Abstract
Lymph nodes are the major site of cell-to-cell transmission and replication of HIV-1. Trafficking of CD4+ T lymphocytes into lymph nodes provides a continual supply of susceptible target lymphocytes, and conversely, recruitment of CD8+ T lymphocytes may be critical for the host response that attempts to control HIV-1 replication. The present study was undertaken as no detailed assessment of lymphocyte subpopulations in HIV-1-infected lymph nodes has previously been reported. Peripheral blood and single-cell suspensions prepared from lymph nodes of patients with HIV-1 and control subjects were analysed using three-colour flow cytometry. Approximately 80% of the lymphocytes in control lymph nodes were CD3+ T lymphocytes, of which over 65% were CD4+. The majority of the CD4+ and CD8+ T lymphocytes obtained from both lymph nodes and blood of control subjects were immunologically naive (CD45RA+). By contrast, in HIV-1-infected patients there was a significant reduction in the proportion of CD4+ T lymphocytes and an expansion of the CD8+ T lymphocyte subset in both lymph nodes and peripheral blood. Furthermore, a high proportion of these T lymphocytes displayed a marker for immunological memory (CD45RO+). T lymphocytes derived from HIV-1-infected lymph nodes also showed altered expression of the adhesion molecules, l-selectin and very late antigen-4 (VLA-4), but not leucocyte function-associated antigen-1 (LFA-1). In an in vitro adhesion assay, lymphocytes from HIV-1-infected nodes were significantly more adhesive than control lymphocytes on fibronectin, as well as recombinant human intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) substrates. This combination of altered lymphocyte subpopulations in the HIV-1-infected lymph nodes, as well as enhanced adhesion phenotype and function, suggests that T lymphocyte traffic to lymph nodes in HIV disease may be an important determinant of pathogenesis.
Keywords: HIV-1, lymph nodes, flow cytometry, lymphocytes, adhesion molecules
INTRODUCTION
The selective recirculation of T lymphocytes through the body is determined at the level of the interaction between lymphocytes and vascular endothelial cells [1]. Naive T lymphocytes recirculate preferentially across high endothelial venules directly from blood into lymph nodes; whereas memory T lymphocytes acquire a predilection, based on the environment in which they first encounter foreign antigen, to home to or recirculate through the same location [2]. In lymph nodes undergoing an immune response, the cell number has been observed to increase by as much as 10-fold over the first 48 h after antigen administration. Over 95% of this effect is due to traffic of cells from blood rather than lymphocyte proliferation within the node [3–5]. At a molecular level, the adhesion molecules, l-selectin, leucocyte function-associated antigen-1 (LFA-1), very late antigen-4 (VLA-4) and their ligands facilitate the recruitment of lymphocytes into lymph nodes during immune responses [6–10].
Lymphoid tissue is the predominant target of HIV infection and is the major site of T lymphocyte destruction throughout the course of HIV disease [11]. Hence, a considerably greater viral burden (5–10-fold) is detected in lymphoid tissue in comparison with peripheral blood [11–13]. The recruitment of circulating lymphocytes to lymph nodes is critical to the immunopathogenesis of HIV/AIDS, as it facilitates initial seeding of the virus into the lymphoid organs, provides a continuing supply of CD4+ target lymphocytes for infection and also establishes cellular immune mechanisms for the potential control of HIV replication via CD8+ T lymphocytes [11, 14].
Immunohistochemistry has previously been used to determine the cellular composition of lymph nodes obtained from HIV-1-infected patients. However, this method is limited by: the heterogeneous distribution of lymphocyte subsets in regions within the lymph nodes; the logistic difficulties of enumeration of cell numbers on tissue sections; and technical constraints, such as the lack of a range of MoAbs that stain formalin-fixed tissues. Hence, a reliable evaluation of leucocyte subpopulations in lymph nodes has not previously been possible.
This study describes the first detailed three-colour flow cytometric analysis of leucocyte phenotypes in lymph nodes and peripheral blood of patients with HIV infection and control subjects. The use of single-cell suspensions prepared from lymph nodes enabled accurate evaluation of the CD4+ and CD8+ T lymphocyte proportions as well as the co-expression of markers of adhesion and immunological memory. An in vitro adhesion assay was utilized to define the functional significance of the altered adhesion molecule expression on the lymphocyte populations.
SUBJECTS AND METHODS
Lymph node and peripheral blood collection
Ten male HIV-1-infected patients underwent excision of a palpable cervical lymph node under local anaesthesia. Apparently normal cervical lymph nodes were obtained during carotid endarterectomy from 10 uninfected male subjects who did not have palpable lymphadenopathy. Peripheral blood was also collected from patients and control subjects. The HIV patients were aged 22–48 years (mean 36 years). The control subjects were aged 48–74 years (mean 62 years). All subjects provided informed consent. The study was approved by the institutional ethics committee.
Preparation of single-cell suspensions
A single-cell suspension from each cervical lymph node was prepared by mincing, digestion, and filtration [15]. A total cell count was enumerated after assessment of viability with trypan blue and the cells resuspended at 2 × 106/ml in PBS.
Histopathological assessment of nodes
Sections of formalin-fixed paraffin-embedded lymph node tissue (4 μm) were stained with haematoxylin and eosin. The slides were then systematically evaluated by an independent experienced pathologist for histopathological diagnoses.
