Abstract
HIV-1 can be neutralized by soluble factors produced and secreted by activated CD8+ T cells. Production of such anti-viral CD8 factors (including chemokines) can be induced with IL-2 or phytohaemagglutinin (PHA). In addition to PHA or IL-2, we have co-stimulated CD8+ T cells with PHA/IL-2 and a mixture of thymic peptides (TP) of molecular weights below 10 kD. For the activation, CD8+ T cells were purified from peripheral blood mononuclear cells of HIV-1− individuals and any resultant anti-viral activity was monitored using an HIV-1 neutralization assay. Using HIV-1 isolates highly resistant to chemokine inhibition we detected significantly higher levels of HIV-1 neutralizing activity in CD8+ T cell culture supernatants which had been co-activated with TP. When the TP-induced anti-viral activity was monitored, neutralization of both non-syncytia-inducing (NSI) and syncytia-inducing (SI) patient isolates was enhanced by 38% (NSI, PHA +/− TP), 66% (SI, PHA +/− TP), 28% (NSI, IL-2 +/− TP), and 57% (SI, IL-2 +/− TP) compared with the anti-viral activity present in supernatants from CD8+ T cell cultures stimulated only with PHA or IL-2. Peptide sequence analysis of purified TP showed that the TP mixture predominantly contains peptides with homology to human histone and collagen sequences. Our data demonstrate that CD8+ T cells are additionally activated by a mixture of TP. In this way, the production of HIV-1 neutralizing CD8 factors can be enhanced.
Keywords: HIV-1, anti-viral factors, CD8+ T cells, thymic peptides
INTRODUCTION
Infection of CD4+ T cells with HIV-1 can be inhibited by soluble factors secreted by activated CD8+ T cells [1, 2]. Chemokines are a group of CD8 factors able to neutralize HIV-1: the CC-chemokines RANTES, MIP-1α, and MIP-1β [3], the CXC-chemokine SDF-1 [4, 5], and MDC-1 [6] are produced and secreted by CD8+ T cells in response to stimuli such as phytohaemagglutinin (PHA) or IL-2. Chemokines are the natural ligands for the cellular receptors, the chemokine receptor seven-transmembrane (7-TM) family. The characterized anti-viral chemokines prevent HIV-1 infection by blocking the interaction of the external HIV-1 glycoprotein (gp120) V3 loop [7] with the chemokine receptors. Besides the primary CD4 receptor, the two chemokine receptors CCR5 [8] and CXCR4 [9] act as the major co-receptors for HIV-1 isolates. HIV-1 isolates using the CCR5 co-receptor are of macrophage tropic (M-tropic) or non-syncytia-inducing (NSI) phenotype [10, 11], whereas HIV-1 isolates using the CXCR4 co-receptor possess the T cell tropic (T-tropic) or syncytia-inducing (SI) phenotype [12]. Since the gp120–chemokine receptor interaction is crucial for viral infectivity, chemokines which bind to the same receptor molecule are able to inhibit viral infection. In this way, the CC-chemokines RANTES, MIP-1α and MIP-1β neutralize NSI strains preferentially using CCR5, whereas the CXC-chemokine SDF-1 has anti-viral activity directed against HIV-1 strains of the SI phenotype preferentially using the CXCR4 molecule.
In addition to the CC- and CXC-chemokines, IL-16 and other non-characterized anti-viral factors have been described in the supernatant of CD8+ T cells [13, 14]. Besides IL-16 and chemokines, all CD8+ cell-derived, but as yet unknown factors are called CD8+ cell anti-viral factors (CAF). CAF blocks viral transcription intracellularly [15] and does not correspond to any known chemokines or cytokines, including interferons (IFNs) and tumour necrosis factor (TNF) [16, 17]. Contrary to the actions of chemokines, CAF is able to neutralize HIV-1 NSI as well as SI isolates [18, 19]. Although CAF has potent anti-viral activity, the amount of CAF produced by CD8+ T cells is low and varies among infected individuals [20, 21].
The aim of our study was to enhance the production of HIV-1 anti-viral factors by CD8+ T cells. It has been reported that thymic peptides have immunomodulatory activity on CD8+ T cells [22]. Therefore, we have used thymic peptides (TP) for CD8+ T cell activation. The anti-viral activity in the supernatants of TP- activated CD8+ T cells was tested against HIV-1 patient isolates of both NSI and SI phenotype. The patient isolates used for the screening of CD8 anti-viral factors were chosen because they were highly resistant against neutralization by one of the two chemokines RANTES and SDF-1.
