Abstract
CD7 co-expression by CD4 T cells has been reported to be higher in the Th1 compared with the Th2 functional subset. Clinical immunodeficiency and immune dysregulation are more prevalent in the advanced stages of B cell chronic lymphocytic leukaemia (B-CLL). To analyse this further 25 patients with B-CLL and 11 healthy subjects were examined for cell surface CD7 and intracellular IFN-γ and IL-4 expression in the peripheral blood CD4+ T helper cell population. Significantly decreased CD7, IFN-γ and IL-4 expression was observed in the patients with B-CLL (P < 0.001). While CD7 negativity and IL-4 expression were more frequent in the later stages of the disease, this did not attain statistical significance. These results suggest a possible explanation for the reduced cellular and humoral immunity in B-CLL.
Keywords: B-CLL, intracellular, cytokines, CD4, CD7
INTRODUCTION
B cell chronic lymphocytic leukaemia (B-CLL) is the most common leukaemia in the western world and is characterized by the slow and progressive accumulation of monoclonal, and apparently mature CD5+ B lymphocytes [1]. Disease progression is associated with secondary immunodeficiency and with an increased risk of infections [2], which may be related to alterations in T cell subsets and cytokine production [3]. Certainly total T cell numbers are increased, CD4 to CD8 ratios are decreased and activation markers such as HLA-DR, CD25 and CD11b are highly expressed with disease progression [4]. Interestingly, both decreased T helper (Th) function [5], and increased suppressor function [6] have been reported in B-CLL. Regardless, increased autoantibody formation and decreased DTH [2] attendant on disease progression suggest that Th2-type responses may dominate the later stages of B-CLL. Whether this reflects impaired functional T cell maturation is unclear.
Autran et al. have recently demonstrated the CD7 antigen to distinguish CD4 T cells with different cytokine production capability [7]. Thus CD4+CD7+ cells favoured type-1 cytokine production, whereas CD4+CD7− cells favoured type-2 cytokine secretion [7]. We therefore examined the CD4+ T cells from patients with B-CLL and healthy controls for intracellular Th1 (IFN-γ) and Th2 (IL-4) cytokines and CD7 co-expression. This was, first, to see if the pattern of Th1/Th2 function differed between patients and controls, and second, to see if disease progression was associated with a shift to Th2 predominance. Finally, in the absence of a single reliable prognostic marker of disease progression we wanted to see if an assessment of T helper cell cytokine profile would provide any prognostic information. This would be important, as disease progression requires active intervention whilst indolent disease is best managed without treatment which may even be detrimental [8].
PATIENTS AND METHODS
B-CLL was diagnosed according to defined clinical, morphological and immunological criteria [9]. Patients were staged according to the guidelines proposed by Binet et al. [10]. Venous blood was collected into EDTA and tested within 6 h of collection. All reagents were obtained from Sigma Chemical Co. (St Louis, MO) and the MoAbs from Becton Dickinson Immunocytometry Systems (Mountain View, CA).
Stimulation
Briefly, whole blood from patients and controls was diluted 1:1 with RPMI 1640 containing 2 mml-glutamine. Cells were stimulated with phorbol myristate acetate (PMA) at 50 ng/ml and ionomycin at 500 ng/ml both diluted in dimethyl sulfoxide (DMSO) in the presence of Brefeldin A at 10 ng/ml and incubated at 37°C in 4% CO2 for 4 h.
Surface staining
Surface staining was performed on whole blood using the Leu-3a (CD4) FITC- and Leu-9 (CD7) PE-conjugated markers and FACS lysis method (Becton Dickinson Immunocytometry Systems) as described by Bossuyt et al. [11]. Expression was assessed on a FACScan according to the manufacturer's instructions (Becton Dickinson Immunocytometry Systems).
