Abstract
Behçet's disease (BD) is a multisystem disorder with oral and genital ulcers, mucocutaneous, ocular, joint, vascular and central nervous system involvement. In this study, the peripheral T cell repertoire was analysed in patients with BD with MoAbs against T cell receptor (TCR) Vβ gene products in CD4+ and CD8+ T cell compartments, and these were compared with rheumatoid arthritis (RA) patients and healthy controls (HC). In the CD4+ T cell compartment, oligoclonal TCR Vβ expression was observed in 56% of BD (10/18), 71% of RA (5/7) patients and 21% (3/14) of HC. In the CD8+ T cell group 50% of BD (9/18), 57% of RA patients and 28% of HC (4/14) had an oligoclonal TCR repertoire. An increase of TCR Vβ5.1 subset was observed in five BD patients among CD8+ T cells. Other elevations of TCR Vβ subsets were heterogeneously distributed with one to three different Vβ subsets. Our results suggest an antigen-driven oligoclonal increase of T cells in BD. There was no overall increase in any Vβ group to suggest a superantigen effect. Analysis of the responsible antigens causing the increase in T cell subsets may give insights into the aetiopathogenesis of BD and immunomodulation of these T cells may lead to new treatments.
Keywords: Behçet's disease, T cell receptor, Vβ usage
INTRODUCTION
Behçet's disease (BD) is a chronic multisystem disorder with mucocutaneous, ocular, arthritic, vascular and central nervous system involvement. The disease has a genetic background with an association with HLA-B51 and an increased incidence in countries around the Mediterranean basin and far east Asia, especially in Japan and Korea [1]. Various microorganisms such as streptococci and herpes simplex virus have been implicated in the aetiopathogenesis of BD [2, 3]. Histological findings in BD suggest a mixed or mainly mononuclear cell infiltration with a predominance of T cells in the inflammatory infiltrates of oral ulcers, erythema nodosum-like lesions and pathergy reactions [4–6]. Changes in peripheral CD4+/CD8+ cell ratio, diminished CD45RA+‘naive’ and increased CD4+16+ and CD4+56+ T cells were also observed [7, 8]. T and B cell responses to peptides derived from mycobacterial and human 65-kD heat shock protein (hsp) were also shown in BD [9–12]. Increases in γδ+ T cells in peripheral blood and cerebrospinal fluid and γδ+ T cell responses to hsp-derived peptides also suggest a role for this T cell subset in the aetiopathogenesis of BD [13–15].
T cells normally recognize antigens as short peptides using their T cell receptors (TCR) specifically binding to MHC molecules on the surface of antigen-presenting cells. TCR recognition of the antigen results in stimulation and clonal proliferation of the activated T cell. In addition, bacteria and viruses also express superantigens which are capable of stimulating T cells having particular Vβ gene segments in both CD4+ and CD8+ T cell compartments, independent of normal MHC TCR recognition [16].
In immune-mediated diseases where the antigen is unknown, studies of the TCR repertoire for any expansion can give clues to the nature of the antigen. MoAbs specific for TCR Vβ gene segments can identify expanded T cell populations. Selective expansions of T cell populations occur in various autoimmune and vasculitic disorders such as psoriasis, multiple sclerosis, temporal arteritis, Kawasaki's disease (KD) and Wegener's granulomatosis [17–21].
In this study peripheral blood T cells were stained with MoAbs against Vβ gene products in patients with active BD and changes in the T cell repertoire was analysed and compared with healthy and diseased controls.
PATIENTS AND METHODS
Patients
Eighteen patients with BD (10 males, eight females, mean age 34.3 ± 6.4 years) classified according to International Study Group Criteria and followed in the multidisciplinary Behçet's Disease Out-patient Clinic in Marmara University Hospital, Istanbul, were studied [22]. All patients had recurrent oral ulcers. Seventeen (94%) patients had cutaneous, 15 (83%) genital, nine (50%) ocular, eight (44%) arthritic, seven (39%) vascular and one (6%) central nervous system involvement. At the time of the study, all patients were clinically active with at least two clinical manifestations. Thirteen patients (72%) were pathergy and 10 (55%) were HLA-B5-positive.