MATERIALS AND METHODS
Thymic peptides
A commercial extract of bovine TP (Thym-Uvocal; Strathmann AG, Hamburg, Germany) [23] was used. This TP extract was further purified by diethylether extraction followed by azeotrope distillation. To produce a TP mixture of low molecular weight (<10 kD), the purified TP mixture was diluted in RPMI 1640 (10 mg/ml) and ultrafiltrated (10 kD cut off; Sartorius, Goettingen, Germany). After sterile filtration (0.22 μm; Sartorius) the TP mixture was used to activate CD8+ T cells.
Activation of CD8+ T cells
Peripheral blood lymphocytes (PBL) from HIV-1− donors were isolated by Ficoll–Paque gradient centrifugation. CD8+ T cells were purified by immunomagnetic beads (Dynal, Oslo, Norway). Purified CD8+ T cells (5 × 105/ml) were cultured in 24-well plates in serum-free RPMI 1640 including 5 μg/ml PHA-L (Sigma, St Louis, MO) or 50 U/ml IL-2. After stimulation for 48 h 0.4 μg/ml of the TP mixture was added. Fetal calf serum (FCS; 10%) was added 12 h post-TP-stimulation. Supernatants from CD8+ T cell cultures were collected at daily intervals and tested for HIV-1 neutralization at a concentration of 50%.
Determination of CD8+ anti-viral activity
The isolation of cell-free neutralization-resistant patient isolates from the serum of AIDS patients has been described before [24]. Virus stocks were grown in peripheral blood mononuclear cells (PBMC) and used for NSI and SI testing and neutralization assays in PBMC. For virus neutralization, 100 μl of CD8+ T cell culture supernatant were mixed with 50 μl containing 2 × 105 PHA- or IL-2-activated PBMC and incubated for 1 h at 37°C. After incubation, 50 μl containing 25 TCID50 of PI-910 or PI-952 virus were added and the cells were incubated for 18 h at 37°C. After viral infection cells were washed four times in RPMI 1640. Viral p24 antigen was measured by a standard p24 antigen ELISA [25] (Aalto Bio Reagents Ltd., Dublin, Ireland).
Determination of chemokines
Levels of RANTES in the supernatants of CD8+ T cell cultures were measured by a commercial ELISA (R&D Systems, Minneapolis, MN). Levels of SDF-1 were measured in a dot blot assay. Cell culture medium (100 μl) was mixed with 1, 10, 50, 100, 200 and 500 ng of SDF-1 (Strathmann Biotech GmbH, Hamburg, Germany) and blotted onto nitrocellulose membrane (BA85; Schleicher & Schuell, Dassel, Germany). Supernatants (200 μl) from CD8+ T cell cultures were also blotted onto nitrocellulose. Bound SDF-1 was detected with goat anti-SDF-1 antibodies (R&D Systems) and secondary rabbit anti-goat horseradish peroxidase (HRP)-conjugated antibodies (Dako A/S, Glostrup, Denmark). Staining was performed with 4-chloro-1-naphthol.
Peptide sequencing
To analyse TP amino acid sequences 1 ml of the TP mixture was fractionated using cationic exchange chromatography (Polysulfoethyl-A column). The prepurified fractions or 20 μl of the crude mixture were applied on a C18 reversed phase high performance liquid chromatography (HPLC) column directly connected on-line to a TSQ7000 triple-stage quadrupole mass spectrometer (Finnigan-MAT). With this procedure the amino acid sequences of 26 peptides (TP1, TP3–TP18, TP21–TP26, TP29–TP31) were identified. Sequences of five additional thymic peptides (TP2, TP19, TP20, TP27, TP28) were analysed by Edman sequencing. The crude peptide mixture (1 ml) was separated using a cationic exchange column (Protein-Pac; Waters, Milford, MA). Distinct and predominant fractions were taken for a second purification on a cationic exchange column (Polysulfoethyl-A). Fractions were further purified on a C18 column (2 × 250 mm; SGE, Weiterstadt, Germany). Purified peptides from this third separation were directly applied onto an automated protein sequencer (476A; Perkin Elmer, Applied Biosystems, Foster City, CA).
