Abstract
Enterovirus-specific cellular immunity was studied in Estonian and in Finnish children at the age of 9 months. The aim was to evaluate the level of responsiveness in two neighbouring countries with different poliovirus immunization practices and striking differences in the incidence of insulin-dependent diabetes mellitus (IDDM), a disease in which early enterovirus infections are an aetiological risk factor. The Estonian children immunized with live attenuated polio vaccine had stronger T cell responses to coxsackievirus B4 and poliovirus type 1 when compared with Finnish children immunized with inactivated polio vaccine (median stimulation indices 10.4 and 6.3 in Estonian children and 1.9 and 2.9 in Finnish children, respectively; P < 0.05). Lymphocytes stimulated by poliovirus type 1 antigen expressed interferon-gamma (IFN-γ) mRNAs, which strongly correlated with the level of proliferation responses. Lymphocytes of Estonian children had a tendency towards stronger expression of IFN-γ upon poliovirus challenge when compared with Finnish children. The number of children who had experienced coxsackievirus B infections, as determined by the presence of neutralizing antibodies, did not differ between Estonian and Finnish children. The results show that Finnish children have weaker cellular immunity against enteroviruses at the age of 9 months compared with Estonian children at the same age. This is most probably due to the difference in polio vaccination schedules; in Estonia live poliovirus vaccine is used and given at earlier ages than the inactivated vaccines in Finland. This leads to stronger T cell immunity which cross-reacts with other enterovirus serotypes. This may explain the lower incidence of IDDM in Estonia by providing effective protection against diabetogenic enterovirus strains in Estonian children.
Keywords: children, enteroviruses, insulin-dependent diabetes mellitus, polio immunization, T cell proliferation
INTRODUCTION
Enteroviruses are small RNA viruses belonging to the family of Picornaviridae. Enteroviruses are divided to five subgroups: Echoviruses, Coxsackie A viruses, Coxsackie B viruses, polioviruses and numbered enteroviruses [1]. They form altogether a group of almost 70 different serotypes. Enteroviral infections are usually subclinical but occasionally cause complications such as meningitis and myocarditis, as well as life-threatening systemic infections in newborns [2–4]. Enterovirus infections are common during infancy and the majority of children have experienced at least one enterovirus infection by the age of 1 year [5, 6].
Protection against enterovirus infections depends on circulating and mucosal neutralizing antibodies [7]. Neutralizing antibodies are serotype-specific by definition, but the bulk of the antibodies are largely cross-reactive, as are T cell responses [8, 9]. T cell epitopes have been found on each of the four capsid proteins (VP1–VP4) [9–12]. The significance of the cellular immune response is largely unknown, but T cell help is needed for antibody production and cross-reactive T cell memory may accelerate the formation of neutralizing antibodies. Experimental data also suggest that cross-reactive cellular immunity may be deleterious in some cases [13].
Enteroviral infections have been linked to the pathogenesis of certain autoimmune diseases such as chronic cardiomyopathies and insulin-dependent diabetes mellitus (IDDM) [6, 14–17]. Recently diagnosed IDDM patients have been observed to be more often positive for enterovirus-specific IgM class antibodies and enterovirus RNA than healthy control subjects [18–20]. Children who later manifest with clinical IDDM have had enterovirus infections more frequently than other children, and seroconversions for islet cell antibody (ICA) positivity are associated with coinciding enterovirus infections in the preclinical period [15, 16]. Intrauterine exposures have also been reported to increase the risk of IDDM in the offspring [15]. These findings imply that enterovirus infections can initiate the β cell-damaging process years before the manifestation of clinical IDDM.
The incidence of IDDM varies considerably from country to country. The highest incidence has been reported in Finland (35.3 per 100 000 under 15-year-old children) [21]. The variation is quite wide even between neighbouring countries. For example, the incidence of IDDM in Estonia just south of Finland is only one third of that in Finland [21]. The reason for this variation is not known, but apparently both environmental and genetic factors could be involved. Regarding the possible role of enterovirus infections in the development of IDDM, one has to note that the vaccination policy against polioviruses differs markedly between Finland and Estonia. The oral administration of three doses of live attenuated poliovirus vaccine (OPV) by the age of 6 months may greatly modify the formation of cellular immunity to enteroviruses in general compared with the one dose of inactivated vaccine (IPV) subcutaneously starting at the age of 6 months in Finland.