At the time of sampling 11 patients were using colchicine, seven an immunosuppressive agent such as azathioprine, cyclophosphamide or methotrexate, six corticosteroids or non-steroidal anti-inflammatory drugs and one patient each was using dapsone and warfarin. Seven patients with rheumatoid arthritis (RA) classified according to 1987 ACR criteria (six females, one male, age 35.4 ± 14.6 years) and 14 healthy controls (HC) (9 males, five females, age 31.5 ± 7.8 years) were also studied [23].
Monoclonal antibodies
To analyse TCR Vβ variable region gene products, MoAbs against Vβ2, Vβ3, Vβ5.1 Vβ5.2, Vβ5.3, Vβ6, Vβ8, Vβ9, Vβ11, Vβ12, Vβ13.1, Vβ13.6, Vβ14, Vβ16, Vβ17, Vβ18, Vβ20, Vβ21, Vβ22 and Vβ23 were used. Among peripheral blood T cells 0.3–10% were described to carry one of these antigens and approximately 40–70% of T cell repertoire is covered by this panel of antibodies in different series. In addition, FITC-labelled CD3, pan-αβ, pan-γδ and PE-labelled CD4 and CD8 antibodies were also used. CD45+CD14+ was the positive control and mouse IgM, IgG1, IgG2a and IgG2b were negative isotypic controls. All antibodies including anti-Vβ antibodies were purchased from Immunotech SA (Marseille, France).
Analysis of lymphocyte subsets by flow cytometry
Immunophenotypic studies were performed using whole blood. Venous blood samples were collected in EDTA-containing vacuum tubes and processed on the same day. After erythrocyte lysis, 3 μl of purified Vβ MoAbs and isotypic controls were incubated with 30 μl of whole blood for 30 min at 4°C, as suggested by the manufacturer. Cells were then washed with PBS twice and then incubated with 2 μl of goat anti-mouse FITC (FITC–GAM). After incubation at 4°C for 30 min, mouse IgG was added in order to block non-specific binding. Cells were then washed and incubated with PE-conjugated anti-CD4 and anti-CD8 antibodies for 30 min. After the last wash with PBS, stained cells were examined on the same day by flow cytometry equipped with Cell Quest software (FACSort; Becton Dickinson, Mountain View, CA). Mononuclear cells were gated on forward and side scatter plot and checked on CD45–FITC versus CD14–PE plot in order to gate only CD45+ cells. The percentage of cells expressing surface antigens of interest was determined by using appropriate isotypic controls. A T cell expansion was defined as a value of 3 s.d. above the mean of healthy controls for the corresponding antibody [24]. Frequency of expansions in the CD4+ and CD8+ subsets was calculated by dividing the number of all expansions by the sum of all the different TCR Vβ CD4+ or CD8+ MoAbs analysed [18].
Statistical analysis
To compare the differences between mean percentages of study groups the Mann–Whitney U-test was used. Fisher's exact test was employed for the comparison of proportions. For Vβ expansion analysis a corrected P value was given with Bonferroni method by multiplying the calculated P values by 20 (the number of Vβ antibodies studied).
RESULTS
Lymphocyte subsets
CD3+CD4+ helper and CD3+CD8+ suppressor cytotoxic T cell ratios were not significantly different between the three groups (CD4+: BD 41.1 ± 8.4%, RA 45.4 ± 7.5%, HC 37.8 ± 7.5%; CD8+: BD 32.6 ± 7.9%, RA 37.0 ± 10.5%, HC 29.8 ± 8.5%). However, αβ+ T cells were significantly higher in BD and RA patients compared with HC (BD 78.2 ± 7.7%, RA 77.4 ± 9.9%, HC 63.7 ± 13.7%; BD versus HC, P = 0.003; RA versus HC, P = 0.04). BD patients also had an elevated ratio of γδ+ T cells but not reaching statistical significance (BD 14.5 ± 5.8%, RA 8.7 ± 4.2%, HC 10.4 ± 4.8%; BD versus HC, P = 0.08).