RESULTS
HIV-1 patient isolates
Two patient isolates, PI-910 and PI-952, obtained from AIDS patient serum were used for testing anti-viral activity in cell culture supernatants. Virus PI-952 showed syncytia formation when tested in PBMC. Virus PI-910 was of the NSI phenotype. V3 loop sequence analysis showed that the N-glycosylation site NNT within the PI-952 V3 loop amino acid sequence was changed to IQK in the PI-910 V3 loop (Fig. 1). Both isolates had a high content of positively charged amino acids in the V3 loop sequences (PI-952, +5; PI-910, +7), but it is the removal of the N-glycan which correlates highly with the NSI phenotype [26].
Fig. 1.
Features of HIV-1 patient isolates 910 and 952. Both patient isolates were isolated from serum samples of AIDS patients. Virus stocks were grown in donor peripheral blood mononuclear cells (PBMC) and both isolates were characterized by V3 sequencing. The non-syncytia-inducing (NSI) and syncytia-inducing (SI) phenotype and neutralization by RANTES and SDF-1 chemokines were tested in donor PBMC. In bold, positively charged amino acids; underlined, N-glycosylation site.
To investigate whether PI-910 or PI-952 could be neutralized by RANTES or SDF-1 chemokines, we studied the action of these chemokines on infection of PBMC (Table 1). PI-910 was neutralized at a 43% level by a combination of 500 ng/ml RANTES and 500 ng/ml SDF-1. RANTES and SDF-1 alone showed no anti-PI-910 activity. PI-952 was more efficiently neutralized by SDF-1 (44%, 500 ng/ml) compared with RANTES (26%, 500 ng/ml). The data demonstrate that both virus isolates were highly resistant against RANTES or SDF-1 inhibition.
Table 1.
Inhibition of PI-910 and PI-952 infection of peripheral blood mononuclear cells (PBMC) by RANTES and SDF-1

–, Neutralizing activity <1%.
* For each of the two chemokines.
CD8+ activation and progress of anti-viral activity
To analyse the progress of anti-viral activity within a given time, CD8+ T cell culture supernatants were collected at daily intervals post-stimulation. CD8+ T cells stimulated with PHA alone showed no increase in anti-viral activity over a period of 5 days in contrast to PHA and TP co-activation. After 5 days, the anti-viral activity present in the supernatants of PHA and TP co-activated cells was enhanced by 50%, as measured by PI-910 neutralization (Fig. 2). We identified the highest levels of anti-viral activity in supernatants 5 days after TP stimulation. Day 5 supernatants were therefore tested for PI-910 and PI952 neutralizing activity in all the following experiments.
Fig. 2.
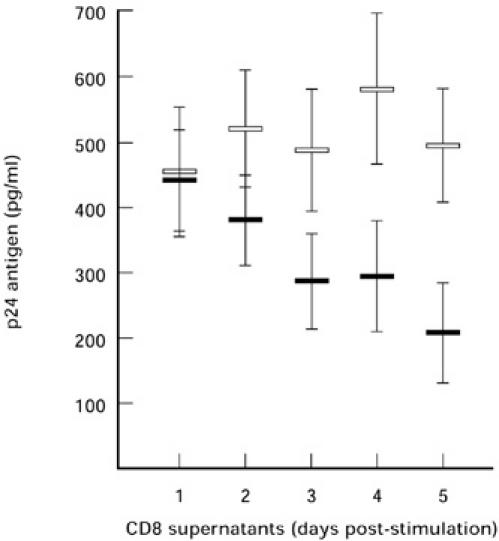
CD8+ activation and progress of anti-viral activity. Anti-viral activity in supernatants of CD8+ T cell cultures was tested after activation of cells with phytohaemagglutinin (PHA; 5 μg/ml, □) or a mixture of PHA and thymic peptides (TP; 0.4 μg/ml, ▪). Anti-viral activity was measured at daily intervals (days 1–5) in a neutralization assay using 25 TCID50 of the non-syncytia-inducing (NSI) patient isolate PI-910. In the neutralization assay virus PI-910 was cultured in PHA- and IL-2-stimulated HIV-1− donor peripheral blood mononuclear cells (PBMC). Each value is the average percent reduction in p24 in triplicate wells; error bars represent s.d.