Permeabilization
Cells were permeabilized by initial incubation in the dark for 20 min at room temperature in 4% formaldehyde in fresh 1% hypertonic saline solution. After incubation the cells were centrifuged and the supernatant discarded. The pellet was then resuspended in 0.5% saponin in PBS containing 1% human serum albumin and 0.1% sodium azide and incubated for 10 min at room temperature in the dark. The cells were then centrifuged and the pellet resuspended for intracellular staining as below.
Intracellular staining
Ten microlitres of Fastimmune IFN-γ FITC/IL-4 PE-conjugated MoAbs (Becton Dickinson) were added to the permeabilized cells, vortexed and incubated at room temperature in the dark for 30 min. Tubes were topped up with 0.5% saponin in PBS, centrifuged and decanted as previously described. The cells were resuspended in 0.5 ml of 1% paraformaldehyde in PBS. Samples were stored at 4°C in the dark and analysed within 24 h. Expression was assessed on a FACScan according to the manufacturer's instructions (Becton Dickinson Immunocytometry Systems).
Statistical analysis
As all the data were non-parametrically distributed, the Mann–Whitney U-test was used in all comparisons.
RESULTS
CD7 co-expression by CD4 cells
The ratio of CD7+CD4+ cells to CD7−CD4+ cells was significantly lower in the patients with B-CLL compared with those in the control group (medians 2.7 versus 5.8; P < 0.002) (Fig. 1). There was no significant difference in the ratio between the B-CLL patients in Binet's stage A compared with those in stage B/C (medians 3.1 versus 2.1; P > 0.3).
Fig. 1.
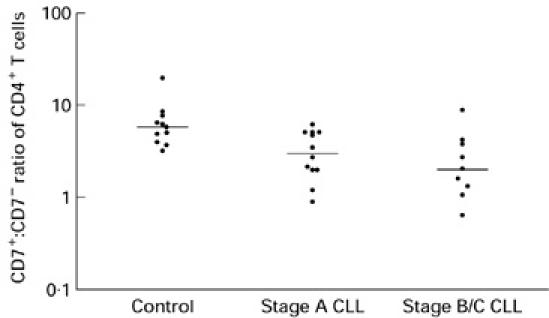
The CD4+CD7+:CD4+CD7− ratio of the control group (n = 11) (median 5.78) compared with all B cell chronic lymphocytic leukaemia (B-CLL) patients (n = 21) (median 2.7) (P < 0.0023), stage A patients (n = 12) (median 3.1) (P < 0.009), and stage B/C patients (n = 9) (median 2.06) (P < 0.001). Stage A B-CLL patients compared with stage B/C was not significant (P > 0.37).
Intracellular IFN-γ and IL-4
The intracellular expression of IFN-γ and IL-4 in the CD4+ T cells of B-CLL patients was significantly reduced compared with the control group (for IFN-γ medians 18.0 versus 51.4, P < 0.0016; for IL-4 medians 1.0 versus 24.0, P < 0.0006) (Fig. 2). The CD4+ intracellular cytokine expression of stage A B-CLL patients was significantly lower than the control group (for IFN-γ medians 19.0 versus 51.4, P < 0.02; for IL-4 medians 2.0 versus 24.0, P < 0.001). Similarly, the CD4+ intracellular cytokine expression of stage B/C-CLL patients was significantly lower than the control group (for IFN-γ medians 12.0 versus 51.4, P < 0.006; for IL-4 medians 1.0 versus 24.0, P < 0.013). While the activated CD4+ cells from stage A patients showed a greater expression of IFN-γ compared with stage B and C patients, this finding was not statistically significant (medians 19.0 versus 12.0; P > 0.25) (Fig. 2). In the case of IL-4 the activated CD4+ cells from stage A patients showed a lower expression of this cytokine compared with stage B and C patients, which was again not statistically significant (medians 2.0 versus 1.0; P > 0.7) (Fig. 3).
Fig. 2.
