Abstract
We have studied the expression of the inducible form of nitric oxide synthase (iNOS) in joints of goats infected with the caprine arthritis encephalitis virus (CAEV). Nitric oxide generated by iNOS is thought to play an important role in the pathogenesis of various types of arthritis, especially rheumatoid arthritis (RA) in humans. Surprisingly, iNOS immunoreactivity was found only in joints of long-term infected goats with severe clinical arthritis, whereas—despite the presence of high numbers of inflammatory cells in the synovial tissue—no iNOS immunoreactivity was detected in mildly arthritic and in short-term experimentally infected goats. Most iNOS-positive cells expressed neither MHC class II nor CD68, which suggests that they were fibroblast-like synoviocytes. In situ hybridization studies showed that there was no correlation between iNOS immunoreactivity and detectable virus expression in the joint. In addition, infection of macrophages in vitro—the major host cells of CAEV in vivo—did not lead to increased iNOS mRNA expression. In response to stimulation, similar levels of iNOS expression were observed in infected and in uninfected macrophages. These findings suggest that the expression of iNOS is a feature of late-stage chronic arthritis and is not involved in the development of the inflammatory lesions. Both the lack of co-localization of iNOS protein and viral transcripts in the joint and the finding that CAEV does not stimulate the expression of iNOS in vitro further suggest that iNOS is not directly induced by the virus or the anti-viral immune response in the joint, that it may well, however, be involved in tissue remodelling or scar formation.
Keywords: caprine arthritis encephalitis virus, rheumatoid arthritis, nitric oxide synthase pathogenesis, goat, lentivirus
INTRODUCTION
Nitric oxide (NO) is a short-lived gaseous radical which is generated enzymatically by nitric oxide synthase (NOS). Three different isoforms of NOS are known. Two forms are constitutively expressed: the neuronal NOS (called nNOS or NOS1) and endothelial NOS (eNOS or NOS3). The inducible isoform of NOS (iNOS or NOS2) is produced by a variety of cells upon stimulation with bacterial endotoxin or inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) [1–3]. Once induced, iNOS can generate large amounts of NO over an extended period of time. NO is rapidly converted to other reactive nitrogen intermediates. Peroxinitrite anion, generated by the reaction of NO with superoxide, is a strong oxidant which can damage proteins, lipids, membranes, DNA and subcellular organelles. In autoimmune and infectious diseases, iNOS is up-regulated and thought to contribute to both immune modulation and tissue damage [3]. In rheumatoid arthritis (RA) patients, increased levels of nitrite, a breakdown product of NO, have been found in serum and synovial fluid [4, 5] and iNOS protein has been detected in inflamed synovial tissue [6–8]. However, it is not known whether iNOS expression is initially involved in the pathogenesis of RA or whether it is just a hallmark of chronic inflammation. The aetiology of RA is unknown, but several infectious agents (viral or bacterial) have been implicated in the initiation of the disease [9–11].
Caprine arthritis encephalitis virus (CAEV) is a lentivirus which causes persistent infection in goats. Clinical symptoms include chronic arthritis, mastitis, pneumonia and, in young animals, encephalitis. Caprine arthritis is characterized by mononuclear infiltration of synovial membranes with lymphocytes, macrophages and plasma cells. In the advanced stage of the disease, necrosis and fibrosis of synovial tissue as well as mineralization and erosion of articular surfaces occur [12–15]. In view of the histopathological similarity, caprine arthritis serves as a naturally occurring model for human RA. We have studied the expression of iNOS in the synovium of goats which had been infected with CAEV for different lengths of time. We have asked whether iNOS is detectable in the early stages of arthritis and therefore conceivably involved in the pathogenesis of arthritis and whether CAEV directly stimulates the expression of iNOS in its major host cell, the macrophage.