Anti-viral activity against SI and NSI patient isolates
The anti-viral activity of CD8+ T cell culture supernatants was tested in HIV-1 inhibition assays using patient isolates of either the NSI (PI-910) or SI (PI-952) phenotype, which are highly resistant to inhibition by chemokines. CD8+ T cells were activated with combinations of PHA and TP or IL-2 and TP. Compared with the PHA controls (0 ± 8% and 0 ± 7%), anti-viral activity was enhanced after PHA and TP activation by 38 ± 11% for PI-910 and was enhanced by 66 ± 12% for PI-952. Activation of CD8+ T cells with TP and IL-2 also showed an increase of anti-viral activity. Compared with the IL-2 controls (0 ± 3% and 0 ± 4%), anti-viral activity was enhanced after IL-2 and TP activation by 28 ± 9% for PI-910 and was enhanced by 57 ± 13% for PI-952 (Fig. 3). Both experiments demonstrate that TP stimulation, in addition to PHA or IL-2, activates CD8+ T cells in such a way that higher levels of anti-viral factors are secreted into the cell culture medium.
Fig. 3.
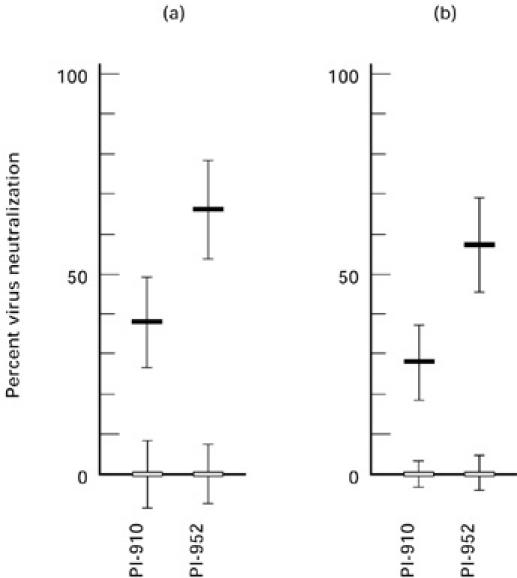
Anti-viral activity against syncytia-inducing (SI) and non-syncytia-inducing (NSI) patient isolates. (a) Anti-viral activity in supernatants of CD8+ T cell cultures was tested after activation of cells with phytohaemagglutinin (PHA; 5 μg/ml, □) or a mixture of PHA and thymic peptides (TP; 0.4 μg/ml, ▪). (b) Anti-viral activity in supernatants of CD8+ T cell cultures was tested after activation of the cells with IL-2 (50 U/ml, □) or a mixture of IL-2 and TP (40 μg/ml, ▪). Each value is the average percent reduction in p24 in triplicate wells; error bars represent s.d.
RANTES and SDF-1 levels in CD8 T cell culture supernatants
Since high concentrations of RANTES and SDF-1 showed an inhibitory effect on PI-910 and PI-952 infection of PBMC, we measured the amount of RANTES and SDF-1 in CD8+ T cell culture supernatants. No significant increase in RANTES was detected in the supernatants of the TP-activated supernatants compared with the PHA and IL-2 controls. In our study, CD8+ T cells from different donors produced RANTES levels varying between 30 and 500 pg/ml. After TP activation, however, only changes < 10% of the background level (amount of RANTES in the PHA, IL-2 controls) were detected (data not shown).
Since no commercial SDF-1 ELISA was available, we measured SDF-1 levels in a dot blot procedure. This assay is suitable to detect levels > 50 ng/ml of SDF-1 in CD8+ T cell culture supernatants. In all the CD8+ T cell supernatants used in our study SDF-1 concentrations were < 50 ng/ml (data not shown). Thus, the activation of CD8+ T cells does not lead to a marked increase of RANTES as well as SDF-1. Neither of these two chemokines was produced at levels > 500 pg/ml (RANTES) or 50 ng/ml (SDF-1). These chemokine concentrations had either no or only a weak neutralizing activity on the PI-952 and PI-910 viruses, as shown in Table 1.
Characterization of the TP mixture
The amino acid sequence of 31 purified peptides was determined by combined LC-MS/MS or Edman sequencing. The sequences of the purified TP as shown in Fig. 4 were compared with the Genbank database. We observed that most of the TP sequences (TP1 to TP18) were identical to partial sequences of human histone (H2A.1, H2B, H3.1, and H4) and collagen proteins (TP19 to TP29). Two peptides were identified as sharing homology with neurexin-1α (TP30) and serum albumin precursor protein sequences (TP31). Altogether, our sequencing data demonstrate that the TP mixture is predominantly composed of peptides highly identical to amino acid sequences of human proteins.