TCR Vβ subtype analysis
Oligoclonal expansions in one or more CD4+ or CD8+ TCR Vβ subset were observed in 67% of BD (12/18) and 86% (6/7) of RA patients. Although oligoclonality was lower in the HC group (43%, 6/14), there were no statistically significant differences between the three groups. Similarly, no significant difference was observed between BD and HC in terms of the mean values of any TCR subset in CD4+ and CD8+ T cell populations (data not shown). In the CD4+ group, oligoclonal TCR Vβ subset elevations were observed in 10 (56%) patients with BD compared with five (71%) RA patients and three (21%) HC, but without a statistically significant difference between the three groups. As seen in Table 1, in the CD4+ T cell group, each subset was increased in one or two patients. The overall frequency of expansions in the CD4+ group was 4% (15/360) in BD compared with 4% (6/139) in RA and 1% (3/276) in HC (BD versus HC, P = 0.06).
Table 1.
T cell receptor (TCR) Vβ expression in peripheral blood CD4+ T cells in Behçet's disease (% of CD4+ T cells)
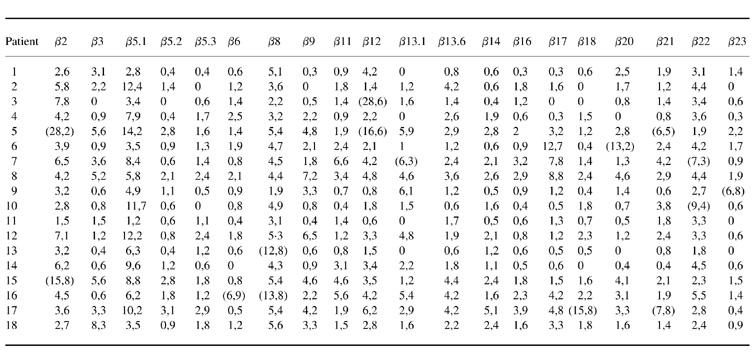
Values in parentheses define positive samples as over mean + 3 s.d. of healthy controls.
In the CD8+ T cell population, nine patients with BD (50%), four (57%) patients with RA and four (28%) HC had at least one oligoclonal expansion (BD versus HC, P = 0.3) (Table 2). In the CD8+ T cell compartment, Vβ5.1 was found to be expanded in five patients. Similarly, a 4% (15/359) frequency of expansion was observed in BD patients compared with 4% (7/139) in RA and 1% (4/279) in HC (BD versus HC, P = 0.11).
Table 2.
T cell receptor (TCR) Vβ expression in peripheral blood CD8+ T cells in Behçet's disease (% of CD8+ T cells)
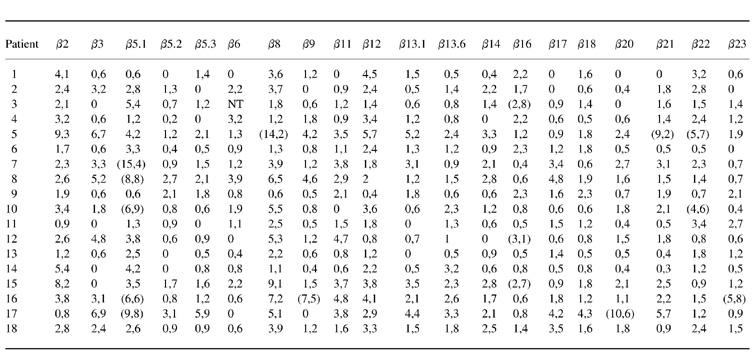
NT, Not tested.
Values in parentheses define positive samples as over mean + 3 s.d. of healthy controls.