MATERIALS AND METHODS
Animals and tissue samples
Goats were experimentally infected with the molecular clone CAEV CO [16]. Fifty percent tissue culture infectious dose (TCID50) (5 × 104) of CAEV CO was injected both intracarpally and intravenously [17, 18]. Six, 12, 33 days or 1 year post-infection, the animals were euthanized by an overdose of pentobarbital. Synovial membranes were fixed overnight in 4% paraformaldehyde in PBS pH 7.4, dehydrated and embedded in paraffin. Three goats which had been experimentally infected with the biological clone CAEV 63 [14, 15] and showed severe clinical arthritis were also studied. Goats naturally infected with CAEV came from infected flocks in Switzerland. They were either serologically CAEV+ as demonstrated by an ELISA [19] and by Western blot [20] or, when only archive material was available, they were diagnosed as CAEV-infected by the detection of viral transcripts in affected joints by in situ hybridization. Two seronegative goats from a CAEV-free herd served as controls.
Immunohistochemistry
Dewaxed paraffin sections were incubated for 30 min with 10 mg/ml human γ immunoglobulin (Berna, Schweizerisches Serum- und Impfinstitut, Bern, Switzerland) in PBS. A rabbit anti-mouse iNOS serum (06-295; Upstate Biotechnology, Lake Placid, NY) or control rabbit serum was diluted 1:200 in PBS containing 0.1 mg/ml saponin and added to the sections for 60 min. After washing with Tris buffer (0.25 m NaCl, 20 mm Tris, pH 7.5, 0.13% Tween 20), the sections were incubated for 45 min with a biotinylated goat anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA) in Tris buffer. After washing in Tris buffer, the sections were incubated with avidin–biotin–peroxidase complex (Vector Labs, Burlingame, CA) for 45 min followed by another wash in Tris buffer and addition of the substrate diaminobenzidine tetrahydrochloride (DAB; Sigma, St Louis, MO) in Tris buffer, 0.01% H2O2. All incubation steps were performed at room temperature. After colour development the slides were washed in Tris buffer for 30 min. For MHC class II staining, VPM54 antibody (Dutia, 1990; kindly donated by B. Blacklaws, Cambridge, UK) was applied overnight at 4°C in Tris buffer. As secondary antibody, a biotinylated goat anti-mouse IgM and IgG (Jackson) was added for 60 min followed by avidin–biotin–alkaline phosphatase complex (Vector) (30 min). Fast Red was used as a substrate (2 mg/ml Fast Red TR salt; Chroma-Gesellschaft, Kongen, Germany), 1 mg/ml naphtol ASMX-phosphate (Sigma), in 100 mm Tris pH 8.0). Staining of CD68 (mouse anti-human CD68, M0718; Dako Diagnostics AG, Zug, Switzerland) was performed similarly but with prior treatment of the tissue with 0.2% trypsin (Difco Labs, Detroit, MI), 14 mm CaCl2, 50 mm Tris, pH 7.5 at 37°C for 15 min on a shaker. After colour development, the tissue was counterstained with Mayer's haematoxylin and mounted in glycerol-gelatine (Sigma).
Riboprobes for slot blot analysis and in situ hybridization
CAEV CO gag (nt 512–1858) was cloned in pBluescript (Stratagene, La Jolla, CA). Caprine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cloned in pSPT19 (Boehringer, Mannheim, Germany) was a gift of Dr G. Quérat (Marseille, France). Bovine iNOS cDNA cloned in pSPT19 was a gift of Dr H. Adler [21].
Anti-sense probes and sense control probes were transcribed in vitro from the T3, T7 or SP6 promoters in the presence of digoxigenin-UTP (Boehringer).
Cell cultures
Mature macrophages were obtained from peripheral blood mononuclear cells (PBMC) as described previously [22]. PBMC were cultured in Teflon bags for 8 days and then seeded in tissue culture flasks in RPMI 1614 medium supplemented with penicillin 100 U/ml, streptomycin 100 μg/ml, l-glutamine 2 mm, 2-mercaptoethanol 50 μm, HEPES (N-2-hydroxyethylpiperazine-N′-2ethanesulfonic acid; 10 mm) and 2% heat-inactivated goat serum (Sigma). Macrophages were infected with CAEV CO at a multiplicity of infection (moi) of 0.01 or 0.0001. Two hours after infection, the cells were rinsed and fresh medium containing 4% goat serum was added. Similarly, mock-infection was performed with UV-inactivated virus stock. The cells were stimulated either with 100 ng/ml lipopolysaccharide (LPS; Escherichia coli serotype 055:B; Sigma) or fixed cells of Staphylococcus aureus Cowan I (SAC; Pansorbin, diluted 1:1000; Calbiochem, La Jolla, CA) or a combination of 200 μg/ml heat-killed Listeria monocytogenes and 100 U/ml recombinant bovine IFN-γ (Ciba Geigy, Basel, Switzerland). Four hours after the addition of the stimulating agents, total RNA was isolated from the cells with Trizol (Gibco BRL, Grand Island, NY).