In the present study we have evaluated the cellular immune responses to enteroviruses in Estonian and Finnish children at the age of 9 months. Lymphocyte proliferation responses to purified coxsackievirus B4 and poliovirus type 1 antigens were tested, as was also interferon-gamma (IFN-γ) and IL-4 mRNA expression of stimulated lymphocytes. Previous coxsackievirus B exposure was estimated by measuring neutralizing antibodies in serum against Coxsackie B serotypes.
SUBJECTS AND METHODS
Subjects
Study subjects were healthy children from the general population aged 9 months. Finnish children (n = 21) were recruited for the Diabetes Prediction and Prevention (DIPP) trial at the University of Turku carrying HLA-DQB1*02/*0302 genotype associated with increased IDDM risk. Estonian children (n = 21) were healthy children from the Tartu area. Heparinized venous blood (2–5 ml) was collected each sampling day from both Estonian and Finnish children, and cells were processed during the same day.
The Finnish children were immunized according to the standard vaccination protocol, including bacille Calmette–Guerin (BCG) immunization to the newborns at the age of a few days and diphtheria, tetanus, pertussis (DTP) vaccination at the age of 3, 4 and 5 months. The Salk type of IPV was given at the age of 6 and 12 months. The Estonian vaccination schedule included also BCG immunization of the newborns, DTP and live attenuated OPV at 3, 4.5 and 6 months of age.
Lymphocyte proliferation assay
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by Ficoll–Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation. The PBMC were washed and resuspended in RPMI 1640 medium supplemented with 10% human AB serum (Finnish Red Cross, Helsinki, Finland), glutamine, HEPES and gentamycin 10 μg/ml and frozen in the same medium containing 10% dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany). Cells were thawed from equal numbers of Estonian and Finnish children for each culture series. Fifty thousand PBMC/well were incubated in quadruplicate with antigens in 200 μl final volume in 96-well round-bottomed microtitre plates for 6 days. Tritiated thymidine (2 μCi/ml; Amersham, Aylesbury, UK) was added 18 h before harvesting. The cultures were harvested on glass fibre filters using a Tomtec 93 Mach III Manual Harvester (Tomtec, Orange, CT) and the incorporated radioactivity was measured with a Micro-Beta scintillation counter (Wallac, Turku, Finland). Stimulation indices (SI) were calculated by dividing the median ct/min value of antigen-stimulated quadruplicate wells by the median ct/min of the quadruplicate control wells. The proliferation response was considered positive when the SI was > 3.
Antigens
Purified poliovirus type 1 and coxsackievirus B4 virions at 1 μg/ml and 0.1 μg/ml concentrations were used to test proliferation responses against enteroviruses. The preparation of purified Coxsackie B4 and poliovirus type 1 antigens was done by sucrose gradient centrifugation. The protein concentrations of the purified antigen preparations were established by the Pierce BCA protein assay reagent (Pierce, Rockford, IL). Responses to purified adenovirus hexon protein (10 μg/ml and 1 μg/ml) [22] and tetanus toxoid (TT) (1 μg/ml; National Public Health Institute, Helsinki, Finland) were also studied. Pokeweed mitogen (PWM) (12.5 μg/ml) was used as a mitogen control.
Virus antibodies
Serotype-specific antibodies against coxsackievirus B serotypes 1–6 were studied using a standard plaque neutralization assay [23]. IgG class antibodies against coxsackievirus B4 and poliovirus were analysed by enzyme immunoassay (EIA) as previously described [15]. Briefly, highly purified viruses (the same as in the T cell proliferation tests) were first incubated at 56°C for 15 min to expose antigenic determinants which are cross-reactive between different enterovirus serotypes. Microtitre plates (Nunc Immunoplate, Nunc, Roskilde, Denmark) were coated by the virus at a concentration of 2 μg/ml in PBS followed by blocking using PBS + 1% bovine serum albumin (BSA) (30 min, room temperature). Sera were incubated at 1:2000 (IgG) dilution in PBS + 1% BSA + 0.05% Tween-20. Peroxidase-conjugated anti-human IgG (Dako, Glostrup, Denmark) was used as the second layer at 1:2000 dilution. Virus antibody levels were expressed in enzyme immunoassay units (EIU), which were calculated according to the formula: 100 × [(ODsample− ODnegative reference serum)/(ODpositive reference serum− ODnegative reference serum)]. An EIU value of > 10 was considered positive.
