MHC, HLA-DR, T cell differentiation
INTRODUCTION
A human thymoma is a neoplasm derived from thymic epithelial cells [1], and is well known for its association with autoimmune diseases, especially with myasthenia gravis. One of its histological characteristics is the coexistence of large numbers of lymphocytes. The ratio of lymphocytes to neoplastic epithelial cells varies greatly from tumour to tumour, thus thymomas are classified as predominantly lymphocytic, predominantly epithelial, or mixed type [2, 3]. The lymphocytes in thymomas are of T cell lineage [4–6], and a considerable proportion are CD4+CD8+, so called double-positive cells, exactly as in the normal thymic cortex [7–9]. In the murine and the human thymus, CD3−CD4−CD8− cells differentiate to CD3+CD4+CD8− or CD3+CD4−CD8+ cells via CD4+CD8+ cells. Recently, we have shown that the neoplastic epithelial cells from thymoma were able to induce the differentiation of CD4+CD8+ cells from CD3−CD4−CD8−CD34+ T cell precursors in an in vitro culture [10]. Furthermore, mature CD4 single-positive cells expressing CD69 have also been found in thymoma and shown to be capable of proliferating in response to a mitogen [11]. These observations suggest that T cell development takes place even in the neoplastic environment of a thymoma.
We and another group have identified an intermediate CD3−CD4+CD8− stage, so called immature CD4 single-positive cells, between the CD3−CD4−CD8− and the CD3−CD4+CD8+ stages [12, 13]. Interestingly, these CD3−CD4+CD8− cells were shown to accumulate more in thymomas than in normal thymus [14, 15], and did not express CD69 [11], a marker of positive selection of thymocytes, suggesting that T cell development is somewhat impaired in thymomas.
Thymic stromal cells are known to govern T cell development. The interaction between the T cell antigen receptor (TCR) on thymocytes and MHC antigen on thymic epithelium is believed to play an important role in selection of mature thymocytes [16–18]. Previous immunohistochemical analyses have demonstrated a reduced expression of MHC class II molecules on thymoma epithelial cells [19–22]. Thus, it seems reasonable to speculate that the abnormal T cell development in thymomas might be accompanied by the altered selection of newly generated T cells, and this in turn might lead to autoimmune disease [15]. To examine the relation between the function of the neoplastic epithelial cells and T cell development in thymomas, we quantitatively evaluated the surface marker expression of lymphocytes and the expression level of the MHC molecules on neoplastic epithelial cells by flow cytometry. The results of this study clearly demonstrate a correlation between the expression level of MHC class II molecules and the maturation of CD4+CD8− cells in the tumour.
PATIENTS AND METHODS
Patients
The characteristics of the thymoma patients studied here are shown in Table 1. All of the cases were classified according to the clinicopathological staging system of Masaoka [23]. None of these patients had been treated with corticosteroids, induction chemotherapy or preoperative irradiation. Various autoantibodies, including anti-acetylcholine receptor antibody (αAChR), were examined in these patients. Although αAChR was positively detected in cases 2, 5, 6 and 7, myasthenic symptoms were found only in case 2. As controls, normal thymi were obtained from 21 children during open cardiac surgery under written informed consent when resection of a part of the thymus was necessary to perform the surgical procedures. The mean age of the control patients was 2.2 ± 4.2 years.
Table 1.
Characteristics of thymoma patients
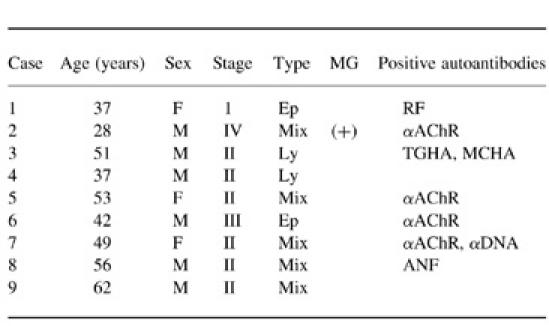
Type, histological type; Ep, predominantly epithelial cell type; Ly, predominantly lymphocytic type; mix, mixed type; MG, myasthenia gravis; RF, rheumatoid factor; αAChR, anti-acetylcholine receptor antibody; TGHA, thyroglobulin haemagglutination; MCHA, microsome haemagglutination; αDNA, anti-DNA antibody; ANF, anti-nuclear factor.