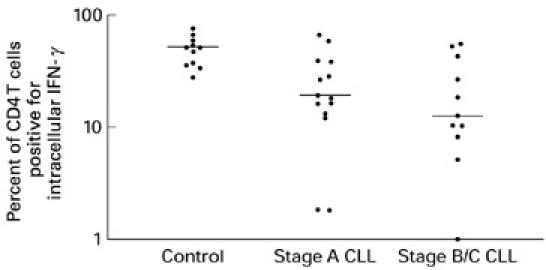
The percentage of intracellular IFN-γ expression from CD4+ cells of the control group (n = 11) (median 51.41) compared with all B cell chronic lymphocytic leukaemia (B-CLL) patients (n = 21) (median 18) (P < 0.0016), stage A patients (n = 14) (median 19) (P < 0.0197), and stage B/C patients (n = 11) (median 12) (P < 0.0059). Stage A patients compared with stage B/C was not significant (P < 0.2874).
Fig. 3.
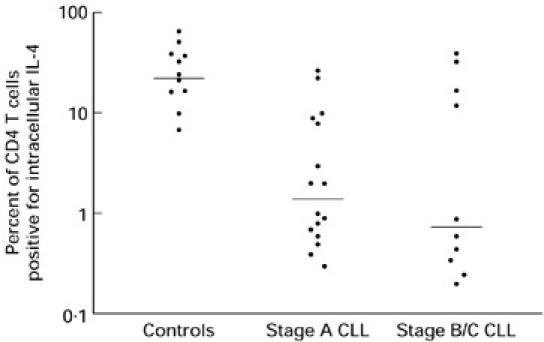
The percentage of intracellular IL-4 expression from the CD4+ cells of the control group (n = 11) (median 24) compared with all B cell chronic lymphocytic leukaemia (B-CLL) patients (n = 21) (median 1) (P < 0.0006), stage A patients (n = 14) (median 2) (P < 0.0009), and stage B/C patients (n = 11) (median 1) (P < 0.0128). Stage A compared with stage B/C patients was not significant (P < 0.7331).
DISCUSSION
Patients with B-CLL are susceptible to a variety of infections. Whereas the pattern of immunodeficiency and subnormal levels of serum immunoglobulins [12] confirms a defect of humoral function, there are cases of impaired cellular immunity leading to severe viral and fungal infections [13]. In many ways subjects with B-CLL appear to behave like patients with the hyper-IgM syndrome, in whom diminished or defective CD40L expression has been documented [14]. Thus patients with B-CLL are susceptible to several categories of infective agents and suffer a variety of autoimmune problems particularly affecting the haematopoietic system [15]. Of interest, impaired CD40L expression by CD4 T cells has recently been documented in patients with B-CLL [16].
It is presently very difficult to establish the balance of cellular (Th1) and humoral (Th2) immunity in a given disease. The best means currently available are based on determining the pattern of cytokine expression by a relevant cell population. Soluble CD30 (sCD30) has recently been proposed as a useful marker in determining the pattern of T cell function. This relates to preferential expression of CD30 by Th2 but not Th1 cells [17]. Thus serum sCD30 has been found to be elevated in advanced HIV infection as well as in patients with allergy [18]. However, CD30 expression is also observed on Th1 cells, suggesting that it may not be as clear a marker of Th2 function as previously proposed [19]. Nevertheless, it would be interesting to see if serum sCD30 is elevated in B-CLL and whether it increases with disease progression.
In the present study we used CD7 co-expression by CD4 T cells and IL-4 and IFN-γ expression as a means of determining the state of cellular and humoral immunity in subjects with B-CLL. CD7 co-expression by CD4 T cells was decreased in the patients with B-CLL compared with the healthy controls (Fig. 1). This difference was evident in both early and intermediate/late stage disease, although comparison of these two latter groups failed to show a difference. Interpretation of these results according to the observations of Autran et al. [7] would suggest impaired cellular immunity arising from reduced numbers of Th1-type CD7/CD4 T cells. This occurred early in the disease and did not appear to worsen with disease progression. For practical purposes, and similar to serum soluble CD30, assessment of CD7 co-expression would be unsuitable as a prognostic marker in individual patients with B-CLL. Thus these cells accounted for < 1% of the total lymphocytes in our patients owing to the large number of CD5 B-CLL cells. Additionally, there was a wide range of values for CD7 expression which overlapped considerably between the healthy controls and patients with B-CLL.