DISCUSSION
Oligoclonal expansions in CD4+ and CD8+ T cell subsets were observed in 67% of clinically active BD patients in this study. Though no preferential Vβ subset elevation in the CD4+ T cell population was found, in the CD8+ group Vβ5.1 was found to be increased in five patients. Among healthy controls, 21% had CD4+ and 28% had CD8+ oligoclonal expansions, but the frequency and the magnitude of these expansions were lower compared with BD. RA patients, however, had similar oligoclonal increases to BD in the peripheral blood T cell compartment.
In two previous studies the unstimulated Vβ TCR repertoire of peripheral blood mononuclear cells (PBMC) in BD patients was investigated. James et al. observed a decrease of Vβ5.1 and Vβ5.2 in the peripheral blood of BD patients compared with healthy controls [25]. This might be explained by a preferential migration of certain pathogenic Vβ subsets to local inflammatory areas and a reciprocal decrease in the peripheral blood. Alternatively, this may reflect a relative decrease due to increases in other TCR subsets. Esin et al., on the other hand, observed oligoclonal increases in 30% (7/23) of BD patients in the CD4+ cell population and this increase was more pronounced in clinically active patients [26]. Our results are in accordance with this study, and the higher frequency of oligoclonal increases of T cell subsets in our study can be explained by the selection of only active cases and using a larger panel of MoAbs. In contrast to Esin et al., however, we also observed increases of some Vβ subsets in the CD8+ T cell compartment.
Although various microorganisms, such as atypical streptococci and herpes simplex virus, have been implicated in the pathogenesis of BD, only a few antigens of these agents were studied in detail. Among these, the 60/65-kD hsp, which is highly conserved among various microorganisms and mammalians, is the most widely studied antigen. T and B cell responses to synthetic peptides derived from mycobacterial and human 60/65-kD hsp were shown to be preferentially increased in BD patients compared with controls in studies from the UK, Japan and Turkey [9–12]. Although a dominant response of γδ T cells was suggested, a synthetic peptide from human 60-kD hsp (peptide 336–51) also caused oligoclonal increases of Vβ5.1, Vβ5.2, Vβ13.6, Vβ18 and Vβ21 in CD4+ T cells following 9-day cultures in Japanese patients [10]. The complexity of TCR analysis was also demonstrated in this study, as only a single peptide caused a variable dominant TCR expansion in different patients. Interestingly, this peptide selectively induces uveitis in rats without other clinical manifestations of BD, suggesting that different antigens may be related to different manifestations of BD [27].
Changes in the Vβ repertoire have been reported in the literature in a variety of autoimmune and vasculitic disorders such as psoriasis, multiple sclerosis, reactive arthritis, temporal arteritis, Wegener's granulomatosis, Takayasu's arteritis and KD [17–21]. In some of these disorders dominant changes of particular TCR subsets (Vβ2, Vβ8, etc.) were accepted as a sign of a superantigen effect of certain microorganisms such as staphylococci in KD and streptococci in psoriasis [19, 21]. A superantigen effect was also suggested in BD when an enhanced interferon-gamma (IFN-γ) response to low doses of staphylococcal enterotoxins was observed in BD patients compared with controls [28]. Our results, however, do not support a superantigen effect on the TCR repertoire, as we found no significant overall increase of any Vβ subset in our study group.
Our results, however, suggest an antigen-driven oligoclonal expansion of T cells in BD. Multisystemic organ manifestations may imply the contribution of multiple antigens using a variable TCR repertoire in each BD patient. Alternatively, a restricted antigen group may use variable MHC molecules in different patients, as suggested for the 60-kD human hsp peptide 336–51. In accordance with the immunohistology studies of oral ulcers showing both CD4+ and CD8+ T cells in the infiltrates, oligoclonal increases of both CD4+ and CD8+ T cells were observed in our study, suggesting a role for both cell types in the aetiology of BD [4].
In conclusion, we have observed an alteration of the T cell repertoire in BD, both in CD4+ and CD8+ T cell populations, indicating an involvement of T cells in the immune pathogenesis of the disease. There was a bias in the usage of certain TCR Vβ families. Further studies on these subsets might delineate their role in the pathogenesis of BD, with a possibility of modulating their reactivity with selective immunotherapies.