Fig. 4.
Thymic peptide (TP) sequence homologies. All peptides were purified from the crude TP mixture by anion exchange chromatography. *Purified peptides sequenced by combined LC-MS/MS; +, purified peptides sequenced by Edman degradation; −, positions of amino acid identity. Dots represent sequence gaps, numbers the position of the amino acid in the human or bovine sequences from the Genbank database (left column). Sequence homology studies were performed using the Genbank database and the Basic Local Alignment Search Tool (BLAST) from the National Centre for Biotechnology Information (NCBI) [49].
DISCUSSION
It is well known that CD8+ T cells can suppress viral infection by direct destruction of infected cells [27]. In HIV-1 infection, however, it is also important that CD8+ T cells are able to suppress viral infection by indirect effects such as the production of anti-viral soluble factors [16]. The family of these HIV-1 suppressor factors is still growing. More and more chemokines are characterized as being soluble anti-viral factors. Additional anti-viral factors, not identical to chemokines, have also been described, but not yet characterized [18]. Since CD8+ T cell responses can be manipulated in vivo by vaccination [28, 29] or low-dose IL-2 therapy [30–32], the new developments in characterizing CD8 factors lead to important questions, such as how to activate and enhance the indirect anti-viral effector activities of CD8+ T cells in vitro and in vivo.
In our study, we have demonstrated that the production and secretion of anti-viral factors into the cell culture medium by CD8+ T cells is significantly enhanced after in vitro stimulation with a mixture of TP. It has been reported that CD8+ T cells also respond in vivo to TP [33] or thymic hormones, like thymosin α1 [34], thymus humoral factor γ2 [35], and thymopentin (TP-5) [36]. However, these studies present only limited data on CD8 proliferation, the CD8 specificity and their direct anti-viral activities. In our studies, we observed no TP effects on CD8 proliferation, as measured by propidium iodide uptake, and also no toxic TP effects on CD8 cells, as measured by alamar blue staining (data not shown). Only the enhancing, indirect, TP effect on the secretion of anti-viral factors was observed.
Besides the direct HIV-1-specific CD8 effects, soluble factors, which are characterized by their indirect anti-viral activity, can be detected in the supernatants of CD8+ T cells from HIV-1− [37] or HIV-1+ [38] individuals. In our study we used CD8+ T cells from HIV-1− individuals because CD8+ T cells from infected individuals show reduced responses to not only HIV-1-specific but also various other antigens [39]. High levels of anti-viral factors are produced by various CD8+ T cell clones isolated from AIDS patients. The anti-viral activity produced by such CD8+ T cell clones is highly HIV-1 type-specific and therefore shows different neutralization effects on HIV-1 laboratory strains or primary isolates [3]. To avoid HIV-specific effects we used mixtures of CD8+ T cells purified from peripheral blood to determine the influence of TP on the production of anti-viral factors. Now, our system can also be used for screening CD8+ T cell clones from HIV-1− or HIV-1+ individuals.
In other studies, CD8+ T cell culture supernatants were tested for anti-viral activity against HIV-1 laboratory adapted isolates grown in cell lines [3]. We, in contrast, used wild-type PI grown in donor PBMC. Our two isolated PI possess all the characteristic features of typical NSI or SI primary isolates [26]. In general, PI are distinct from cell culture-adapted laboratory strains because they are neutralization-resistant [40] and because they are able to use more than one co-receptor [41]. In addition, the wild-type PI used in our study were highly resistant to RANTES and SDF-1 chemokines, the natural ligands for the CCR5 and CXCR4 co-receptors, responsible for blocking HIV-1 infection. Therefore, measuring anti-viral activity in a system based on PI and PBMC is a more stringent assay compared with viral neutralization assays using cell culture-adapted laboratory strains and laboratory cell lines.
The effects of TP on CD8+ T cells [42] and in AIDS therapy [33] have already been published, but the in vivo effects reported could not be adequately reproduced by CD8-specific in vitro assays [36, 43, 44]. We have now demonstrated that TP have direct effects on CD8+ T cells. In addition to crude thymus extracts, thymus hormones are proposed to play a role in the activation of CD8+ T cells [35, 36]. In general, crude extracts of cells or tissue samples contain high amounts of proteins and peptides of the major cellular components, such as actin, collagen, etc. The TP mixture used in our experiments was also a crude extract of thymus tissue that had been proteolytically degraded and only fractionated by ultrafiltration to generate TP of low molecular weight (< 10 kD) [23]. Our peptide analysis of the crude TP mixture showed that the mixture contains peptides highly homologous to human histone and collagen sequences. We might speculate that the major components of the TP mixture cause the enhancing effect but, to prove this, fractions of the TP mixture, histone-specific or collagen-specific peptides, should be identified which induce CD8 anti-viral activity.