RNA slot blot analysis
Total RNA isolated from macrophages was blotted on positively charged nylon membranes (Boehringer) with a slot blot apparatus (Schleicher & Schuell, Keene, NH). The blots were hybridized with digoxigenin-labelled riboprobes according to standard procedures. CDP-Star (Tropix, Bethesda, MD) was used as the chemiluminescent substrate. Chemiluminescent signals were scanned with a Molecular Imager (BioRad, Hercules, CA) and values were normalized to GAPDH.
In situ hybridization
Paraffin-embedded tissue samples were dewaxed and hybridized with digoxigenin-labelled riboprobes specific for CAEV gag RNA as described previously [17, 23, 24].
RESULTS
iNOS is detected only in goats with severe clinical arthritis
Histological analysis of synovial membrane tissues from goats naturally or experimentally infected with CAEV showed varying degrees of synovial lining cell hyperplasia and infiltration with lymphocytes, macrophages and plasma cells [17, 18]. Tissue sections from different stages of arthritis were tested for immunoreactivity for iNOS using a polyclonal serum raised against murine iNOS. Positive cells were detected only in tissue samples from goats with long-term severe clinical arthritis. In contrast, no iNOS protein was found in mildly arthritic goats or animals experimentally infected for 1 year or less, whereas large numbers of inflammatory cells were also present in the latter two groups of animals (Table 1).
Table 1.
Detection of inducible nitric oxide synthase (iNOS) and viral transcripts in caprine arthritis encephalitis virus (CAEV)-infected goats
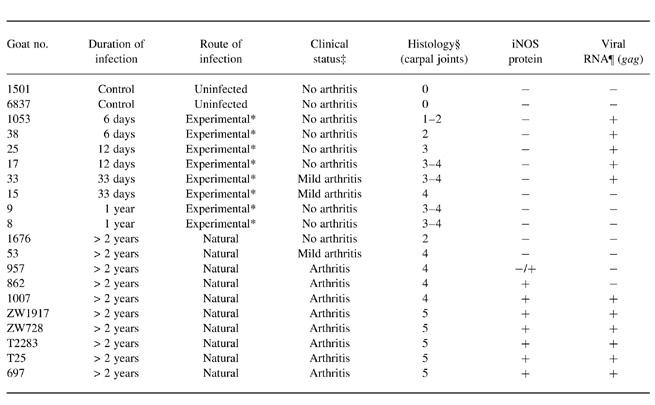
*Experimentally infected with CAEV CO.
‡Clinical status was assessed by the measurement of the carpal/metacarpal (C/MC) circumference ratio of carpal joints. An arbitrary threshold for clinical arthritis was set at C/MC = 1.80. Goats with mild arthritis had C/MC ratios of 1.71–1.79.
§Evaluation of haematoxylin–eosin (H–E)-stained tissue sections of synovial membranes from carpal joints was performed independently by two examiners. 0 = no inflammation; 1 = some inflammatory cell aggregates, synovial lining layer hyperplasia; 2 = dispersed mononuclear infiltrates in villi, few perivascular infiltrates; 3 = infiltrates in villi, subintima and around vessels; few small lymphoid-like follicles; 4 = as 3; with lymphoid-like follicles, large plasma cell aggregates; 5 = intense mononuclear infiltration of intima and subintima, numerous plasma cells, necrosis/fibrosis.
¶ Viral RNA in synovial membranes of carpal joints was detected by in situ hybridization of gag RNA.
No iNOS+ cells were detected in synovial membranes of seronegative control goats (not shown). Incubation of tissue sections of arthritic goats with control rabbit serum did not result in positive staining (Fig. 1c).
Fig. 1.