Flow cytometry
The lymph node-derived single-cell suspensions and peripheral blood obtained from the patients and control subjects were stained with commercially available antibodies. MoAbs directed against CD3, CD4, CD8, CD19, CD14 and CD56 (Becton Dickinson, Mountain View, CA) were used to quantify lymphocyte subsets. Antibody markers for activation (CD38, HLA-DR), and naive/memory phenotype (CD45RA/CD45RO) were purchased from Becton Dickinson. Co-expression of the adhesion molecules CD11a, CD11b, CD54 (Serotec, Sydney, Australia), CD49d and CD62L (Endogen, Haem Pty Ltd, Sydney, Australia) on the T lymphocyte subsets was also analysed. Isotype-matched irrelevant MoAbs conjugated to each fluorochrome were used as negative controls (Becton Dickinson). All tubes contained 2 × 105 cells per 100 μl of PBS to ensure saturation binding of cell surface markers at a constant reaction volume in each tube for each specimen. A combination of CD45–FITC and CD14–PE (leucogate) was used for gating (Becton Dickinson). Data acquisition and analysis were performed using Becton Dickinson FACScan and LYSYS II software, respectively (Becton Dickinson). Ten thousand events were acquired from each tube. To illustrate how the flow cytometric data were obtained, representative dot plots from lymph node cells of an HIV-1-infected patient and a control subject are shown in Fig. 1.
Fig. 1.
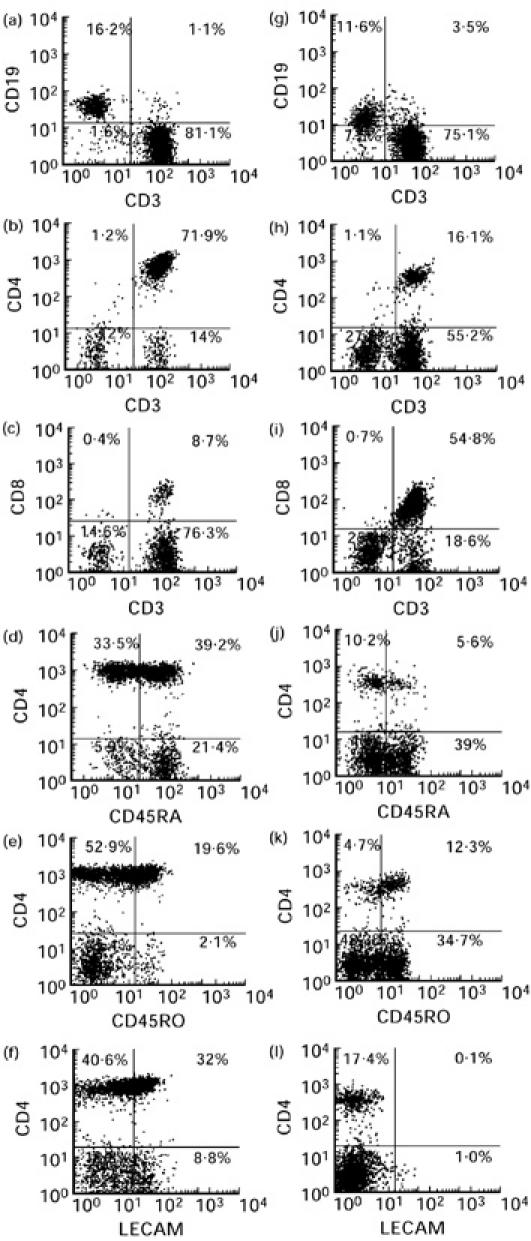
Representative dot plots illustrating the FACS analysis of lymph node cells derived from a control subject (a–f) and an HIV-1-infected individual (g–l) on the lymphocyte gate as defined by the Leucogate. The numerical values indicated on each quadrant represent the proportions of cells that are single-positive (upper left and lower right quadrants), double-positive (upper right quadrant) and double-negative (lower left quadrant) for the phenotypic markers designated on the abscissa and ordinate of each plot. The cursors for the quadrants were set based on negative control antibodies IgG1–FITC, IgG2a–PE and IgG2b–PerCp conjugated antibodies.
Mononuclear cell separation from blood and single-cell suspensions
A standard density gradient centrifugation (Histopaque; Sigma, Sydney, Australia) was used to purify mononuclear cells from lymph node-derived single-cell suspensions and peripheral blood. Following the purification of the lymph node-derived mononuclear cells (LNMC) and peripheral blood mononuclear cells (PBMC), contaminating monocytes were depleted to < 3% by repeated adherence to plastic. The average percentage of lymphocytes in the purified samples was > 92% (± 3%) as determined by FACS analysis. These lymphocyte preparations were used for adhesion assays (see below).
Adhesion assay
Flat-bottomed 96-well microtitre plates were coated with sheep anti-human IgG (Silenius, Sydney, Australia) followed by recombinant human (rh) intercellular adhesion molecule (ICAM)–IgG or vascular cell adhesion molecule (VCAM)–IgG fusion protein, as described previously [16]. In parallel, plates were similarly coated with human fibronectin (Gibco BRL, Melbourne, Australia) at 4°C overnight. Following a blocking step with 3% bovine serum albumin (BSA)/PBS, wells were washed with RPMI 1640/5% fetal calf serum (FCS) and 2 × 105 peripheral blood lymphocytes (PBL) or lymph node-derived lymphocytes (LNL) were added in triplicate wells for the adhesion in which the plates were placed at 37°C in a 5% CO2 incubator for 1 h. Wells were then washed three times using a standardized washing procedure to remove non-adherent cells. Plates were dried on absorbent towels and 100 μl of 0.25% (w/v) rose Bengal solution was added. The stain was uniformly aspirated using low pressure (2–4 mmHg) suction after a 5-min incubation at room temperature. Following two standard washes, 200 μl of ethanol:PBS (1:1) solution were added to each well. When total release of stain from the cells had occurred after approx. 30 min, quantification of adherent cells was performed using a colorimetric read-out at 540 nm [17]. Optical density (OD) readings obtained from cells plated onto uncoated wells or wells coated with sheep anti-human IgG alone were close to the background and were automatically deducted from the readings for fibronectin or rhICAM-1/VCAM-1, respectively. The percentage of residual adherent cells was estimated by running a standard curve of rose Bengal uptake and release from serially diluted LNL or PBL.