Cytokine assays
PBMC were incubated in duplicate wells (100 000 per well). The IFN-γ and IL-4 mRNAs were detected from poliovirus type 1 and TT-stimulated cells after 3 days of incubation and from PWM-stimulated cells after a 1-day incubation. The expression of IFN-γ and IL-4 mRNAs was also analysed after 1 and 3 days' incubation from non-stimulated PBMC. Time-resolved fluorometry was used in the detection of reverse transcriptase-polymerase chain reaction (RT-PCR)-amplified mRNAs with a method described in detail elsewhere [24]. In short, RNA was extracted and reverse transcribed to cDNA. Cytokine cDNAs were amplified by multiplex-PCR using one biotinylated primer per primer pair. The amplified cDNAs were collected onto streptavidin-coated microtitration plates. After denaturation with NaOH, single-stranded cDNAs captured onto the wells were hybridized with oligonucleotide probes specific for each target sequence and specifically labelled with various lanthanide chelates. Finally, lanthanide labels were detached from the probes by incubation in enhancement solution and measured using a time-resolved fluorometer. Signal to noise values (s/n) were calculated by dividing the signal of cell-containing wells by the signal of PCR-aqua-containing wells.
Statistical analysis
The two-tailed Mann–Whitney U-test was used for comparisons of the SI and s/n values between the two groups. χ2 test and Fisher's exact test were used to compare frequencies of positive responses.
RESULTS
Lymphocyte proliferation responses
The responsiveness to PWM was used as a positive control. All subjects responded and the levels were similar in both groups (data not shown). Lymphocyte proliferation responses against TT were frequent in both Estonian and Finnish children. Altogether 57% (23/42) of these children had positive T cell responses (SI > 3) to TT and there were no significant differences between the Estonian and Finnish children (Fig. 1). Proliferation responses to both poliovirus type 1 and coxsackievirus B4 were stronger (P < 0.05) in Estonian children compared with Finnish children (median SI 10.4 and 6.3 in Estonian children and 1.9 and 2.9 in Finnish children, respectively) (Fig. 1). Proliferation responses to poliovirus type 1 and coxsackievirus B4 correlated very strongly in Finnish children (r = 0.720, P = 0.0001), whereas there was no significant correlation between these responses in Estonian children (r = 0.198, P = NS) (Fig. 2). Responses against adenovirus hexon protein were detected only in 17% (7/42) of the children and the strengths of responses were similar between the two groups (Fig. 1).
Fig. 1.

T cell responses to tetanus toxoid (TT), poliovirus type 1, coxsackievirus B4 and adenovirus hexon protein in Estonian children (□) and Finnish children (hatched). The horizontal line in the box shows the median value. The outlines of the boxes show the 25% and 75% percentiles, while the bars outside the boxes represent the 10% and 90% percentiles. ○, Values outside this range. P values for the differences between the stimulation index (SI) values were calculated using the Mann–Whitney U-test.
Fig. 2.
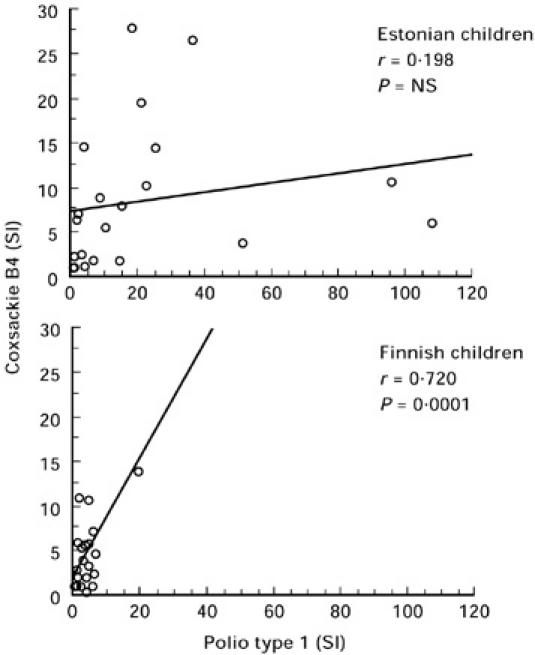
Correlation between the T cell responses (in stimulation index (SI) values) to poliovirus type 1 and coxsackievirus B4 in the Estonian and Finnish children.