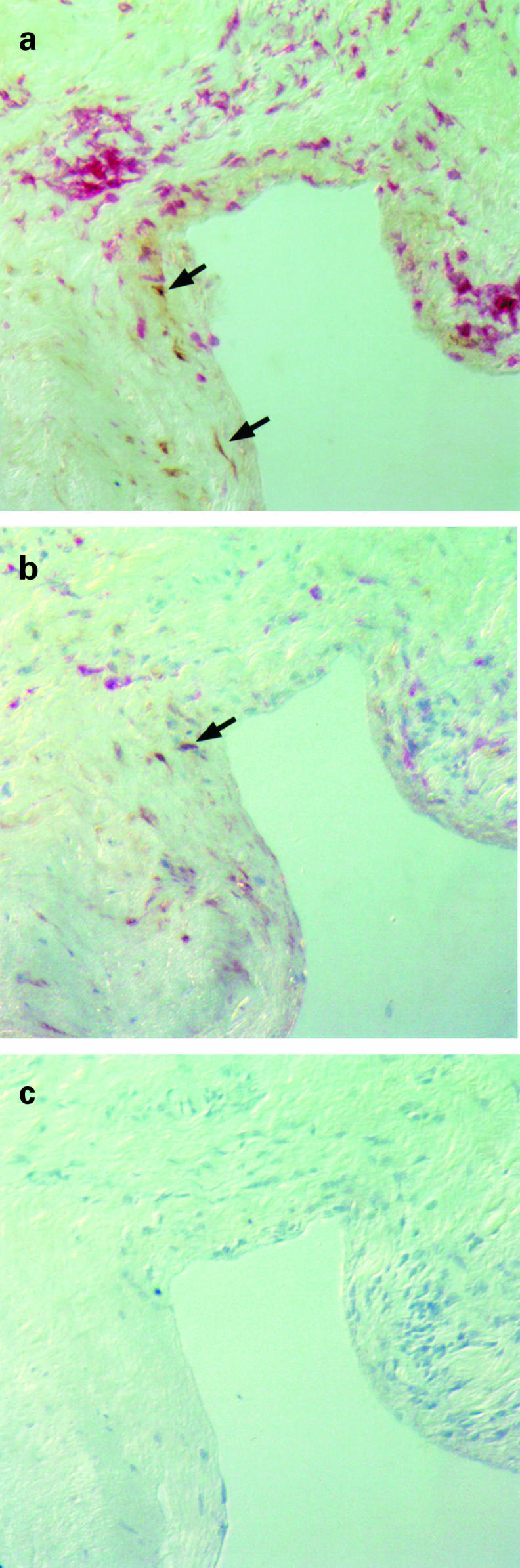
Detection of inducible nitric oxide synthase (iNOS), MHC class II and CD68 in the joint of an arthritic goat by immunohistochemistry. Serial synovial membrane tissue sections from a goat with clinical arthritis (no. 1007) were incubated with antibodies specific for iNOS, MHC class II and CD68. (a) Immunoreactivity for iNOS (brown) was detected in a fibrotic area adjacent to a mononuclear infiltrate strongly expressing MHC class II (red). (b) iNOS staining (brown) and CD68 staining (red) were detected in different non-overlapping cell populations in the inflamed joint. (c) No specific staining was obtained with a control rabbit serum.
Most iNOS+ cells do not express MHC class II or CD68
iNOS immunoreactivity was not evenly distributed throughout the inflammatory lesions but confined to distinct areas. Most iNOS+ cells were located in clusters in the subintimal layer, often in fibrotic areas which contained only few inflammatory cells (Fig. 1a). Occasional positive cells were also found in the synovial lining layer as well as in vascular smooth muscle cells of a minority of synovial vessels. Perivascular infiltrates consisting predominantly of T and B lymphocytes as well as macrophages were practically devoid of iNOS+ cells.