Statistical analysis
A computer software program, Microsoft Excel, version 5.0 (Microsoft Corp., Redmond, WA), was used to calculate means and s.d. Two-tailed Mann–Whitney U-tests were performed to assess the level of statistical significance using SPSS for Windows, version 6.0 (SPSS Inc., Chicago, IL). As several comparisons were performed appropriate Bonferroni adjustments of the significance level were made.
RESULTS
Clinical characteristics and lymph node pathology
A total of 11 lymph nodes was collected from 10 patients with HIV-1 infection. One patient (patient 1) donated a lymph node on two occasions, 3 years apart. At the time of this study, all patients with the exception of patients 1 and 2 were receiving anti-retroviral therapy with AZT (Zidovudine; Glaxo Wellcome). Patients 3, 4, 5 and 6 were receiving combination therapy with AZT and DDI (Videx; Roche, Molecular Biochemical, Basel, Switzerland). The clinical details and the histopathological patterns observed in the lymph nodes of each patient are summarized in Table 1. The patient classified as Centres for Disease Control (CDC) stage IVa had persistent diarrhoea, and the other two patients designated as stage IVc presented with disseminated Mycobacterium avium complex (MAC). These latter two patients (patients 3 and 4 in Table 1) with a type III pattern of lymph node pathology [18] had moderate numbers of long rods consistent with mycobacteria, detected by immunofluorescence staining (figure not shown). In one patient (patient 5 in Table 1) sections of the lymph node showed architectural effacement by a vascular spindle cell lesion with features characteristic of Kaposi's sarcoma. Morphological assessment of lymph nodes obtained from control subjects showed varying degrees of hyperplasia. Lymph nodes obtained from six subjects had mild hyperplasia, two showed moderate hyperplasia, one showed mild sinus histocytosis and one had prominent sinus histocytosis.
Table 1.
Clinical profile and histopathological patterns of the lymph nodes obtained from patients with HIV/AIDS
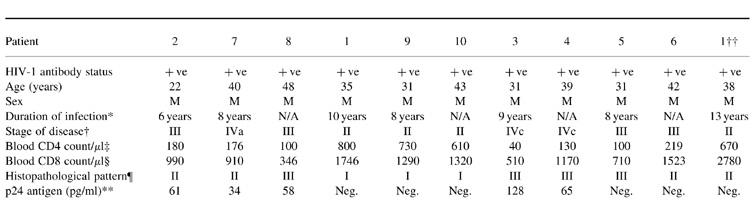
*Approximate time since seroconversion. N/A indicates patients who did not recall a seroconversion illness, but had a positive serological result for more than 6 years.
†Centers for Disease Control (CDC) classification of HIV-1 disease (MMWR 1986; 35:334).
‡Normal range (700–1200 cells/μl).
§Normal range (550–650 cells/μl).
¶Histopathological patterns [37–39]; Pattern I, germinal centre hyperplasia and expansion; II, germinal centre loss, neovascularization; III, lymphocyte depletion, node atrophy; secondary diseases such as mycobacteriosis, lymphoma or Kaposi's sarcoma.
**p24 antigen level in serum at the time of biopsy (Innogenetics, Gent, Belgium).
‡‡This patient donated a lymph node on two occasions 3 years apart.
HIV-1-infected lymph nodes have a decreased proportion of lymphocytes and an increase in the proportion of monocytes
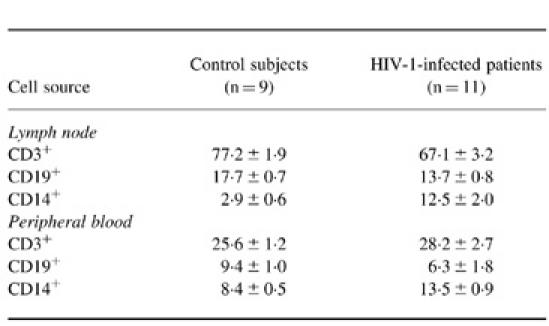
In the ungated cell populations, CD3+ T lymphocytes accounted for over 75% of the cells in the lymph nodes obtained from control subjects (Table 2). When compared with the corresponding peripheral blood samples, the ungated population in the control lymph nodes had a significantly higher proportion of CD3+ T lymphocytes (P < 0.001) and CD19+ B lymphocytes (P < 0.001), but had a lower percentage of CD14+ cells (P < 0.001), indicating that lymphocytes were the principal cellular components of the lymph nodes. In HIV-1-infected lymph nodes, the proportions of T and B lymphocytes were significantly lower than the control nodes (P < 0.01; Table 2). In contrast, the proportion of CD14+ cells was significantly increased in HIV-1-infected lymph nodes (12.5%) compared with control subjects (2.9%; P < 0.01).