Monoclonal antibodies
For the analyses of lymphocytes, FITC-conjugated control mouse IgG and anti-CD3, PE-conjugated anti-κ-chain and anti-CD4, and biotin-conjugated anti-CD8 antibodies were purchased from Becton Dickinson (San Jose, CA). For the analyses of stromal cells, anti-HLA-ABC antibody was purchased from Pharmingen (San Diego, CA). Anti-HLA-DP/DQ/DR (CR3/43), anti-cytokeratin (MNF116) and FITC-conjugated anti-cytokeratin (MNF116) antibodies were purchased from Dako (Glostrup, Denmark).
Histological evaluation
Surgical specimens were fixed in 10% formaldehyde and then paraffin-embedded. They were classified as predominantly lymphocytic, predominantly epithelial, or mixed type according to the ratio of lymphocytes to epithelial cells by haematoxylin–eosin staining. By definition, the ratio of lymphocytes to epithelial cells was at least 2:1 in the predominantly lymphocytic thymomas and below 1:2 in the predominantly epithelial thymomas. Intermediate cases were classified as the mixed type.
Preparation of lymphocytes
Lymphocytes in the thymoma or thymus were isolated by mechanical teasing and pressing the tissue against stainless steel mesh. The viable lymphocytes were separated on a Ficoll–Hypaque (Lymphoprep; Nycomed, Oslo, Norway) density gradient to remove any erythrocytes and dead cells. These isolated lymphocytes were then counted and applied to the flow cytometric analyses.
Preparation of stromal cells
Stromal cells were isolated from the thymoma or normal child thymus tissue by 2-deoxyguanosine (2-dGuo; Sigma, St Louis, MO) treatment and trypsin–EDTA dispersion [24, 25]. Briefly, fresh thymoma or thymus tissue was minced and the fragments were washed in PBS repeatedly. The tissue fragments were cultured for 5 days in the presence of 1.35 mm 2-dGuo in complete medium (RPMI 1640 in 10% fetal calf serum (FCS), 2 mm glutamine, 5 μm 2-mercaptoethanol) to deplete the lymphocytes. Five days later, the 2-dGuo-treated fragments were rinsed with Ca2+Mg2+-free PBS and incubated in 0.25% trypsin–0.02% EDTA for 30 min at 37°C. The digested fragments were suspended vigorously, and trypsinization was stopped by the addition of an equal volume of 10% FCS–RPMI. After the dispersed cells were cultured for 4 days in complete medium, the adherent stromal cells were removed, followed by a further culture for 2 days in the presence of IFN-γ (1000 U/ml) in complete medium. The adherent cells were collected by vigorous suspension with 0.02% cold EDTA and then washed and applied to flow cytometric analysis.
Flow cytometry
For staining surface antigens on lymphocytes, 1 × 106 lymphocytes suspended in 300 μl PBS were mixed with a combination of 5 μl each of FITC-conjugated control mouse IgG or anti-CD3, PE-conjugated anti-CD4, and biotin-conjugated anti-CD8 antibodies. After incubation for 30 min at 4°C, the cells were washed and suspended in 300 μl PBS. One microlitre of streptavidin-red 670 (Life Technologies, Gibco BRL, Gaithersburg, MD) was added for three-colour flow cytometry. After incubation for 30 min at 4°C, the cells were washed and subjected to flow cytometric analysis. The cytometer used in this study was a FACScan (Becton Dickinson); 1 × 105 events were collected and analysed by the Cell Quest program.