Acknowledgments
The authors thank Professor Hasan Yazici and Dr David D'Cruz for their valuable contributions, and Turkish Scientific and Technical Research Council (TÜBITAK-Project no. SBAG-Ü-16/11) for supporting the project.
REFERENCES
- 1.Yazici H, Yurdakul S, Hamuryudan V. Behçet's syndrome. In: Klippel JH, Dieppe PA, editors. Rheumatology. Vol. 7. London: Mosby; 1998. pp. 1–7. [Google Scholar]
- 2.Emmi T, Brugnolo F, Salvati G, Marchione T. Immunopathological aspects of Behçet's disease. Clin Exp Rheumatol. 1995;13:687–91. [PubMed] [Google Scholar]
- 3.Lehner T, Fortune F, Lavery E. Recent advances in T-cell immunoregulation and in the microbial causes of Behçet's disease. In: O'Duffy JD, Kökmen E, editors. Behçet's disease. New York: Marcel Dekker Inc; 1991. pp. 463–75. [Google Scholar]
- 4.Poulter LW, Lehner T. Immunohistology of oral lesions from patients with recurrent oral ulcers and Behçet's syndrome. Clin Exp Immunol. 1989;78:189–95. [PMC free article] [PubMed] [Google Scholar]
- 5.Gül A, Esin S, Dilsen N, Koniçe M, Wigzell H, Biberfeld P. Immunohistology of skin pathergy reaction in Behçet's disease. Br J Dermatol. 1995;132:901–7. doi: 10.1111/j.1365-2133.1995.tb16946.x. [DOI] [PubMed] [Google Scholar]
- 6.Ergun T, Gürbüz O, Harvell J, Jorizzo J, White W. The histopathology of pathergy: a chronological study of skin hyperreactivity in Behçet's disease. Int J Dermatol. 1998;37:929–33. doi: 10.1046/j.1365-4362.1998.00474.x. [DOI] [PubMed] [Google Scholar]
- 7.Demiralp EE, Direskeneli H, Sahin S, Karsli F, Ergun T, Fresko I, Akoglu T. Increased CD4+CD16+, CD4+CD56+ and CD8+ CD11b+ T lymphocytes in Behçet's disease, 7th International Conference on Behçet's Disease; Revue du Rheumatisme; 1996. p. 530. (Abstr.) [Google Scholar]
- 8.Kahan A, Hamzaoui K, Ayed K. Abnormalities of T lymphocyte subsets in Behçet's disease demonstrated with anti-CD45RA and anti-CD29 monoclonal antibodies. J Rheumatol. 1992;19:742–6. [PubMed] [Google Scholar]
- 9.Pervin K, Childerstone A, Shinnick T, Mizushima Y, Van der Zee R, Hasan A, Vaughan R, Lehner T. T cell epitope expression of mycobacterial and homologous human 65-kilodalton heat shock protein peptides in short term cell lines from patients with Behçet's disease. J Immunol. 1993;151:2273–82. [PubMed] [Google Scholar]
- 10.Kaneko S, Suzuki N, Yamashita N, Nagafuchi N, Nakajima T, Wakisaka S, Yamamoto S, Sakane T. Characterization of T cells specific for an epitope of human 60 kD heat shock protein (hsp) in patients with Behçet's disease in Japan. Clin Exp Immunol. 1997;108:204–11. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Direskeneli H, Hasan A, Shinnick T, Mizushima Y, Van Der Zee R, Fortune F, Stanford MR, Lehner T. Recognition of B cell epitopes of the 65 kD heat shock protein in Behçet's disease. Scand J Immunol. 1996;43:464–71. doi: 10.1046/j.1365-3083.1996.d01-53.x. [DOI] [PubMed] [Google Scholar]