Table 2.
The proportion of T cells (CD3+), B cells (CD19+) and monocytes (CD14+) in lymph node and peripheral blood samples from patients with HIV-1 and control subjects (mean ± s.e.m.)
Lymph nodes from HIV-1 infected patients contain a decreased proportion of CD4+ and an increased proportion of CD8+ T lymphocytes
In the lymphocyte gate, the mean proportion of CD3+ T lymphocytes in the lymph nodes and peripheral blood of control subjects were 79.9% and 75.6%, respectively. In comparison with the peripheral blood, lymph nodes from control subjects contained a significantly higher (P < 0.001) proportion of CD4+ lymphocytes (68.6% compared with 45.5%) and a significantly lower (P < 0.001) proportion of CD8+ lymphocytes (8.5% compared with 21.0%). The mean CD4:CD8 ratio in the blood of control subjects was 2.4, whereas in the lymph node it was 8.5 (Table 3). The percentage of CD19+ B lymphocytes was also significantly higher (P < 0.005) in the control lymph nodes compared with peripheral blood. Furthermore, there was a significantly lower (P < 0.001) proportion of natural killer (NK) cells in the control nodes (range 0.5–2.4%) in comparison with peripheral blood (range 5.4–19.3%).
Table 3.
The subpopulations of lymphocytes in lymph nodes and peripheral blood in the lymphocyte gate (mean ± s.e.m.)
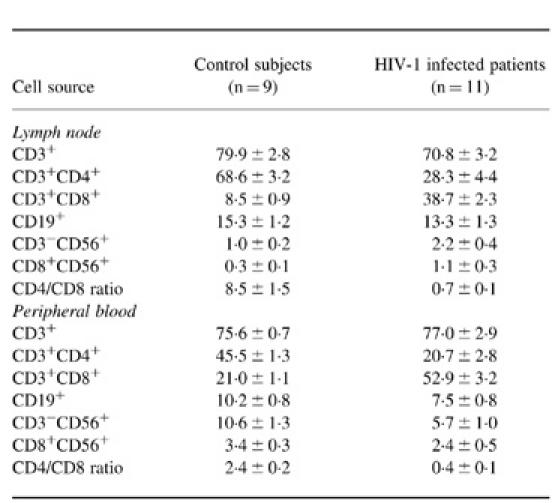
In both the lymph nodes and peripheral blood of HIV-1-infected patients, there was a decrease in the proportion of CD4+ and CD19+ lymphocytes (Table 3). The degree of CD4+ T lymphocyte depletion in the HIV-1-infected lymph nodes was statistically significant (P < 0.005). In contrast to the marked depletion in CD4+ T lymphocytes, there was a prominent expansion of CD8+ lymphocytes in both the nodes (P < 0.005) and peripheral blood samples (P < 0.001) obtained from HIV-1-infected patients. A significant proportion of the CD8+ T cells in the lymph nodes and peripheral blood obtained from HIV-1-infected individuals showed markers of activation as determined by CD38 and HLA-DR (data not shown). The apparent decrease in the proportion of CD19+ B lymphocytes in the HIV-infected lymph nodes was not statistically significant. The proportion of NK cells in the nodes was similar in HIV-1-infected and control subjects, although this level was consistently lower than that in the corresponding peripheral blood samples (Table 3).
The proportion of naive T lymphocytes is decreased and that of memory T lymphocytes is increased in lymph nodes in patients with HIV-1 infection
In both the CD4+ and CD8+ T lymphocyte subsets derived from the control lymph nodes, the proportion of CD45RA+ (naive) lymphocytes was significantly higher (P < 0.001) than that of the CD45RO+ (memory) phenotype (Fig. 2). The range of expression of CD45RO in control nodes was wide (5.2–38.2% for the CD4+ subset, and 5.4–45.6% for the CD8+ subset).
Fig. 2.
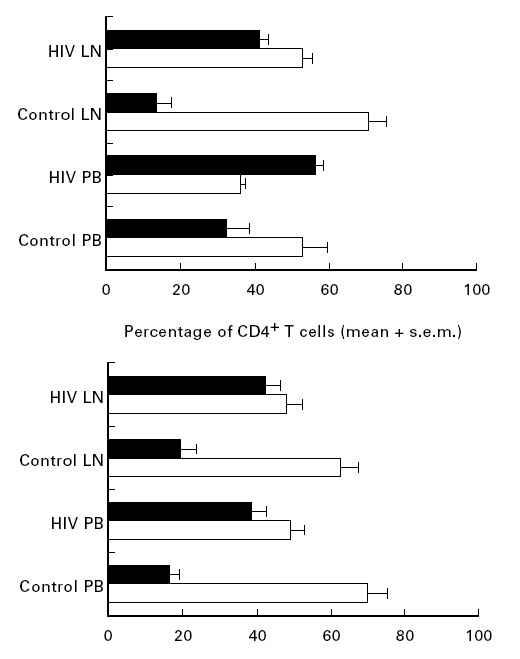
The proportion of T lymphocyte subsets expressing CD45RA (□) or CD45RO (▪) in blood and lymph node of control subjects (n = 9) and patients (n = 11) with HIV (mean ± s.e.m.).