For analysis of the stromal cells, 1 × 105 stromal cells suspended in 200 μl PBS were mixed with 5 μl anti-HLA-ABC or HLA-DP/DQ/DR MoAbs. After incubation for 30 min at 4°C, the cells were washed and suspended in 200 μl PBS. PE-conjugated anti-κ-chain antibody (5 μl) was added as a secondary antibody. After incubation for 30 min at 4°C, the cells were washed and suspended in 200 μl PBS. The suspended cells were blocked with normal mouse serum for 10 min and fixed with 0.25% formaldehyde for 30 min at room temperature, washed and suspended in 200 μl PBS. After permeabilization of the cells with 0.025% saponin (Sigma), 5 μl of FITC conjugated anti-cytokeratin antibody were added and incubated for 30 min at 4°C; 3 × 104 events were collected and analysed by the Cell Quest program. The MHC expression level was judged by the D value, with the p value in Kolmogorov–Smirnov statistics. Pearson's correlation coefficient was calculated to examine the relationship between two variables.
RESULTS
Phenotypic characterization of the lymphocytes in thymomas
The result of the characterization of lymphocytes in thymomas is shown in Table 2. CD4+CD8+ cells accounted for 61.4 ± 8.3% of all lymphocytes on average in nine thymomas. The mean proportions of CD4+CD8− cells and CD4−CD8+ cells were 23.4 ± 7.0% and 8.1 ± 4.4%, respectively. In 17 normal child thymi, CD4+CD8+ cells accounted for 80.5 ± 4.2% of all lymphocytes on average. An example of the CD3 expression in each subset is shown in Fig. 1. In this case, the CD4−CD8+ subset consisted almost exclusively of mature CD3+ cells. In contrast, the proportion of mature CD3+ cells in the CD4+CD8− subset was only 48%, and, typically, CD3-negative and -positive peaks were observed in the CD4+CD8− subset. This bimodal pattern is not usually seen in normal thymocytes. As shown in Table 2, a major proportion of the lymphocytes in the thymomas had a phenotype of cortical thymocytes, and immature CD3−CD4+CD8− cells accumulated compared with the normal thymus samples, which is consistent with our previous report [14].
Table 2.
Phenotypic characterization of the lymphocytes in thymomas and normal infant thymi
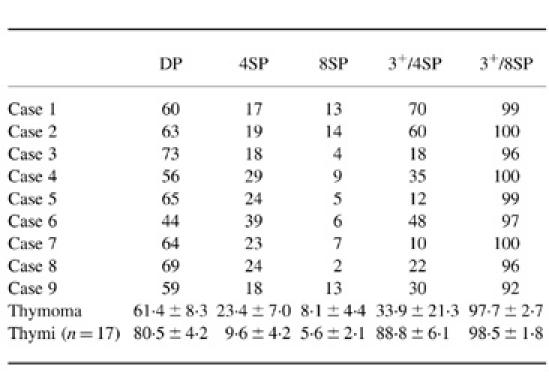
The number indicates the percentage of cells in each population.
Results of all are shown as mean ± s.d.
DP, CD4+CD8+ cell; 4SP, CD4+CD8− cell; 8SP, CD4−CD8+ cell; 3+/4SP, CD3+ cells among CD4+CD8− cells; 3+/8SP, CD3+ cells among CD4−CD8+ cells.
Fig. 1.
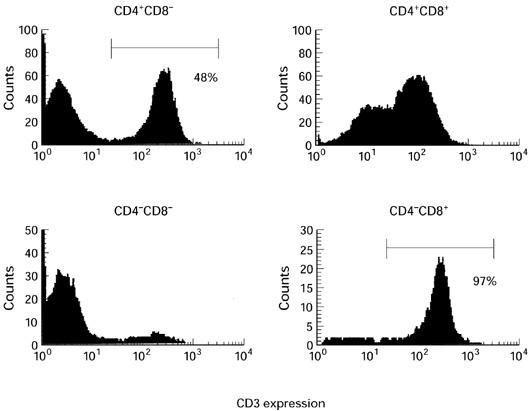
A representative result of the CD3 expression in each subset defined by CD4 and CD8 expressions (case 6).