Interestingly, anti-viral activity was enhanced by TP not only in conjunction with PHA. Enhancement of anti-viral activity was also observed with a combination of TP and IL-2. Our data also showed that the TP-induced anti-viral activity against the PI-952 SI phenotype was greater than neutralization of the PI-910 NSI phenotype. This leads to the suggestion that higher levels of CD8 anti-viral factors blocking SI primary isolates were produced. During the progression of HIV-1 infection, the switch from NSI to SI viruses is a key determinant in AIDS progression [45] and might be triggered by immune escape mechanisms. In HIV-1-infected individuals higher serum levels of RANTES and MIP-1α are detected. This observation is correlated with the presence of an increased proportion of activated CD8+CD38+ T cells in the peripheral blood [46]. High RANTES serum levels might be able to suppress NSI strains, but under the selective force of NSI-specific β-chemokines (RANTES, MIP-1α, MIP-1β) SI escape mutants might be selected. The production of higher amounts of anti-viral factors after TP activation might explain the higher anti-viral activity observed against the PI-952 SI phenotype. However, in our study we demonstrate that TP activation had no significant effect on the activation of RANTES or SDF-1 production. Both viruses were highly resistant to neutralization by these chemokines. High amounts of 500 ng/ml RANTES and SDF-1 were needed to observe a 26–43% neutralization. Moreover, such chemokine concentrations were not found in CD8+ T cell culture supernatants and are usually not present at these high levels in plasma of HIV-1− or HIV-1+ individuals. Since neutralization of both PI was enhanced by TP stimulation, other chemokines or anti-viral factors like CAF might be induced responsible for neutralizing the RANTES- and SDF-1-resistant isolates.
In our experiments, TP activation increased the amount of anti-viral factors reactive against both NSI and SI strains. One aim in HIV-1 therapy is to ‘hit early and hard’. In this regard, it is conceivable that, especially in the early stage of the disease, the anti-viral potential of CD8+ T cells could be enhanced to produce higher levels of all the anti-viral factors, the well known chemokines as well as the unknown CD8 factors, to suppress HIV-1 replication as effectively and as soon as possible. Since HIV-specific CD8+ cells are lost during disease progression [47], the indirect effects of the remaining CD8+ T cells are important not only in the early stage of the disease, but they might also become more and more important over time. Developments in HIV-1 therapy clearly point toward activation of CD8+ T cells and immune restoration [48]. Therefore, the activation of CD8+ T cells by TP seems to be a new strategy to manipulate CD8+ T cells and to induce new and unknown HIV anti-viral factors in vitro and in vivo.
Acknowledgments
We wish to acknowledge the technical assistance of Sonja Ziegelmaier. This work was supported in part by grant FKZ:01 KI 9714/6 to M.S. from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (BMBF).