To characterize further the cells which produce iNOS, double staining of tissue sections was performed. Following iNOS staining, the sections were incubated with either an anti-MHC class II antibody or an anti-CD68 antibody. Over 95% of iNOS+ cells expressed neither MHC class II antigen nor the tissue macrophage-specific marker CD68, which suggests that, in caprine arthritis, the majority of iNOS-expressing cells are not inflammatory cells but fibroblast-like synoviocytes (Fig. 1a,b). Similar findings were reported in a study of synovial tissue obtained from RA patients, where most iNOS+ cells in the joint were found to be negative for CD68 and resembled fibroblasts [7]. Two other studies, however, suggested that other cell types such as macrophages and chondrocytes also produced iNOS in RA [6, 8].
iNOS immunoreactivity and expression of viral RNA do not locally correlate
To test whether NOS immunoreactivity correlates with the expression of viral transcripts in the joint, subjacent tissue sections of synovial tissue were stained with the iNOS antibody or hybridized with a digoxigenin-labelled probe specific for CAEV gag RNA. In contrast to iNOS protein, which was found only in joints of long-term infected goats, viral transcripts were detected both at early time-points following experimental infection (6–33 days post-infection) as well as in goats with severe clinical arthritis. No viral transcripts were found in animals with mild arthritis (Table 1). Moreover, in tissues of arthritic goats, where both iNOS and viral transcripts were detected, the areas with iNOS+ cells and the areas with virus-expressing cells did not overlap (not shown). It thus appears that the expression of iNOS does not directly depend on detectable virus replication in the joint but is rather a feature of late-stage chronic arthritis.
CAEV does not induce iNOS in macrophage cell cultures
Macrophages are the major host cells of CAEV in vivo [25–28]. To test whether CAEV is able to directly increase iNOS expression in this cell type, blood-derived macrophages were infected in vitro with CAEV at a moi of 0.01 or 0.0001. Seven days post-infection, the expression of iNOS transcripts was analysed by RNA slot blot analysis. Without the addition of stimulating agents, very low amounts of iNOS mRNA were detected in infected and mock-infected macrophages. No difference in iNOS expression was noted between infected and mock-infected cells, although the intensities of the chemiluminescent signals were too low to be scanned and quantified (not shown). Following stimulation with LPS (100 ng/ml), iNOS mRNA was enhanced both in infected and in mock-infected cells. However, in infected as opposed to mock-infected cultures the stimulated expression of iNOS was reduced. This was especially the case when the cells were infected with a higher multiplicity of infection (moi = 0.01) (Fig. 2). Similar results were obtained when the cells were stimulated with fixed SAC or heat-killed L. monocytogenes and IFN-γ. Again, CAEV infection led to a reduction of the levels of stimulated iNOS mRNA (not shown). These in vitro findings are in accordance with the results obtained from the studies of tissue sections, where no correlation was found between detectable expression of virus and the presence of iNOS immunoreactivity in the joint (Table 1).
Fig. 2.
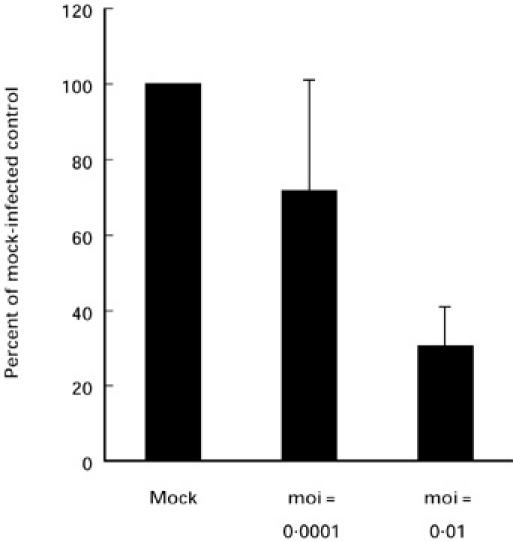
Expression of inducible nitric oxide synthase (iNOS) mRNA in macrophages stimulated with lipopolysaccharide (LPS). Macrophages were mock-infected or infected with CAEV CO at a multiplicity of infection (moi) of 0.01 or 0.001. Seven days post-infection, the cells were stimulated with LPS (100 ng/ml) for 4 h and total RNA was isolated. RNA slot blots were hybridized with probes specific for bovine iNOS or caprine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and scanned values for iNOS were normalized to GAPDH. Results from three experiments are shown. In order to compare the different experiments (n = 3), results are expressed as percentages of the value of the mock-infected cells. Standard deviations are represented by error bars.