MHC expression on the neoplastic epithelial cells in vitro
MHC expression on the neoplastic epithelial cells in vitro in response to exogenously administered IFN-γ was examined by flow cytometry. Using two-colour flow cytometry, the epithelial cells from the entire sample of stromal cells were identified by anti-cytokeratin antibody and gated as cytokeratin-positive cells. Four normal child thymi were examined as positive controls. Figure 2a shows the HLA-ABC (MHC class I) expression and the percentage of CD3+ cells among CD4−CD8+ cells of a normal thymus and thymomas. MHC class I was distinctly expressed and most CD4−CD8+ cells expressed CD3 in these cases. The results of all cases are shown in Fig. 2b. Thus, MHC class I expression of thymoma epithelial cells was comparable to normal thymus. By contrast, HLA-DP/DQ/DR (MHC class II) expression and the percentage of CD3+ cells among CD4+CD8− cells of a normal thymus and typical thymoma cases are shown in Fig. 3a. MHC class II expression on thymoma epithelial cells varied greatly from case to case. The results of all cases are shown in Fig. 3b. MHC class II expression on neoplastic thymic epithelial cells was significantly lower than normal infant thymus.
Fig. 2.
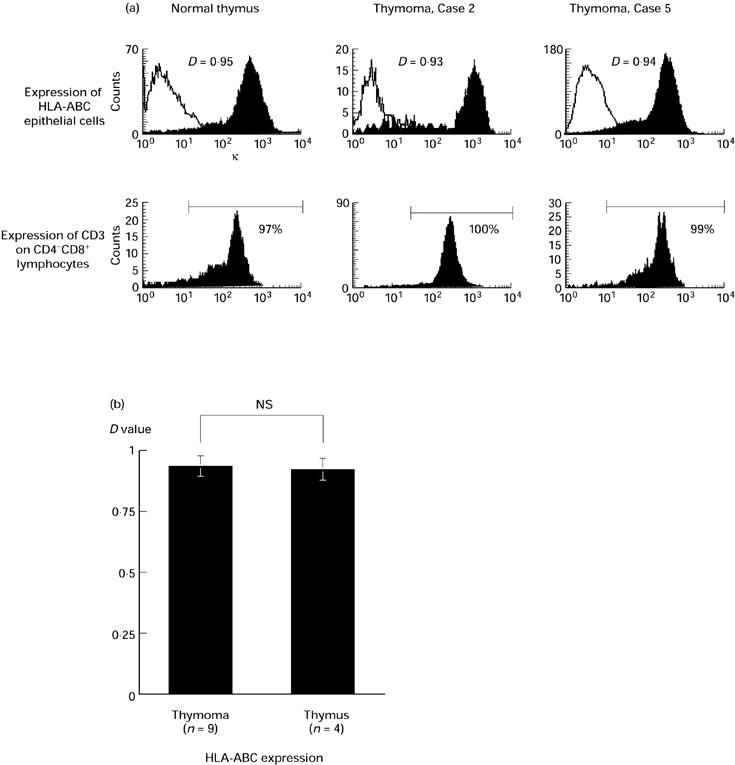
(a) HLA-ABC (MHC class I) expression on epithelial cells (upper) and CD3 expression among CD4−CD8+ lymphocytes (lower) in typical cases. Solid lines show the negative control stains. The expression level was judged by D value in Kolmogorov–Smirnov statistics for MHC and the percentage for CD3. A normal thymus is presented as a positive control. (b) HLA-ABC expression of all thymoma cases and normal thymi. Thymoma: D = 0.93 ± 0.05 versus normal thymus: D = 0.92 ± 0.05, not significant (NS).
Fig. 3.