Cytokine mRNA expression in antigen stimulated PBMC
The expression of either IFN-γ or IL-4 in TT- or pokeweed-stimulated PBMC did not differ between the groups (Table 1). Stimulation with TT induced mainly the expression of IFN-γ mRNA. The PBMC stimulated with PWM expressed significant amounts of both IFN-γ and IL-4 mRNAs. The expression of IFN-γ in poliovirus type 1-stimulated PBMC was frequent in both groups, similar to that observed in TT stimulation. Estonian children produced more IFN-γ mRNA than Finnish children (median s/n 195.2 in Estonian children and 5.8 in Finnish children; P = 0.06) (Table 1). Lymphocyte proliferation responses and the amount of expressed IFN-γ mRNA correlated moderately but significantly in poliovirus type 1-stimulated PBMC (Fig. 3). The expression of IL-4 mRNA in poliovirus-stimulated PBMC was low in both Estonian and Finnish children (median s/n 1.2 in Estonian children and 1.5 in Finnish children) and no difference was observed between the two groups (Table 1).
Table 1.
Median and interquartile range (IQR) of IFN-γ and IL-4 mRNA expression (signal to noise value (s/n)) by antigen-stimulated T cells in Estonian and Finnish children
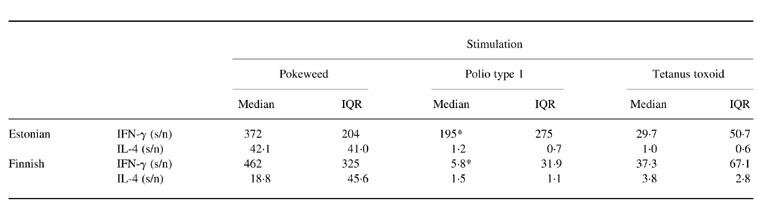
*P = 0.06 by Mann–Whitney U-test.
Fig. 3.
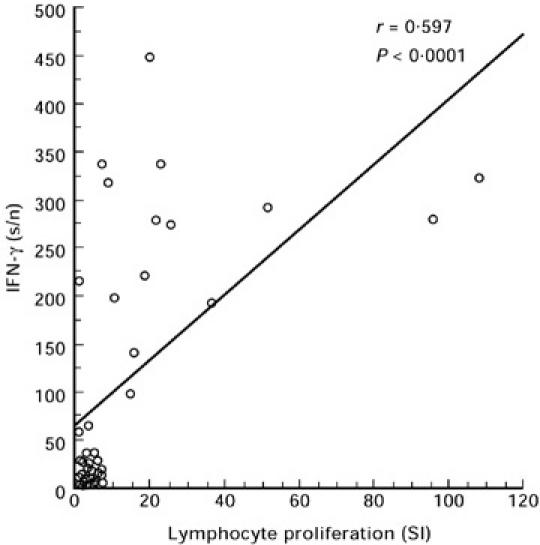
Correlation between lymphocyte proliferation and IFN-γ mRNA expression of poliovirus type 1 antigen-stimulated peripheral blood mononuclear cells (PBMC). Both Estonian and Finnish children are included. s/n, Signal to noise values.
Virus antibodies
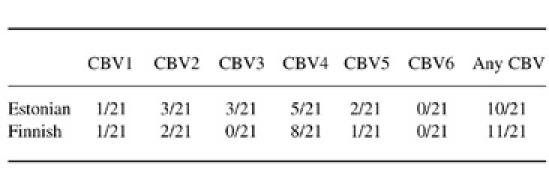
Children were analysed for serotype-specific antibodies against all coxsackievirus B serotypes using the standard plaque neutralization assay. Neutralizing antibodies to at least one of the serotypes were found in the sera of 50% (21/42) of study subjects. The proportion of children with Coxsackie B group neutralizing antibodies did not differ between Estonian and Finnish children (Table 2). When a largely cross-reactive EIA test was used to measure antibodies to coxsackievirus B4, 30% (7/21) of Estonian children had measurable IgG in their sera, compared with only 5% (1/21) of the Finnish children (P = 0.02, Fisher's exact test). The median level of IgG class antibodies to poliovirus were 7.0 EIU in Estonian children and 0 EIU in Finnish children (P = 0.0001) and the median level of IgG class antibodies to Coxsackie B4 4.0 EIU in Estonian children and 0 EIU in Finnish children (P = 0.007). There was a correlation between antibody levels measured using these two antigens (r = 0.35, P = 0.02).