REFERENCES
- 1.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–6. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 2.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ T lymphocytes control of HIV replication in cultured CD4+ cells varies among infected individuals. Cell Immunol. 1989;119:470–5. doi: 10.1016/0008-8749(89)90259-1. [DOI] [PubMed] [Google Scholar]
- 3.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 4.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 5.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 6.Pal R, Garzino-Demo A, Markham PD, Burns J, Brown M, Gallo RC, DeVico AL. Inhibition of HIV-1 infection by the beta-chemokine MDC. Science. 1997;278:695–8. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 7.Speck RF, Wehrly K, Platt EJ, Atchison RE, Charo IF, Kabat D, Chesebro B, Goldsmith MA. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–9. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 10.Alkhatib G, Broder CC, Berger EA. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–94. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger EA, Doms RW, Fenyo EM, et al. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 12.Berson JF, Long D, Doranz BJ, Rucker J, Jirik FR, Doms RW. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–95. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baier M, Bannert N, Werner A, Adler HS, Otteken A, Beer B, Norley S, Kurth R. Chemoattractant factors and the control of human immunodeficiency virus replication. Pathobiology. 1998;66:128–30. doi: 10.1159/000028008. [DOI] [PubMed] [Google Scholar]
- 14.Leith JG, Copeland KF, McKay PJ, Richards CD, Rosenthal KL. CD8+ T-cell-mediated suppression of HIV-1 long terminal repeat-driven gene expression is not modulated by the CC chemokines RANTES, macrophage inflammatory protein (MIP)-1 alpha and MIP-1 beta. AIDS. 1997;11:575–80. doi: 10.1097/00002030-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mackewicz CE, Blackbourn DJ, Levy JA. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci USA. 1995;92:2308–12. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackewicz CE, Barker E, Levy JA. Role of beta-chemokines in suppressing HIV replication. Science. 1996;274:1393–5. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 17.Brinchmann JE, Gaudernack G, Vartdal F. In vitro replication of HIV-1 in naturally infected CD4+ T cells is inhibited by rIFN alpha 2 and by a soluble factor secreted by activated CD8+ T cells, but not by rIFN beta, rIFN gamma, or recombinant tumor necrosis factor-alpha. J Acquir Immune Defic Syndr. 1991;4:480–8. [PubMed] [Google Scholar]
- 18.Moriuchi H, Moriuchi M, Combadiere C, Murphy PM, Fauci AS. CD8+ T-cell-derived soluble factor(s), but not beta-chemokines RANTES, MIP-1 alpha, and MIP-1 beta, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–5. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 20.Walker CM, Levy JA. A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunol. 1989;66:628–30. [PMC free article] [PubMed] [Google Scholar]
- 21.Mackewicz C, Levy JA. CD8+ cell anti-HIV activity: nonlytic suppression of virus replication. AIDS Res Hum Retrovir. 1992;8:1039–50. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 22.Hadden JW, Saha A, Sosa M, Hadden EM. Immunotherapy with natural interleukins and/or thymosin alpha 1 potently augments T-lymphocyte responses of hydrocortisone-treated aged mice. Int J Immunopharmacol. 1995;17:821–8. doi: 10.1016/0192-0561(95)00069-e. [DOI] [PubMed] [Google Scholar]
- 23.Birr C, Bohn B, Jaeger KH. Biochemical characterization and immunomodulatory action of thymic components as determined by flow cytometry on human lymphocytes. Thymus. 1987;10:159–68. [PubMed] [Google Scholar]
- 24.Schreiber M, Petersen H, Wachsmuth C, Müller H, Hufert FT, Schmitz H. Antibodies of symptomatic human immunodeficiency virus type 1-infected individuals are directed to the V3 domain of noninfectious and not of infectious virions present in autologous serum. J Virol. 1994;68:3908–16. doi: 10.1128/jvi.68.6.3908-3916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama EE, Shioda T, Tatsumi M, et al. Importance of the N-glycan in the V3 loop of HIV-1 envelope protein for CXCR-4- but not CCR-5-dependent fusion. FEBS Letters. 1998;426:367–72. doi: 10.1016/s0014-5793(98)00375-5. [DOI] [PubMed] [Google Scholar]