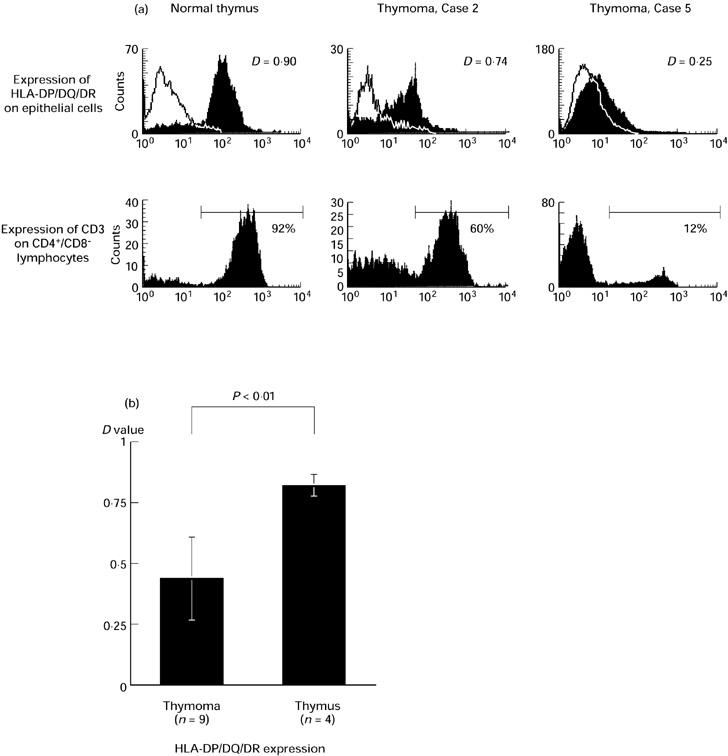
(a) HLA-DP/DQ/DR (MHC class II) expression on epithelial cells (upper) and CD3 expression among CD4+CD8− lymphocytes (lower) in typical cases. Solid lines show the negative control stains. The expression level was judged by D value in Kolmogorov–Smirnov statistics for MHC and the percentage for CD3. A normal thymus is presented as a positive control. (b) HLA-DP/DQ/DR expression of all thymoma cases and normal thymi. Thymoma: D = 0.44 ± 0.17 versus normal thymus: D = 0.82 ± 0.08; P < 0.01, significant by unpaired t-test.
Relationship between HLA-DP/DQ/DR expression and the maturity of the CD4+CD8− subset
Since there was a wide variation in the proportion of CD3+ cells among the CD4+CD8− subset as well as in MHC class II expression on neoplastic epithelial cells, we examined the relationship between these two variables. Interestingly, there was a significant positive correlation between MHC class II expression and the proportion of CD3+ cells among the CD4+CD8− subset, as shown in Fig. 4. The patients with autoantibodies are indicated with open circles and there is no definitive tendency.
Fig. 4.
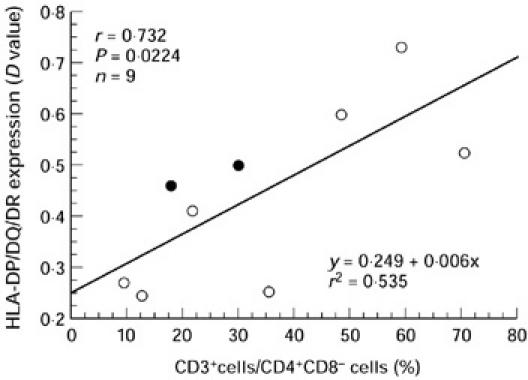
The relationship between HLA-DP/DQ/DR expression and the proportion of CD3+ cells among the CD4+CD8− subset. ○, Patients with positive autoantibodies.
DISCUSSION
The subset of CD3−CD4+CD8− cells has recently been recognized as an intermediate population between CD3−CD4−CD8− and CD4+CD8+ cells during T cell development in the thymus [12, 13]. One of the characteristic features of lymphocytes in thymoma is a prominent accumulation of these immature CD4 single-positive cells, compared with a normal thymus [14, 15]. These observations seem to indicate inefficient differentiation of T cells from CD3−CD4+CD8− cells to CD4+CD8+ cells, or from CD4+CD8+ cells to CD3+CD4+CD8− cells in thymomas. Because MHC molecules have an important role in T cell development in the thymus, we quantified their expression level on the neoplastic epithelial cells in response to exogenous IFN-γ. A preliminary experiment revealed that MHC class II expression on the neoplastic epithelial cells in vitro was reduced without stimulation by IFN-γ and restored with an administration of exogenous IFN-γ.
In our previous study, we evaluated MHC class II expression by immunohistochemistry and could not find a distinct difference [14]. MHC class II expression of all cases was also examined by immunohistochemistry in this study (data not shown), but we could not histologically detect a significant difference between case 2, which showed the highest expression of MHC class II, and case 5, which showed the lowest in vitro by flow cytometric analysis. A proportion, but not all, of the neoplastic epithelial cells was positively stained with anti-HLA-DP/DQ/DR antibody in both of these cases, suggesting a heterogeneous expression of MHC class II molecules. Thus, it is difficult to analyse MHC class II expression in thymoma quantitatively using immunohistochemistry.