In contrast to the control subjects, there was an increased proportion of CD4+CD45RO+ and CD8+CD45RO+ lymphocytes in both the lymph nodes and the corresponding peripheral blood samples of patients infected with HIV-1 (Fig. 2). There was also a significantly lower proportion of naive (P < 0.01), and a significantly higher proportion of memory (P < 0.001) CD4+ lymphocytes in HIV lymph nodes. For the CD8+ subset, although the increase in the proportion of memory lymphocytes was significant in HIV lymph nodes (P < 0.005), the trend towards decreased naive CD8+ lymphocytes did not reach statistical significance.
The expression of adhesion molecules on CD4+ and CD8+ lymphocyte subsets is altered during HIV-1 infection
The expression of l-selectin was lower in both CD4+ and CD8+ T lymphocyte subsets in control lymph nodes compared with the corresponding peripheral blood (Fig. 3; P < 0.001). The most striking finding, however, was the significant down-regulation in the expression of l-selectin on both CD4+ and CD8+ T lymphocytes from HIV-1-infected lymph nodes compared with either the corresponding peripheral blood, or the levels detected in control lymph nodes (Fig. 3; P < 0.005 for each).
Fig. 3.
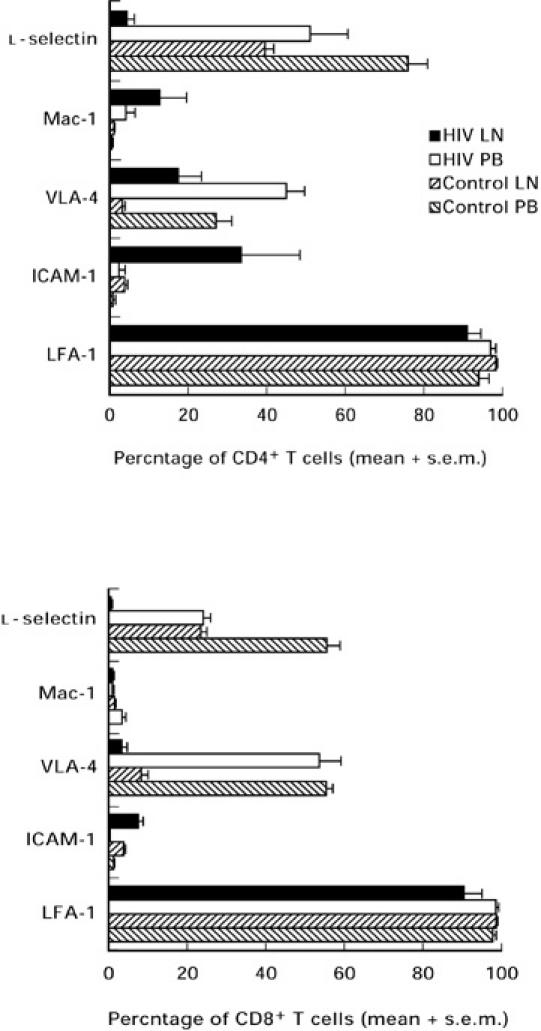
The mean ± s.e.m. proportion of T lymphocytes expressing leucocyte adhesion molecules in lymph nodes and peripheral blood obtained from patients infected with HIV-1 (n = 5) and control subjects (n = 10). VLA-4, Very late antigen-4; ICAM-1, intercellular adhesion molecule-1; LFA-1, leucocyte function-associated antigen-1.
Over 95% of T lymphocytes in the peripheral blood and lymph nodes of control subjects expressed LFA-1 (Fig. 3). Similarly, in patients with HIV-1 infection the mean expression of LFA-1 on T lymphocytes remained consistently high (Fig. 3). By contrast, the expression of VLA-4 on both CD4+ and CD8+ T lymphocyte subsets in the peripheral blood obtained from control subjects was significantly higher than that observed in the corresponding lymph node lymphocytes (Fig. 3; P < 0.001). Expression of VLA-4 by CD4+ lymphocytes derived from HIV-1-infected lymph nodes was higher than the level found in control subjects (Fig. 3).
As expected, the mean expression of Mac-1 was < 3% on CD4+ and CD8+ lymphocyte subsets both in peripheral blood and lymph nodes obtained from control subjects (Fig. 3). The range of Mac-1 expression in HIV-1-infected lymph node and peripheral blood, particularly in CD4+ T lymphocytes, however, was more varied (Fig. 3). The expression of ICAM-1 by CD4+ and CD8+ lymphocytes obtained from control peripheral blood and lymph nodes was < 5% (Fig. 3).
Adherence of PBL and LNL to fibronectin, rhICAM-1 and rhVCAM-1 is increased in HIV-1 infection
LNL and PBL from four HIV-1-infected donors and four control subjects were available for the adhesion assays. HIV LNL showed significantly higher adherence to fibronectin (P < 0.02), rhICAM-1 (P < 0.02) and rhVCAM-1 (P < 0.02) when compared with control LNL (Fig. 4). Although HIV LNL were consistently more adherent to fibronectin and rhICAM-1 than the corresponding HIV PBL in all four patients (Fig. 4), the differences in adherence were not statistically significant. LNL from control nodes were not significantly more adherent to these substrates than the corresponding control PBL (Fig. 4). By contrast, the adhesion of LNL to rhVCAM-1 was significantly higher (P < 0.02) than corresponding PBL in both HIV-1-infected patients and control subjects.
Fig. 4.