Table 2.
The proportion of children having neutralizing antibodies to coxsackievirus serotypes B1, B2, B3, B4, B5 and B6
DISCUSSION
The present study shows that 9-month-old children in Estonia and Finland differ significantly in their immune responsiveness to enteroviruses. This was observed not only in poliovirus-specific responses induced by different vaccination protocols, but also when coxsackievirus B4 was used as an antigen. No difference was observed between Estonian and Finnish children in PWM-induced responses or in responses to TT or adenovirus hexon protein, indicating that the phenomenon is specific to enteroviruses and does not reflect a common difference in cellular immunity.
Finnish children in this study share the same HLA-DQB1 genotype, which might effect the immune responses against enteroviruses. This particular genotype, carrying a strongly increased risk of IDDM, combines DR3-associated DQB1*02 and DR4-associated DQB1*0302. These two haplotypes have been reported to be associated either with low (DR3) or high (DR4) responsiveness to enteroviral antigens. Heterozygosity for both haplotypes, as was the case in the present series, produces an intermediate level of responsiveness [25, 26]. This has also been observed in our own studies (Juhela et al., unpublished data), suggesting that the findings with the Finnish children in the present study are quite representative of the general population in Finland as well, and that the comparison between Estonian and Finnish children is not affected by this bias in HLA genotypes.
The number of children who had experienced coxsackievirus B infections was quite high in this study, as half of the children had neutralizing antibodies to coxsackievirus B serotypes. This confirms our earlier studies, in which an estimation of the proportion of children experiencing enterovirus infections by the age of 9 months varied from 50% to 80% [5]. The lack of a major difference in the proportion of children having neutralizing antibodies against group B coxsackieviruses suggests that the rate of these infections is quite similar in Estonian and in Finnish children.
The difference in polio vaccination protocols in Finland and Estonia is of apparent importance when evaluating the differences in cellular immunity against enteroviruses in these two populations. The Estonian children had received OPV at the ages of 3, 4.5 and 6 months, whereas the Finnish children had received only one dose of IPV at the age of 6 months. In our previous study we showed that IPV vaccination induces cross-reactive T cell response against enteroviruses [5]. Due to this cross-reactivity the markedly different polio vaccination protocols in Estonia and Finland may lead to the observed difference in proliferation responses to both polio type 1 and Coxsackie B4 viruses between Estonian and Finnish children. As the proportion of children with previous Coxsackie B infection did not differ between the groups, the stronger T cell responses to coxsackievirus B4 antigen in Estonian children are probably due to the earlier and more frequent polio vaccinations.
We could confirm our earlier results of the correlation between poliovirus and coxsackievirus B4 lymphocyte proliferation responses in Finnish children [5]. To our surprise this correlation was not found in Estonian children. The lack of correlation in Estonian children may indicate that the OPV vaccination schedule in Estonia is able to induce strong T cell responses to polioviruses even without a history of infections by other enteroviruses. Only one dose of IPV instead is not as efficient in inducing strong polio-specific T cell responses, and most of the responsiveness to poliovirus type 1 antigen in Finnish children is due to the cross-reactive response elicited by past enteroviral infections in addition to the previous IPV immunization.
We found a strong correlation between enterovirus antigen-induced lymphocyte proliferation responses and IFN-γ mRNA expression, indicating the parallel induction of these phenomena. This correlation has not been found in some studies using blood samples collected from adult subjects, which is to be expected because they share strong immunity induced by multiple cross-reactive infections and vaccinations, but demonstrate that different clones of cross-reactive activated memory T cells may be responsible for these phenomena [11, 27]. It has been shown that even a change of one amino acid in the sequence of a T cell epitope may lead to a significant change in either T cell proliferation or in IFN-γ production [28].