Although MHC class I molecules were expressed on the thymoma neoplastic epithelial cells at a level comparable to that on normal thymic epithelial cells, expression of MHC class II molecules was generally decreased and varied from case to case, consistent with previous reports [19–22]. In addition, interestingly, there was a significant correlation between the expression of MHC class II molecules and the proportion of CD3+ cells among the CD4+CD8− subset in a thymoma. This result suggests that the proportion of CD3+ cells in the CD4+CD8− subset reflects the expression level of MHC class II molecules on the thymoma neoplastic epithelial cells.
Recently, an affinity-avidity model was proposed to explain the mechanism of positive and negative selection processes of the T cell repertoire, based on genetically engineered murine models which enabled manipulation of the expression level of the MHC molecules [26–30]. According to this hypothesis, T cell that would normally be deleted by the normal level of MHC class II on the thymic epithelial cells might be positively selected when this level is reduced on the neoplastic epithelial cells. Thus, the T cells newly generated in a thymoma might include an altered TCR repertoire which can react to self-antigens and therefore might cause autoimmune diseases. This hypothesis would be supported if low MHC class II expression on thymoma epithelial cells correlated with the autoimmune associations. Since the presence of AChR-related antigen was reported, it is possible that AChR-specific T cells might be positively selected in the tumour [31]. Also, there must be many non-myasthenic patients with an elevated αAChR. However, we suppose that this autoimmunity is not restricted to neuromyopathy, because thymoma patients have various autoantibodies, as shown in Table 1.
Thymoma lacks a medullary structure and there may be insufficient contact with bone marrow-derived dendritic cells and macrophages that are believed to serve as the negatively selecting elements for autoreactive T cells generated in normal thymus. It has been reported that mice whose MHC class II antigen is expressed in thymic cortex only, and relB-deficient mice which lack thymic medulla, show impairment of thymic negative selection [32, 33]. When the single-positive T cells are generated in the thymoma and exported to the periphery, they may contain autoreactive T cells.
Moreover, cell–cell interactions between thymocytes and thymic stromal cells through several adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1)/fibronectin (FN) to very late antigen-4,5 (VLA-4,5) or intercellular adhesion molecule-1 (ICAM-1) to leucocyte function-associated antigen-1 (LFA-1), have been shown to be involved in T cell development in the thymus [34]. Soluble factors such as IL-7 also participate in T cell development in thymus [35, 36]. We cannot exclude defects in these accessory factors.
The signal transduction cascade of the IFN-γ receptor has been studied extensively, and Jak-1, Jak-2, Stat-1, and CIITA have been shown to be involved in the induction of MHC class II molecules [37, 38]. The existence of a CIITA-independent class II regulatory pathway has also been reported [39]. Experiments to identify the molecules responsible for impairment of the IFN-γ receptor signal transduction cascade in thymoma neoplastic epithelial cells are currently being conducted in our laboratory.
In conclusion, MHC class II expression on neoplastic epithelial cells was generally lower than that on normal thymic epithelial cells in vitro. The level of MHC class II expression had a significant correlation with the proportion of CD3+ cells among the CD4+CD8− subset. These results suggest that the degree of maturation of the CD4+CD8− subset reflects the activity of the neoplastic cells in a thymoma. We suppose that the immaturity of the CD4+CD8− subset in thymomas is caused by the lower expression of MHC class II molecules on neoplastic epithelial cells. It is expected that this impaired expression of MHC class II molecules on thymoma neoplastic epithelial cells might be a factor of pathogenesis of thymoma-associated autoimmune diseases [15]. Further investigation of the mechanism may elucidate the molecular pathogenesis of thymoma-associated autoimmune diseases.
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, and a grant provided by the Ichiro Kanehara Foundation.