- 26.Moore JP, McKeating JA, Weiss RA, Sattentau QJ. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–42. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 27.Riviere Y, Tanneau-Salvadori F, Regnault A, et al. Human immunodeficiency virus-specific cytotoxic responses of seropositive individuals: distinct types of effector cells mediate killing of targets expressing gag and env proteins. J Virol. 1998;63:2270–7. doi: 10.1128/jvi.63.5.2270-2277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toda S, Ishii N, Okada E, et al. HIV-1-specific cell-mediated immune responses induced by DNA vaccination were enhanced by mannan-coated liposomes and inhibited by anti-interferon-gamma antibody. Immunology. 1997;92:111–7. doi: 10.1046/j.1365-2567.1997.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson SA, Sherritt MA, Medveczky J, Elliott SL, Moss DJ, Fernando GJ, Brown LE, Suhrbier A. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–23. [PubMed] [Google Scholar]
- 30.Khatri VP, Baiocchi RA, Bernstein ZP, Caligiuri MA. Immunotherapy with low-dose interleukin-2: rationale for prevention of immune-deficiency-associated cancer. Cancer J Sci Am. 1997;1:S129–S36. [PubMed] [Google Scholar]
- 31.Jacobson EL, Pilaro F, Smith KA. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity. Proc Natl Acad Sci USA. 1996;93:10405–10. doi: 10.1073/pnas.93.19.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landay AL, Clerici M, Hashemi F, Kessler H, Berzofsky JA, Shearer GM. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J Infect Dis. 1996;173:1085–91. doi: 10.1093/infdis/173.5.1085. [DOI] [PubMed] [Google Scholar]
- 33.Valesini G, Barnaba V, Benvenuto R, Balsano F, Mazzanti P, Cazzola P. A calf thymus acid lysate improves clinical symptoms and T-cell defects in the early stages of HIV infection: second report. Eur J Cancer Clin Oncol. 1987;23:1915–9. doi: 10.1016/0277-5379(87)90059-9. [DOI] [PubMed] [Google Scholar]
- 34.Garaci E, Mastino A, Favalli C. Enhanced immune response and antitumor immunity with combinations of biological response modifiers. Bull N Y Acad Med. 1989;65:111–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Maggiolo F, Taras A, Pravettoni MG, Leone M, Ingrosso A, Suter F. Zidovudine and thymus humoral factor gamma-2 in the treatment of HIV infection: preliminary clinical experience. Infection. 1997;25:35–38. doi: 10.1007/BF02113505. [DOI] [PubMed] [Google Scholar]
- 36.Mascart-Lemone F, Huygen K, Clumeck N, Brenez D, Bolla K, Duchateau J. Stimulation of cellular function by thymopentin (TP-5) in three AIDS patients. Lancet. 1983;24:735–6. doi: 10.1016/s0140-6736(83)92271-7. [DOI] [PubMed] [Google Scholar]
- 37.Rosok B, Voltersvik P, Larsson BM, Albert J, Brinchmann JE, Asjo B. CD8+ T cells from HIV type 1-seronegative individuals suppress virus replication in acutely infected cells. AIDS Res Hum Retrovir. 1997;13:79–85. doi: 10.1089/aid.1997.13.79. [DOI] [PubMed] [Google Scholar]
- 38.Clerici M, Balotta C, Trabattoni D, et al. Chemokine production in HIV-seropositive long-term asymptomatic individuals. AIDS. 1996;10:1432–3. doi: 10.1097/00002030-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Geretti AM, Dings ME, van Els CA, van Baalen CA, Wijnholds FJ, Borleffs JC, Osterhaus AD. Human immunodeficiency virus type 1 (HIV-1) and Epstein–Barr virus-specific cytotoxic T lymphocyte precursors exhibit different kinetics in HIV-1-infected persons. J Infect Dis. 1996;174:34–45. doi: 10.1093/infdis/174.1.34. [DOI] [PubMed] [Google Scholar]
- 40.Moore JP, Cao Y, Qing L, et al. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–9. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bazan HA, Alkhatib G, Broder CC, Berger EA. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–91. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadden JW. T-cell adjuvants. Int J Immunopharmacol. 1994;16:703–10. doi: 10.1016/0192-0561(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 43.Costigliola P, Ricchi E, Colangeli V, Pintori C, Boni P, Chiodo F. Thymopentin (TP-5) therapy during lymphadenopathy syndrome (LAS/ARC): preliminary report. J Exp Pathol. 1987;3:705–12. [PubMed] [Google Scholar]
- 44.Clumeck N, Cran S, Van de Perre P, Mascart-Lemone F, Duchateau J, Bolla K. Thymopentin treatment in AIDS and pre-AIDS patients. Surv Immunol Res. 1985;4:58–62. doi: 10.1007/BF02919057. [DOI] [PubMed] [Google Scholar]
- 45.Glushakova S, Grivel JC, Fitzgerald W, Sylwester A, Zimmerberg J, Margolis LB. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat Med. 1998;4:346–9. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 46.Müller P, Engelstadter M, Werner A, et al. Increased serum and mRNA levels of RANTES associated with elevated levels of activated CD8+CD38+ T cells in HIV-1 infected individuals. Intervirology. 1997;40:263–70. doi: 10.1159/000150556. [DOI] [PubMed] [Google Scholar]
- 47.Zerhouni B, Sanhadji K, Touraine JL. Loss of T-cell cytotoxic responses in the course of HIV-1 infection. Thymus. 1997;24:203–19. [PubMed] [Google Scholar]
- 48.Ferbas J. Perspectives on the role of CD8+ cell suppressor factors and cytotoxic T lymphocytes during HIV infection. AIDS Res Hum Retrovir. 1998;14:S153–S60. [PubMed] [Google Scholar]
- 49.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]