- 12.Tasçi B, Direskeneli H, Serdaroglu P, Akman-Demir G, Eraksoy M, Saruhan-Direskeneli G. Humoral immune response to mycobacterial heat shock protein (hsp) 65 in the cerebrospinal fluid of neuro-Behçet's patients. Clin Exp Immunol. 1998;113:100–4. doi: 10.1046/j.1365-2249.1998.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortune F, Walker J, Lehner T. The expression of γδ T cell receptor and the prevalence of primed, activated and IgA-bound T cells in Behçet's syndrome. Clin Exp Immunol. 1990;82:326–32. doi: 10.1111/j.1365-2249.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamzaoui K, Hamzaoui A, Hentati F, Kahan A, Ayed K, Chabbou A, Hamida MB, Hamza M. Phenotype and functional profile of T cells expressing γδ receptor from patients with active Behçet's disease. J Rheumatol. 1994;21:2301–6. [PubMed] [Google Scholar]
- 15.Hasan A, Fortune F, Wilson A, et al. Role of γδ T cells in pathogenesis and diagnosis of Behçet's disease. Lancet. 1996;347:789–94. doi: 10.1016/s0140-6736(96)90868-5. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SM, Posnett DN, Tumang JR, Cole BC, Crow MK. A potential role for microbial superantigens in the pathogenesis of systemic autoimmune disease. Arthritis Rheum. 1991;34:468–80. doi: 10.1002/art.1780340412. [DOI] [PubMed] [Google Scholar]
- 17.Giscombe R, Grunewald J, Nityanand S, Lefvert AK. T cell receptor (TCR) V gene usage in patients with systemic vasculitis. Clin Exp Immunol. 1995;101:213–9. doi: 10.1111/j.1365-2249.1995.tb08341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nityanand S, Giscombe R, Srivastava S, Hjelmström P, Sanjeevi CB, Sinha N, Grunewald J, Lefvert AK. A bias in the αβ T cell receptor variable region gene usage in Takayasu's arteritis. Clin Exp Immunol. 1997;107:261–8. doi: 10.1111/j.1365-2249.1997.295-ce1186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung DYM. Kawasaki disease. Curr Opin Rheumatol. 1993;5:41–51. doi: 10.1097/00002281-199305010-00007. [DOI] [PubMed] [Google Scholar]
- 20.Allen RL, Gillespie GMA, Hall F, Edmonds S, Hall MA, Wordsworth BP, McMichael AJ, Bowness P. Multiple T cell expansions are found in the blood and SF of patients with reactive arthritis. J Rheumatol. 1997;29:1750–7. [PubMed] [Google Scholar]
- 21.Leung DYM, Travers JB, Giorno R, et al. Evidence for a streptoccocal superantigen-driven process in acute guttate psoriasis. J Clin Invest. 1995;96:2106–12. doi: 10.1172/JCI118263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to ‘benign monoclonal gammopathy’. J Exp Med. 1994;179:609–18. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James TE, Bhakta B, Clarke G, Lancaster F, Boylston AW, Noble BA, Chamberlain MA. Behçet's disease: a diagnostic test? Br J Rheumatol. 1994;25:44. (Abstr.) [Google Scholar]
- 26.Esin S, Gül A, Hodara V, Jeddi-Tehrani M, Dilsen N, Koniçe M, Andersson R, Wigzell H. Peripheral blood T cell expansions in patients with Behçet's disease. Clin Exp Immunol. 1997;107:520–7. doi: 10.1046/j.1365-2249.1997.d01-947.x. [DOI] [PubMed] [Google Scholar]
- 27.Stanford MR, Kasp E, Whiston R, et al. Heat shock protein peptides reactive in patients with Behçet's disease are uveitogenic in Lewis rats. Clin Exp Immunol. 1994;97:226–31. doi: 10.1111/j.1365-2249.1994.tb06072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirohata S, Hashimoto T. Abnormal T cell responses to bacterial superantigens in Behçet's disease. Clin Exp Immunol. 1998;112:317–24. doi: 10.1046/j.1365-2249.1998.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