REFERENCES
- 1.Rosai J, Levine GD. Atlas of tumor pathology. Washington DC: Armed Forces Institute of Pathology; 1976. Tumors of the thymus. [Google Scholar]
- 2.Bernatz PE, Harrison EG, Clagett OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg. 1961;42:424–44. [PubMed] [Google Scholar]
- 3.Salyer WR, Eggleston JC. Thymoma: a clinical and pathological study of 65 cases. Cancer. 1976;37:229–49. doi: 10.1002/1097-0142(197601)37:1<229::aid-cncr2820370133>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Pedraza MA. Thymoma immunological and ultrastructural characterization. Cancer. 1977;39:1455–61. doi: 10.1002/1097-0142(197704)39:4<1455::aid-cncr2820390417>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Lauriola L, Piantelli M, Carbone A, Dina MA, Scoppetta C, Musiani P. Subpopulations of lymphocytes in human thymomas. Clin Exp Immunol. 1979;37:502–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Lauriola L, Maggiano N, Marino M, Carbone A, Piantelli M, Musiani P. Human thymoma: Immunologic characteristics of the lymphocytic component. Cancer. 1981;48:1992–5. doi: 10.1002/1097-0142(19811101)48:9<1992::aid-cncr2820480914>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Palestro G, Geuna M, Novero D, Godio L, Ciccone G, Azzoni L. Immunophenotype of thymoma-associated lymphoid cell component of T-cell type. A new analytic procedure in keeping with structural heterogeneities. Virchows Arch B, Cell Pathol. 1990;59:297–304. doi: 10.1007/BF02899417. [DOI] [PubMed] [Google Scholar]
- 8.Fujii Y, Hayakawa M, Inada K, Nakahara K. Lymphocytes in thymoma: association with myasthenia gravis is correlated with increased number of single-positive cells. Eur J Immunol. 1990;20:2355–8. doi: 10.1002/eji.1830201029. [DOI] [PubMed] [Google Scholar]
- 9.Fujii Y, Hayakawa M, Nakahara K. Thymus cells in myasthenia gravis: a two-colour flow cytometric analysis of lymphocytes in the thymus and thymoma. J Neurol. 1992;239:82–88. doi: 10.1007/BF00862978. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Fujii Y, Okumura M, Takeuchi Y, Shiono H, Miyoshi S, Matsuda H, Shirakura R. Neoplastic thymic epithelial cells of human thymoma support T cell development from CD4−CD8− cells to CD4+CD8+ cells in vitro. Clin Exp Immunol. 1998;112:419–26. doi: 10.1046/j.1365-2249.1998.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue M, Fujii Y, Okumura M, Takeuchi Y, Shiono H, Miyoshi S, Matsuda H, Shirakura R. Mature CD4 single positive thymocytes in human thymoma: T cells may differentiate in the thymic epithelial cell tumor. Pathobiol. 1997;65:216–22. doi: 10.1159/000164126. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Vallina L, Gonzales A, Gambon F, Kreisler M, Diaz-Espada F. Delimitation of the proliferative stages in the human thymus indicates that cell expansion occurs before the expression of CD3 (T cell receptor) J Immunol. 1993;150:8–16. [PubMed] [Google Scholar]
- 13.Takeuchi Y, Fujii Y, Okumura M, Inada K, Nakahara K, Matsuda H. Characterization of CD4+ single positive cells that lack CD3 in the human thymus. Cell Immunol. 1993;151:481–90. doi: 10.1006/cimm.1993.1257. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi Y, Fujii Y, Okumura M, Inada K, Nakahara K, Matsuda H. Accumulation of immature CD3−CD4+CD8− single-positive cells that lack CD69 in epithelial cell tumors of the human thymus. Cell Immunol. 1995;161:181–7. doi: 10.1006/cimm.1995.1025. [DOI] [PubMed] [Google Scholar]
- 15.Müller-Hermelink HK, Wilisch A, Schultz A, Marx A. Characterization of the human thymic microenvironment: Lymphoepithelial interaction in normal thymus and thymoma. Arch Histol Cytol. 1997;60:9–28. doi: 10.1679/aohc.60.9. [DOI] [PubMed] [Google Scholar]
- 16.Sprent J. T lymphocytes and the thymus. In: Paul WE, editor. Fundamental immunology. 3. New York: Raven Press; 1993. pp. 75–109. [Google Scholar]