DISCUSSION
In this study, we have compared the expression of iNOS in early-stage infection with CAE virus with that in animals infected chronically. The analysis of the early stage was done using an experimental model of infection in which we previously analysed the kinetics of inflammation, the cell types involved and the expression of cytokines and viral RNA. The type of inflammation that develops within 33 days of infection is similar to that of the chronic form of infection, except that the degenerative changes typically seen in severely arthritic goats are missing [17, 18]. Although we observed tissue hyperplasia and abundant inflammatory cells in the synovium of early stage and mildly arthritic goats, iNOS was detected only in severely arthritic goats that had been infected for > 2 years. However, we are aware of the limitations of iNOS detection by immunohistochemistry, which suggests that minor amounts of nitric oxide may be produced even in situations where iNOS may be too low to be detected. The presence of iNOS protein in the synovium has been convincingly demonstrated in RA patients [6, 7]. However, in most cases RA tissue samples were obtained at the time of synovectomy, i.e. in the late stages of chronic arthritis. Although it has been assumed that NO participates in the pathogenesis of RA, it remains unknown if NO is actually involved in the development of the inflammatory lesions. Our results show that severe joint inflammation and tissue destruction may develop without clear induction of iNOS protein. This was not due to a lack of expression of inflammatory cytokines, which were detected by in situ hybridization at all stages of the disease [17]. The localization of iNOS protein in fibrotic areas rather than in inflammatory cell aggregates suggests that the expression of iNOS is a secondary event which may be involved in tissue remodelling or scar formation. Although NO has been shown to be involved in pathways that lead to the generation of cartilage- and matrix-degrading enzymes [29, 30], there are reports suggesting that NO may also have protective functions by inhibiting catabolic effects of IL-1 on cartilage [31], by suppressing lymphocyte proliferation [32–34] and by inhibition of MHC class II expression [35]. In various experimental animal models of arthritis, increased iNOS transcripts or protein have been noted, and in many instances inhibition of NOS activity by chemical compounds has been shown to reduce the severity of arthritis (reviewed in [36]). However, evidence that iNOS is not always involved in the formation of chronic inflammatory lesions has also been provided. Thus, both iNOS knock-out mice and wild-type control mice immunized with myelin oligodendrocyte glycoprotein develop chronic autoimmune encephalomyelitis with demyelination [37, 38]. In addition, both iNOS− and iNOS+ MRL-lpr/lpr mice develop spontaneous arthritis [39].
The aetiology of RA is unknown. Viruses and bacteria have been suspected to be involved in the pathogenesis by either inducing immune responses against viral or bacterial antigens in the joint and/or by stimulating cross-reactive autoimmune responses. Accordingly, CAEV transcripts or antigens are difficult to detect in the joints of naturally infected goats before severe clinical signs have developed ([40] and Table 1). Following experimental infection of the joint, however, viral transcripts are readily detected by in situ hybridization during the first weeks post-infection. The lack of iNOS immunoreactivity in joints of short-term experimentally infected goats as well as the different location of iNOS protein and viral transcripts in chronically infected goats argue against the hypothesis that iNOS is directly induced by CAEV or the anti-viral immune response. The hypothesis that iNOS is needed for the establishment of chronic inflammation is not supported either. Additional in vitro studies corroborated these in vivo findings. Infection of cultured macrophages with CAEV neither increased iNOS mRNA expression nor led to enhanced iNOS transcription following stimulation. In contrast, HIV—which is related to CAEV—has been shown to increase iNOS mRNA expression and NO generation in monocyte cultures and to prime for increased NO production upon stimulation [41].
In conclusion, our findings suggest that virus-induced arthritis can develop in the absence of pronounced iNOS up-regulation and that the expression of iNOS is a feature of the late stage of chronic inflammation rather than a result of local virus replication or an anti-viral immune response.