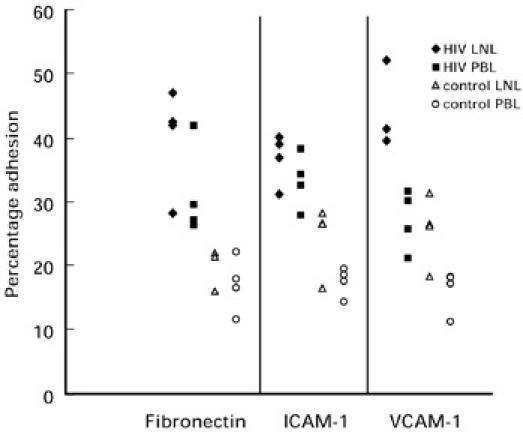
Scatter diagram showing the level of adhesion to fibronectin, rh intercellular adhesion molecule-1 (ICAM-1) or rh vascular cell adhesion molecule-1 (VCAM-1) by lymph node-derived lymphocytes (LNL) and peripheral blood lymphocytes (PBL) obtained from individual HIV-1-infected patients and control subjects (n = 4). Each point represents the mean of triplicate samples and the horizontal bars represent the mean values for each group. The differences among the triplicate wells of each sample were < ± 5%.
DISCUSSION
These data provide the first sound reference values for comparative analysis of lymphocyte phenotypes in human cervical lymph nodes and corresponding peripheral blood samples, including in patients with HIV-1. Previous studies have attempted to document the distribution of lymphocyte subpopulations in lymph nodes using rosette formation [19], indirect immunofluorescence [20–23], and flow cytometric techniques [24, 25]. However, little attention was paid in these studies to the anatomical location of the nodes, or to the peri-mortem changes in nodes collected from cadavers [19, 21, 22, 24, 25]. To address these variables, the control data presented in this study were derived from the examination of cervical nodes collected from living, essentially healthy subjects. Furthermore, the use of three-colour flow cytometry, directly conjugated antibodies, as well as controls that included isotype negative control antibodies and leucogate, allowed accurate identification of lymphocyte subpopulations.
The mean age of 62 years in the control subjects was significantly higher than that of the patients. This age difference might have contributed to the differences in the T cell populations observed between the groups. However, several reports on the effects of ageing on the proportions of T cells in the peripheral blood indicate that age has little [26] or no effect [27] on the percentages and absolute counts of lymphocyte subsets and monocytes. There are no reported data on the effect of ageing on the proportions of T cell subsets in human lymph nodes.
In this study, we have confirmed that T cells represent approx. 80% of lymph node lymphocytes in healthy subjects [20, 21, 24, 25]. Of these, approx. 70% are CD4+ T lymphocytes, as has been demonstrated in a previous study of non-cadaveric [21], and cadaveric lymph nodes [24, 25]. The proportion of CD8+ lymphocytes found here was approx. 10% lower than in all four published reports [20, 21, 24, 25]. Such variance may be related to the specific location of the nodes studied, the status of nodal reactivity at the time of harvest, the technique used to make the lymphocyte suspensions, as well as the methods of staining and analysis. The proportions of CD3+, CD4+, CD8+, CD19+, CD56+ lymphocytes in the peripheral blood of control subjects reported here correspond closely to the normal ranges established in other laboratories [28–31].
Although lymph node sampling can only provide a single ‘snap-shot’ of the dynamic trafficking and proliferation events in human lymph nodes, our data allow a direct comparison of the lymphocyte subpopulations in peripheral blood and lymph node in healthy subjects (Table 3). The differentials found here highlight the existence of active mechanisms to regulate accumulation of some lymphocyte subsets (notably CD4+ T cells), and not others (notably NK cells), in lymph nodes.
We have demonstrated a progressive depletion of CD4+ and CD19+ lymphocytes in both lymph node and peripheral blood samples of HIV-1-infected patients corresponding to the clinical and histopathological stages of disease (Table 1). By contrast, there was prominent expansion of CD8+ lymphocytes in all nodes, as has been reported in previous immunohistochemical studies [18, 32, 33]. The persistently high levels of CD8+ lymphocytes in the peripheral blood may have contributed to the accumulation of this lymphocyte subset in the lymph nodes. However, the absence of a shift in the relative proportion of this subset (in comparison with CD4+ lymphocytes) in blood and lymph node suggests an expansion of the CD8+ subset in both compartments, presumably due to proliferation. In contrast to control subjects, the majority of CD8+ T cells (> 90%) in the peripheral blood and lymph nodes obtained from HIV-1-infected individuals were highly activated (CD38 and HLA-DR+). The higher proportion of CD14+ cells observed in HIV-1-infected lymph nodes might not be attributable only to HIV-1 infection, but may be due to the presence of concomitant infections or due to the relative decrease of T cell populations in HIV-infected lymph nodes.
As expected, the majority of CD4+ and CD8+ lymphocytes in control lymph nodes express CD45RA, a marker for naive phenotype (Fig. 2; [34–37]), consistent with the notion of preferential homing of naive lymphocytes into lymph nodes [35]. When compared with the corresponding populations in the control lymph nodes, there were higher proportions of memory CD4+ T lymphocytes in the blood than in the node, but there was no significant difference between blood and lymph node for the memory and naive subsets of CD8+ T lymphocytes. This finding supports the evidence for an extranodal migration pathway of CD4+ memory lymphocytes [35, 38] in the physiological state.
In HIV-1-infected subjects, the proportion of CD4+ and CD8+ T lymphocytes bearing the CD45RO+ (memory) marker was significantly higher than those of the control subjects, both in lymph nodes and peripheral blood (Fig. 2). This accumulation of memory lymphocytes in HIV-1-infected nodes could be explained by chemokine-mediated recruitment of these cells into HIV-1-infected lymph nodes [39], or by activation in situ leading to conversion of naive cells to the memory phenotype.