While the reduced CD7 co-expression by CD4 T cells suggests impaired cellular immunity, a combined defect of cellular and humoral immunity is suggested by the studies on intracellular IFN-γ and IL-4 expression (Figs 2 and 3). This is in agreement with the pattern of clinical immunodeficiency observed in this condition. For both cytokines the decrease in expression was greater for the intermediate/late stages of disease compared with early disease. As this difference did not attain statistical significance a larger study would be necessary to determine whether a decline in IL-4 and IFN-γ is a feature of disease progression. Owing to the large variance of the IL-4 and IFN-γ expression, it is extremely unlikely that these tests would offer any useful prognostic information for individual patients.
Whether a defect of CD4 T cell function predisposes individuals to develop B-CLL is unclear. Additionally, the mechanism by which T cell function is altered by the malignant proliferation of CD5+ B cells is also unclear. There is certainly evidence suggesting a down-regulation of T cell and natural killer (NK) cell function by soluble factors such as the IL-2 receptor produced by B-CLL cells [20]. Conversely, T cells in B-CLL may also be active in promoting disease progression and immunodeficiency. Thus some of the cytokines produced by T cells may inhibit CD5+ B-CLL cell apoptosis. However, this effect is produced by both IL-4 and IFN-γ [21]. Interpreting our results in this context suggests that the B-CLL cells become independent of cytokine requirements as the disease progresses. Interestingly, the reduced IL-4 and IFN-γ expression with more advanced disease is also associated with significantly impaired autologous and allogeneic mixed lymphocyte reactions [22]. Furthermore, increased fas receptor expression has been observed in the CD4 T cells of patients with B-CLL [23]. This was associated with expression of a functionally active fas ligand by the CD5 B-CLL cells, suggesting a mechanism for the CD4 T cell numerical, and possibly functional, decline with disease progression. Such a decline may clearly explain the reduced IL-4 and IFN-γ expression observed in our study. Nonetheless, these several studies confirm a general impairment of T cell function by the malignant CD5+ B cells. In the case of the reduced T cell CD40L expression in B-CLL [16], the CD40L mRNA levels were similar to controls, suggesting impaired translation. It would therefore be interesting to compare the mRNA levels for IL-4 and IFN-γ between patients with B-CLL and healthy subjects.
In conclusion, we have found reduced IL-4 and IFN-γ expression by the CD4 T cells in B-CLL. This adds to the growing list of abnormalities of B-CLL T cells and offers another possible explanation for the combined cellular and humoral immune deficiency observed in this condition.
Acknowledgments
Special thanks to the Research & Development Foundation at the St Helier NHS Trust for their support with this work. Additional thanks to Dr Mark Larche, Dr Matthew Helbert, Petra Parlo and Dave Turner for their much appreciated guidance.