Acknowledgments
We are grateful to Achilles Tontis for providing tissue blocks taken from chronically infected goats. We thank Heiko Adler for the gift of the bovine iNOS probe and Ulrike Sahrbacher for help with the photographs. Our thanks go to Ruth Parham for linguistic improvements. This work was supported by the Swiss National Science Foundation grants no. 3139-041859.94 and no. 3100-09733.93\1 and by the Union Bank of Switzerland on behalf of a client.
REFERENCES
- 1.Moncada S, Higgs EA, Hodson HF, et al. The L-arginine–nitric oxide pathway. J Cardiovasc Pharmacol. 1991;17:S1–S9. [Google Scholar]
- 2.MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–50. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C. Inducible nitric oxide synthase: what difference does it make. J Clin Invest. 1997;100:2417–23. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–22. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueki Y, Miyake S, Tominaga Y, Eguchi K. Increased nitric oxide levels in patients with rheumatoid arthritis. J Rheumatol. 1996;23:230–6. [PubMed] [Google Scholar]
- 6.Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, Kanno K, Saito I, Miyasaka N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357–63. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McInnes IB, Leung BP, Field M, et al. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–24. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski PS, Wright PK, van ‘t Hof RJ, Helfrich MH, Ohshima H, Ralston SH. Immunolocalization of inducible nitric oxide synthase in synovium and cartilage in rheumatoid arthritis and osteoarthritis. Br J Rheumatol. 1997;36:651–5. doi: 10.1093/rheumatology/36.6.651. [DOI] [PubMed] [Google Scholar]
- 9.Moreland LW, Koopman WJ. Infection as a cause of arthritis. Curr Opin Rheumatol. 1991;3:639–49. doi: 10.1097/00002281-199108000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Wilder RL. Hypothesis for retroviral causation of rheumatoid arthritis. Curr Opin Rheumatol. 1994;6:295–9. doi: 10.1097/00002281-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kalden JR, Gay S. Retroviruses and autoimmune rheumatic diseases. Clin Exp Immunol. 1994;98:1–5. doi: 10.1111/j.1365-2249.1994.tb06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodard JC, Gaskin JM, Poulos PW, MacKay RJ, Burridge MJ. Caprine arthritis-encephalitis: clinicopathologic study. Am J Vet Res. 1982;43:2085–96. [PubMed] [Google Scholar]
- 13.Crawford TB, Adams DS. Caprine arthritis-encephalitis: clinical features and presence of antibody in selected goat populations. JAVMA. 1981;178:713–9. [PubMed] [Google Scholar]
- 14.Wilkerson MJ, Davis WC, Cheevers WP. Peripheral blood and synovial fluid mononuclear cell phenotypes in lentivirus induced arthritis. J Rheumatol. 1995;22:8–15. [PubMed] [Google Scholar]
- 15.Wilkerson MJ, Davis WC, Baszler TV, Cheevers WP. Immunopathology of chronic lentivirus-induced arthritis. Am J Pathol. 1995;146:1433–43. [PMC free article] [PubMed] [Google Scholar]
- 16.Saltarelli M, Querat G, Konings DAM, Vigne R, Clements JE. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990;179:347–64. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- 17.Lechner F, Vogt HR, Seow HF, Bertoni G, Cheevers WP, von Bodungen U, Zurbriggen A, Peterhans E. Expression of cytokine mRNA in lentivirus-induced arthritis. Am J Pathol. 1997;151:1053–65. [PMC free article] [PubMed] [Google Scholar]
- 18.von Bodungen U, Lechner F, Pfister H, Vogt HR, Cheevers WP, Bertoni G, Jungi TW, Peterhans E. Immunohistology of the early course of lentivirus-induced arthritis. Clin Exp Immunol. 1998;111:384–90. doi: 10.1046/j.1365-2249.1998.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanoni RG, Vogt HR, Pohl B, Bottcher J, Bommeli W, Peterhans E. An ELISA based on whole virus for the detection of antibodies to small-ruminant lentiviruses. J Vet Med Series B. Infectious Diseases, Immunobiology, Food Hygiene, Public Health. 1994;41:662–9. doi: 10.1111/j.1439-0450.1994.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 20.Zanoni R, Krieg A, Peterhans E. Detection of antibodies to caprine arthritis-encephalitis virus by protein G enzyme-linked immunosorbent assay and immunoblotting. J Clin Microbiol. 1989;27:580–2. doi: 10.1128/jcm.27.3.580-582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler H, Frech B, Thöny M, Pfister H, Peterhans E, Jungi TW. Inducible nitric oxide synthase in cattle. Differential regulation of nitric oxide synthase in bovine and murine macrophages. J Immunol. 1995;154:4710–8. [PubMed] [Google Scholar]