Comparison of the adhesion molecule phenotype of lymphocytes in peripheral blood and lymph node of control subjects revealed a significant down-regulation of l-selectin and VLA-4, but not LFA-1 expression (Fig. 3). There was an even more marked down-regulation of l-selectin expression on T lymphocytes obtained from HIV-1-infected human lymph nodes. l-selectin is known to be shed upon binding and transmigration of lymphocyte subpopulations across the specialized high endothelial venules (HEV) of peripheral lymph nodes [40]. Furthermore, VLA-4 expression was mildly increased on T lymphocytes in HIV-1-infected lymph nodes in comparison with control lymph nodes. This finding is also consistent with a phenotype of migrating T lymphocytes.
Lymphocytes derived from HIV-1-infected lymph nodes showed a significantly higher adhesive capacity than lymphocytes obtained from the lymph nodes of control subjects, despite the absence of substantially increased expression of adhesion molecules on HIV-1 LNL (Fig. 4). Furthermore, PBL obtained from both HIV-1-infected patients and control subjects showed a trend towards decreased adhesive capacity to fibronectin, rhICAM-1 and rhVCAM-1 when compared with the corresponding LNL, despite higher levels of VLA-4 expression and similar levels of LFA-1 expression. These findings suggest that qualitative changes in the affinity status of the integrin molecules are more important than quantitative changes in expression. Affinity modulation of both LFA-1 and VLA-4 is thought to be the dominant mode of regulation of the adhesion properties of T lymphocytes [16, 41]. Alternatively, adhesion molecules other than those studied here may be involved in these interactions. Based on these observations, we suggest that in HIV LNL, altered expression of adhesion molecules and enhanced adhesive phenotype may facilitate a substantially increased trafficking of T lymphocytes into lymph nodes in HIV disease. Regulation of this lymphocyte traffic may provide a useful target to abrogate CD4 T lymphocyte recruitment (and minimize viral propagation), as well as to enhance trafficking of CD8+ T lymphocytes and promote anti-viral activities.
Acknowledgments
We are grateful to Drs Mark Kelly and Susan Adams for critical appraisal of the manuscript and members of the Division of Surgery, Prince of Wales Hospital for their assistance in collection of lymph nodes from control subjects. This work was supported in part by the Japanese Foundation for the Prevention of AIDS.
REFERENCES
- 1.Young AJ, Hay JB, Mackay CR. Lymphocyte recirculation and life span in vivo. Curr Topics Microbiol Immunol. 1993;184:161–73. doi: 10.1007/978-3-642-78253-4_13. [DOI] [PubMed] [Google Scholar]
- 2.Cahill RNP, Poskitt DC, Frost H, et al. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J Exp Med. 1977;145:420–8. doi: 10.1084/jem.145.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall JG, Morris B. The origin of the cells in the efferent lymph from a single lymph node. J Exp Med. 1965;121:901–10. doi: 10.1084/jem.121.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill RN, Frost H, Trnka Z. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J Exp Med. 1976;143:870–88. doi: 10.1084/jem.143.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackay CR, Marson WL, Dudler L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur J Immunol. 1992;22:2205–10. doi: 10.1002/eji.1830220904. [DOI] [PubMed] [Google Scholar]
- 6.Christensen JP, Andersson C, Scheynius A, et al. α4 integrin directs virus-activated CD8+ T cells to sites of infection. J Immunol. 1995;154:5293–301. [PubMed] [Google Scholar]
- 7.Andersson EC, Christensen P, Marker O, et al. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–45. [PubMed] [Google Scholar]
- 8.Hou S, Hyland L, Bradley LM, et al. Subverting lymph node trafficking by treatment with the Mel-14 MoAb to l-selectin does not prevent an effective host response to Sendai virus. J Immunol. 1995;155:252–8. [PubMed] [Google Scholar]
- 9.Pals ST, DenOtter A, Miedema F, et al. Evidence that leucocyte function-associated antigen-1 is involved in recirculation and homing of lymphocytes via high endothelial venules. J Immunol. 1988;140:1851–3. [PubMed] [Google Scholar]
- 10.Hamann A, Jablonski-Westrich D, Scholz KU, et al. Regulation of lymphocyte homing. I. Alterations in homing receptor expression and organ-specific high endothelial venule binding of lymphocytes upon activation. J Immunol. 1988;140:737–43. [PubMed] [Google Scholar]
- 11.Pantaleo G, Graziosi C, Demarest JF. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 12.Blattner W, Gallo RC, Temin HM. HIV causes AIDS. Science. 1988;241:515–6. doi: 10.1126/science.3399881. [DOI] [PubMed] [Google Scholar]
- 13.Emberston J, Zupancic M, Ribas L. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–62. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 14.Landay AL, Mackewicz CE, Levy JA. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–16. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 15.Schnizlein CT, Kosco MH, Szakal AK, et al. Follicular dendritic cells in suspension: identification, enrichment, and initial characterization indicating immune complex trapping and lack of adherence and phagocytic activity. J Immunol. 1985;134:1360–8. [PubMed] [Google Scholar]