The significance of the stronger cellular immunity at a very young age induced by early introduced OPV remains open, although the high incidence of IDDM in Finland and Sweden using inactivated poliovirus vaccination might invite speculation. The ongoing follow-up studies revealing the order of specific enterovirus infections in children eventually developing IDDM-associated antibodies might throw light on this question in future [29]. In conclusion, this study reveals major differences in cellular immunity against enteroviruses in Estonian and Finnish children at the age of 9 months. This difference is most probably mainly due to polio immunization schedules which significantly differ between these countries. This supports the hypothesis presented by Hiltunen et al. [30] that the late polio immunization by IPV in Finland may lead to a weak immune responsiveness to enteroviruses and thus make Finnish children vulnerable to diabetogenic enterovirus strains during the first months of life.
Acknowledgments
The expert technical assistance of Mrs Maria Kivivirta, Ms Anne Suominen and Ms Eeva Jokela is gratefully acknowledged. Grant support is acknowledged from Sigrid Jusélius Foundation, the Academy of Finland, Foundation for Diabetes Research in Finland, Juvenile Diabetes Foundation International (grants no. 395019 and no. 197114), Estonian Science Foundation (grant 3045) and European Commission (INCO-Copernicus grant).
REFERENCES
- 1.Melnick JL. Enteroviruses: Polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Fields BM, Knipe DM, Howley PM, editors. Fields virology. 3. Philadelphia: Lippincott-Raven; 1995. pp. 655–713. [Google Scholar]
- 2.Goren A, Kaplan M, Glaser J, et al. Chronic neonatal coxsackie myocarditis. Arch Dis Child. 1989;64:404–6. doi: 10.1136/adc.64.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abzug MJ, Levin MJ, Rotbart HA. Profile of enterovirus disease in the first two weeks of life. Ped Inf Dis J. 1993;12:820–4. doi: 10.1097/00006454-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Alexander JP, Chapman LE, Pallanch MA, et al. Coxsackievirus B2 infection and aseptis meningitis: a focal outbreak among members of a high school football team. J Infect Dis. 1993;167:1201–5. doi: 10.1093/infdis/167.5.1201. [DOI] [PubMed] [Google Scholar]
- 5.Juhela S, Hyöty H, Lönnrot M, et al. Enterovirus infections and enterovirus specific T-cell responses in infancy. J Med Virol. 1998;54:226–32. doi: 10.1002/(sici)1096-9071(199803)54:3<226::aid-jmv14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Grist R, Bell E, Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114–57. [PubMed] [Google Scholar]
- 7.Dagan R, Prather SL, Powell KR, et al. Neutralizing antibodies to non-polio enteroviruses in human immune serum globulin. Ped Inf Dis J. 1983;15:67–71. doi: 10.1097/00006454-198311000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Beck MA, Tracy SM. Evidence for a group-specific enteroviral antigen(s) recognized by human T cells. J Clin Microbiol. 1990;28:1822–7. doi: 10.1128/jcm.28.8.1822-1827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham S, Wang E, Jenkins O, et al. Analysis of the human T-cell response to picornaviruses: identification of T-cell epitopes close to B-cell epitopes in poliovirus. J Virol. 1993;67:1627–36. doi: 10.1128/jvi.67.3.1627-1637.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck MA, Tracy S, Coller B, et al. Comoviruses and enteroviruses share a T cell epitope. Virology. 1992;186:238–46. doi: 10.1016/0042-6822(92)90078-4. [DOI] [PubMed] [Google Scholar]
- 11.Cello J, Strannegård Ö, Svennerholm B. A study of the cellular immune response to enteroviruses in humans: identification of cross-reactive T cell epitopes on the structural proteins of enteroviruses. J Gen Virol. 1996;77:2097–108. doi: 10.1099/0022-1317-77-9-2097. [DOI] [PubMed] [Google Scholar]
- 12.Tracy S, Chapman NM, Rubocki RJ, et al. Host immune responses to enterovirus infections. In: Rotbart HA, editor. Human enterovirus infections. Washington, DC: American Society for Microbiology; 1995. pp. 175–91. [Google Scholar]