- 17.Hugo P, Kappler JW, Marrack PC. Positive selection of TcRαβ thymocytes: is cortical thymic epithelium an obligatory participant in the presentation of major histocompatibility complex protein? Immunol Rev. 1993;135:133–55. doi: 10.1111/j.1600-065x.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 18.Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 19.Willcox N, Schluep M, Ritter MA, Schuurman HJ, Newsom-Davis J, Christensson B. Myasthenic and nonmyasthenic thymoma. An expansion of a minor cortical epithelial cell subset? Am J Pathol. 1987;127:447–60. [PMC free article] [PubMed] [Google Scholar]
- 20.Chilosi M, Iannucci A, Fiore-Donati L, et al. Myasthenia gravis: immunohistological heterogeneity in microenvironmental organization of hyperplastic and neoplastic thymuses suggesting different mechanisms of tolerance breakdown. J Neuroimmunol. 1986;11:191–204. doi: 10.1016/0165-5728(86)90003-2. [DOI] [PubMed] [Google Scholar]
- 21.van der Kwast TH, van Vliet E, Cristen E, van Ewijk W, van der Heul RO. An immunohistologic study of the epithelial and lymphoid components of six thymomas. Hum Pathol. 1985;16:1001–8. doi: 10.1016/s0046-8177(85)80277-x. [DOI] [PubMed] [Google Scholar]
- 22.Savino W, Manganella G, Verley J-M, et al. Thymoma epithelial cells secrete thymic hormone but do not express class II antigens of the major histocompatibility complex. J Clin Invest. 1985;76:1140–6. doi: 10.1172/JCI112069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–92. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Anderson G, Owen JJT, Moore NC, Jenkinson EJ. Characteristics of an in vitro system of thymocyte positive selection. J Immunol. 1994;153:1915–20. [PubMed] [Google Scholar]
- 25.Jenkinson EJ, Anderson G, Owen JJT. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med. 1992;176:845–53. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashton-Rickardt PG, van Kaer L, Schumacher TN, Ploegh HL, Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+ T cells in the thymus. Cell. 1993;73:1041–9. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 27.Ashton-Rickardt PG, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today. 1994;15:362–6. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 28.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 29.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1994;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 30.Fukui Y, Ishimoto T, Utsuyama M, et al. Positive and negative CD4+ thymocyte selection by a single MHC class II/peptide ligand affected by its expression level in the thymus. Immunity. 1997;6:401–10. doi: 10.1016/s1074-7613(00)80283-6. [DOI] [PubMed] [Google Scholar]
- 31.Kirchner T, Tzartos S, Hoppe F, Schalke B, Wekerle H, Müller-Hermelink HK. Pathogenesis of myasthenia gravis. Acetylcholine receptor-related antigen determinants in tumor-free thymus and thymic epithelial tumors. Am J Pathol. 1988;130:268–80. [PMC free article] [PubMed] [Google Scholar]
- 32.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 33.Dekoning J, DiMolfetto L, Reilly C, Wei Q, Havran WL, Lo D. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J Immunol. 1997;158:2558–66. [PubMed] [Google Scholar]
- 34.Crisa L, Cirulli V, Ellisman MH, Ishii JK, Elices MJ, Salomon DR. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: the roles of fibronectin, VLA4, and VLA5. J Exp Med. 1996;184:215–28. doi: 10.1084/jem.184.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He W, Kabelitz D. Cytokines involved in intrathymic T cell development. Int Arch Allergy Immunol. 1994;105:203–10. doi: 10.1159/000236759. [DOI] [PubMed] [Google Scholar]
- 36.Watson JD, Morrissey PJ, Namen AE, Conlon PJ, Widmer MB. Effect of IL-7 on the growth of fetal thymocytes in culture. J Immunol. 1989;143:1215–22. [PubMed] [Google Scholar]
- 37.Bach EA, Aguet M, Schreiber RD. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–91. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 38.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 39.Zhou H, Su HS, Zhang X, Douhan J, 3, Glimcher LH. CIITA-dependent and -independent class II MHC expression revealed by a dominant negative mutant. J Immunol. 1997;158:4741–9. [PubMed] [Google Scholar]