- 16.Lloyd AR, Oppenheim JJ, Kelvin DJ, et al. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156:932–8. [PubMed] [Google Scholar]
- 17.Gamble JR, Vadas MA. A new assay for the measurement of the attachment of neutrophils and other cell types to endothelial cells. J Immunol Methods. 1988;109:175–84. doi: 10.1016/0022-1759(88)90240-2. [DOI] [PubMed] [Google Scholar]
- 18.Brynes RK, Gill PS. Clinical characteristics, immunologic abnormalities, and hematopathology of HIV infection. In: Vijay VJ, editor. Pathology of AIDS and other manifestations of HIV infection. New York: Igaku-Shoin; 1990. pp. 21–41. [Google Scholar]
- 19.Ermin O, Plumb D, Coombs RA. T and B lymphocyte populations in human normal lymph nodes. Int Arch Allergy Appl Immunol. 1976;52:277–90. doi: 10.1159/000231693. [DOI] [PubMed] [Google Scholar]
- 20.Black RB, Leong AS, Crowed PA, et al. Lymphocyte subpopulations in human lymph nodes: a normal range. Lymphol. 1980;13:86–90. [PubMed] [Google Scholar]
- 21.Lindelman C, Mellstedt H, Biverfeld P. Blood and lymph node T-lymphocyte subsets in non-Hodgkin lymphomas. Scand J Haematol. 1983;30:68–78. doi: 10.1111/j.1600-0609.1983.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 22.Verma RC, Balakrishmas K, Vasudevan DM. Lymphocytes bearing immunoglobulin determinants in normal human lymph nodes and in patients with lepromatous leprosy. Int J Leprosy. 1971;39:20–24. [PubMed] [Google Scholar]
- 23.Aisenberg AC, Long JC. Lymphocyte surface characteristics in malignant lymphoma. Am J Med. 1975;58:300–6. doi: 10.1016/0002-9343(75)90595-1. [DOI] [PubMed] [Google Scholar]
- 24.Kelly-Williams S, Zmijewski M, Tomaszewski E. Lymphocyte subpopulations in ‘normal’ lymph nodes harvested from cadavers. Lab Med. 1989;20:487–90. [Google Scholar]
- 25.Bryan CF, Eastman J, Conner B, et al. Clinical utility of a lymph node normal range obtained by flow cytometry. Ann NY Acad Sci. 1993;667:404–6. doi: 10.1111/j.1749-6632.1993.tb38799.x. [DOI] [PubMed] [Google Scholar]
- 26.Negoro S, Hara H, Miyata S, Saiki O, Tanaka T, Yoshizaki K, Nishimoto N, Kishimoto S. Age-related changes of the function of T cell subsets: predominant defect of the proliferative response in CD8 positive T cell subset in aged persons. Mech Ageing Dev. 1987;39:263–79. doi: 10.1016/0047-6374(87)90066-2. [DOI] [PubMed] [Google Scholar]
- 27.Matour D, Melnicoff M, Kaye D, Murasko DM. The role of T cell phenotype in decreased lymphoproliferation of the elderly. Clin Immunol Immunopathol. 1989;50:82–89. doi: 10.1016/0090-1229(89)90224-9. [DOI] [PubMed] [Google Scholar]
- 28.Landay A, Ohlsson-Wilhelm B, Giorgi J. Application of flow cytometry to the study of HIV infection. AIDS. 1990;4:479–97. doi: 10.1097/00002030-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Strauss K, Hannet I, Hulstaert F, et al. Immune system monitoring and the management of HIV patients. Clinical Notes from Becton Dickinson. 1992:1–15. [Google Scholar]
- 30.Beck R, Lam-Po-Tang R. Comparison of cord blood and adult blood lymphocyte normal range: a possible explanation for decreased severity of graft versus host disease after cord blood transplant. Immunol Cell Biol. 1994;72:440–4. doi: 10.1038/icb.1994.65. [DOI] [PubMed] [Google Scholar]
- 31.Giorgi JV, Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989;52:10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 32.Chan WC, Brynes RK, Spira TJ. Lymphocyte subsets in lymph nodes of homosexual men: correlation of morphology and blood changes. Arch Pathol Lab Med. 1985;109:128–32. [PubMed] [Google Scholar]
- 33.Frizzera G, Seo S. Histopathology of non-malignant lymphadenopathies. In: Pangalis G, Pollack A, editors. Benign and malignant lymphadenopathies: clinical and laboratory diagnosis. 1. Chur: Harwood Academic Publishers; 1993. pp. 131–57. [Google Scholar]
- 34.Ziegler-Heitbrock W, Stachel D, Schunk T, et al. Class II (DR) antigen expression on CD8+ lymphocyte subsets in acquired immune deficiency syndrome (AIDS) J Clin Immunol. 1988;8:473–8. doi: 10.1007/BF00916953. [DOI] [PubMed] [Google Scholar]
- 35.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–17. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackay CR. Migration pathways and immunologic memory among T lymphcytes. Semin Immunol. 1992;4:51–58. [PubMed] [Google Scholar]
- 37.Westermann J, Pabst R. How organ specific is the migration of ‘naive’ and ‘memory’ T cells? Immunol Today. 1996;17:278–82. doi: 10.1016/0167-5699(96)80545-7. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y, Newman W, Tanaka Y, et al. Lymphocyte interactions with endothelial cells. Immunol Today. 1992;13:106–12. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 39.Tedla N, Palladinetti P, Kelly M, et al. Chemokines and T cell recruitment to lymph nodes in HIV infection. Am J Pathol. 1996;148:1367–73. [PMC free article] [PubMed] [Google Scholar]
- 40.Gallatin WM, Weissman L, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 41.Carlos TM, Harlan JM. Leucocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]