- 22.Lechner F, Machado J, Bertoni G, Seow HF, Dobbelaere DAE, Peterhans E. Caprine arthritis encephalitis virus dysregulates the expression of cytokines in macrophages. J Virol. 1997;71:7488–97. doi: 10.1128/jvi.71.10.7488-7497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graber HU, Meyer RK, Fatzer R, Vandevelde M, Zurbriggen A. In situ hybridization and immunohistochemistry for prion protein (Pr P) in bovine spongiform encephalopathy (BSE) J Vet Med A. 1995;42:453–9. doi: 10.1111/j.1439-0442.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 24.Lechner F, Vogt HR, Seow HF, von Bodungen U, Bertoni G, Zurbriggen A, Peterhans E. Expression of TNF alpha in arthritis caused by caprine arthritis encephalitis virus. Vet Immunol Immunopathol. 1996;54:281–9. doi: 10.1016/s0165-2427(96)05701-7. [DOI] [PubMed] [Google Scholar]
- 25.Narayan O, Wolinsky JS, Clements JE, Standberg JD, Griffin DE, Cork LC. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982;59:345–56. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- 26.Gendelman HE, Narayan O, Molineaux S, Clements JE, Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci USA. 1985;82:7086–90. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gendelman HE, Narayan O, Kennedy-Stoskopf S, Kennedy PGE, Ghotbi Z, Clements JE, Stanley J, Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986;58:67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorrell MD, Brandon MR, Sheffer D, Adams RJ, Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992;66:2679–88. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murrell GA, Jang D, Williams RJ. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995;206:15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 30.Moulton PJ. Inflammatory joint disease: the role of cytokines, cyclooxygenases and reactive oxygen species. Br J Biomed Science. 1996;53:317–24. [PubMed] [Google Scholar]
- 31.Stefanovic-Racic M, Morales TI, Taskiran D, McIntyre LA, Evans CH. The role of nitric oxide in proteoglycan turnover by bovine articular cartilage organ cultures. J Immunol. 1996;156:1213–20. [PubMed] [Google Scholar]
- 32.Wei X, Charles IG, Smith AUJ, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–11. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 33.Albina JE, Abate JA, Henry WL. Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T-cell proliferation—role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991;147:144–8. [PubMed] [Google Scholar]
- 34.Mills CD. Molecular basis of ‘suppressor’ macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991;146:2719–23. [PubMed] [Google Scholar]
- 35.Sicher SC, Vazquez MA, Lu CY. Inhibition of macrophage Ia expression by nitric oxide. J Immunol. 1994;153:1293–300. [PubMed] [Google Scholar]
- 36.Cochran FR, Selph J, Sherman P. Insights into the role of nitric oxide in inflammatory arthritis. Med Res Rev. 1996;16:547–63. doi: 10.1002/(SICI)1098-1128(199611)16:6<547::AID-MED3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940–6. [PubMed] [Google Scholar]
- 38.Sahrbacher UC, Lechner F, Eugster HP, Frei K, Lassmann HFA. Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1332–8. doi: 10.1002/(SICI)1521-4141(199804)28:04<1332::AID-IMMU1332>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Gilkeson GS, Mudgett JS, Seldin MF, Ruiz P, Alexander AA, Misukonis MA, Pisetsky DS, Weinberg JB. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J Exp Med. 1997;186:365–73. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams DS, Crawford TB, Klevjer-Anderson P. A pathogenetic study of the early connective tissue damage of viral arthritis-encephalitis. Am J Pathol. 1980;99:257–78. [PMC free article] [PubMed] [Google Scholar]
- 41.Bukrinsky MI, Nottet HS, Schmidtmayerova H, Dubrovsky L, Flanagan CR, Mullins ME, Lipton SA, Gendelman HE. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–45. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]