- 13.Beck MA, Chapman NM, McManus BM, et al. Secondary enterovirus infection in the murine model of myocarditis. Pathologic and immunologic aspects. Am J Pathol. 1990;136:669–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlquist GG, Ivarsson S, Lindberg B, et al. Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM. A population-based case-control study. Diabetes. 1995;44:408–13. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 15.Hyöty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Diabetes. 1995;44:652–7. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 16.Hiltunen M, Hyöty H, Knip M, et al. Islet cell antibody seroconversion in children is temporally associated with enterovirus infections. J Infect Dis. 1997;175:554–60. doi: 10.1093/infdis/175.3.554. [DOI] [PubMed] [Google Scholar]
- 17.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101:425–84. [PMC free article] [PubMed] [Google Scholar]
- 18.Clements GB, Galbraith DN, Taylor KW. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–3. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 19.D'Àlessio DJ. A case-control study of group B Coxsackievirus immunoglobulin M antibody prevalence and HLA-DR antigens in newly diagnosed cases of insulin-dependent diabetes mellitus. Am J Epidemiol. 1992;135:1331–8. doi: 10.1093/oxfordjournals.aje.a116244. [DOI] [PubMed] [Google Scholar]
- 20.Andreoletti L, Hober D, Hober-Vanderberghe C, et al. Coxsackie B virus infection and beta cell autoantibodies in newly diagnosed IDDM adult patients. Clin Diag Virol. 1998;9:125–33. doi: 10.1016/s0928-0197(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 21.Karvonen M, Tuomilehto J, Libman I, et al. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organisation DIAMOND Project Group. Diabetologia. 1993;36:883–92. doi: 10.1007/BF02374468. [DOI] [PubMed] [Google Scholar]
- 22.Waris M, Halonen P. Purification of adenovirus hexon protein by high-performance liquid chromatography. J Chromatography. 1987;397:321–5. doi: 10.1016/s0021-9673(01)85015-9. [DOI] [PubMed] [Google Scholar]
- 23.Roivainen M, Knip M, Hyöty H, et al. Several different enterovirus serotypes can be associated with prediabetic autoimmune episodes and onset of overt IDDM. J Med Virol. 1998;56:74–78. doi: 10.1002/(sici)1096-9071(199809)56:1<74::aid-jmv12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 24.Halminen M, Sjöroos M, Mäkelä M, et al. Simultaneous detection of IFN-γ and IL-4 mRNAs using RT-PCR and time-resolved fluorometry. Cytokine. 1999;11:87–93. doi: 10.1006/cyto.1998.0392. [DOI] [PubMed] [Google Scholar]
- 25.Bruserud O, Stenerson M, Thorsby E. T lymphocyte responses to coxsackie B4 and mump virus. II. Immunoregulation by HLA-DR3 and -DR4 associated restriction elements. Tissue Antigens. 1985;26:179–92. doi: 10.1111/j.1399-0039.1985.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 26.Bruserud O, Jervell J, Thorsby E. HLA-DR3 and -DR4 control T-lymphocyte responses to mumps and coxsackie B4 virus: studies on patients with type 1 (insulin-dependent) diabetes and healthy subjects. Diabetologia. 1985;28:420–6. doi: 10.1007/BF00280884. [DOI] [PubMed] [Google Scholar]
- 27.Hecht TT, Longo DL, Matis LA. The relationship between immune interferon production and proliferation in antigen specific, MHC restricted T-cells and clones. J Immunol. 1983;131:1049–55. [PubMed] [Google Scholar]
- 28.Kozovska M, Zang YCQ, Aebischer I, et al. T cell recognition motifs of an immunodominant peptide of myelin basic protein in patients with multiple sclerosis: structural requirements and clinical implications. Eur J Immunol. 1998;28:1894–901. doi: 10.1002/(SICI)1521-4141(199806)28:06<1894::AID-IMMU1894>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 29.Hyöty H, Hiltunen M, Lönnrot M. Enterovirus infections and insulin dependent diabetes mellitus—evidence for causality. Clin Diagn Virol. 1998;9:77–84. doi: 10.1016/s0928-0197(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 30.Hiltunen M, Lönnrot M, Hyöty H. Immunisation and insulin-dependent diabetes mellitus: is there a link? Drug Safety. 1999;20:207–12. doi: 10.2165/00002018-199920030-00001. [DOI] [PubMed] [Google Scholar]