REFERENCES
- 1.Dighiero GP, Travade P, Chevret S, Fenaux P, Chastang C, Binet JL. B-cell chronic lymphocytic leukaemia: present status and future directions. Blood. 1991;78:1901–14. [PubMed] [Google Scholar]
- 2.Dianzani U, Omede P, Marmont F, et al. Expansion of T cells expressing low CD4 or CD8 levels in B-cell chronic lymphocytic leukemia; correlation with disease status and neoplastic phenotype. Blood. 1994;83:2198–205. [PubMed] [Google Scholar]
- 3.Freedman AS. Immunology of chronic lymphocytic leukaemia. Haematol Oncol Clinics North Am. 1990;4:405–29. [PubMed] [Google Scholar]
- 4.Peller S, Kaufman S. Decreased CD45RA T cells in B-cell chronic lymphocytic leukaemia: correlation with disease stage. Blood. 1991;78:1569–73. [PubMed] [Google Scholar]
- 5.Chiorazzi N, Fu SM, Montazert G, Kunkel HG, Rai K, Gee T. T-cell helper defect in patients with chronic lymphocytic leukaemia. J Immunol. 1979;122:1087–90. [PubMed] [Google Scholar]
- 6.Kay NE. Abnormal T-cell subpopulation function in CLL. Express suppressor (T gamma) and deficient helper (Tmu) activity with respect to B cell proliferation. Blood. 1981;57:418–20. [PubMed] [Google Scholar]
- 7.Autran B, Legac E, Blanc C, Debre' P. A Th0/Th2-like function of CD4+CD7− T helper cells from normal donors and HIV-infected patients. J Immunol. 1995;154:1408–17. [PubMed] [Google Scholar]
- 8.Benichou (writing committee) French Cooperative Group on Chronic Lymphocytic Leukeamia: effects of chlorambucil and therapeutic decision on initial forms of chronic lymphocytic leukeamia (stage A); results of a randomised clinical trial on 612 patients. Blood. 1990;75:1414–21. [PubMed] [Google Scholar]
- 9.Bennet JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposal for the classification of chronic (mature) B and T lymphoid neoplasms. J Clin Pathol. 1989;42:567–84. doi: 10.1136/jcp.42.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukaemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Bossuyt X, Marti GE, Fleisher TA. Comparative analysis of whole blood lysis methods for flow cytometry. Cytometry. 1997;30:124–33. [PubMed] [Google Scholar]
- 12.Rozman C, Montserrart E, Vinolas N. Serum immunoglobulin levels in B chronic lymphocytic leukaemia. Cancer. 1988;61:279–83. doi: 10.1002/1097-0142(19880115)61:2<279::aid-cncr2820610215>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Fulkerson W, Spickard A, Davis BW. Opportunistic infections in chronic lymphocytic leukaemia. Southern Med J. 1979;72:187–8. doi: 10.1097/00007611-197911000-00046. [DOI] [PubMed] [Google Scholar]
- 14.DiSanto JP, Bonnefoy JY, Gauchet JF, Fischer A, de Saint Basile G. CD40 ligand mutations in X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–3. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 15.Hamblin TJ, Oscier DG, Young BJ. Autoimmunity in chronic lymphocytic leukaemia. J Clin Pathol. 1986;39:713–6. doi: 10.1136/jcp.39.7.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantwell M, Tinh H, Pappas J, Kipps TJ. Acquired CD40-ligand deficiency in chronic lymphocytic leukaemia. Nature Med. 1997;3:984–9. doi: 10.1038/nm0997-984. [DOI] [PubMed] [Google Scholar]
- 17.Del Prete GF, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2 cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- 18.Del Prete G, Maggi E, Pizzolo G, Romagnani S. CD30, Th2 cytokines and HIV infection: a complex and fascinating link. Immunol Today. 1995;16:76–80. doi: 10.1016/0167-5699(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 20.Burton J, Kay NE. Does IL-2 receptor expression and secretion in chronic B-cell leukemia have a role in down regulation of the immune system. Leukemia. 1994;8:92–96. [PubMed] [Google Scholar]
- 21.Nowell PC, Moore JS. Aberrant responses of human lymphocytic neoplasms to cytokine regulation. Immunologic Res. 1998;17:171–17. doi: 10.1007/BF02786442. [DOI] [PubMed] [Google Scholar]
- 22.Ayanlar-Batuman O, Ebert E, Hauptman SP. Defective interleukin-2 production and responsiveness by T cells in patients with chronic lymphocytic leukaemia of B cell variety. Blood. 1986;67:279–94. [PubMed] [Google Scholar]
- 23.Tinhofer I, Marschitz I, Kos M, Henn T, Egle A, Villunger A, Greil R. Differential sensitivity of CD4+ and CD8+ T lymphocytes to the killing efficacy of Fas (Apo-1/CD95) ligand+ tumor cells in B chronic lymphocytic leukemia. Blood. 1998;91:4273–81. [PubMed] [Google Scholar]


