Abstract
Nonmuscle cells have almost ubiquitously evolved a mechanism to detect and prevent Ca2+ store depletion—store operated calcium entry. No such mechanism has, as yet, been reported in cardiac myocytes. However, it is conceivable that such a mechanism may play an important role in cardiac Ca2+ homeostasis to ensure the availability of sufficient stored Ca2+ to maintain normal excitation contraction coupling. We present data that confirms the presence of a mechanism that is able to monitor the Ca2+ load of the SR and initiate a signaling process to accelerate Ca2+ uptake by the SR when store depletion is detected. Depletion of SR Ca2+ activates a protein kinase, the principal SR substrate of which is phospholamban. Phosphorylation of this SR protein promotes Ca2+ pump activity and therefore store refilling. Furthermore, a protein kinase activity associated with the SR that is inhibited by Ca2+ ions has been identified. We have measured lumenal [Ca2+] by using a fluorescent Ca2+ indicator and found that by initiating Ca2+ uptake and increasing Ca2+ load, we can inhibit the protein kinase activity associated with the SR. This confirms that a protein kinase, that is regulated by lumenal [Ca2+], has been identified and represents part of a previously unidentified signalling cascade. This local feedback mechanism would allow the myocyte to detect and prevent SR Ca2+ load depletion.
The calcium required for cardiac muscle contraction is derived from two sources, the intracellular store [the sarcoplasmic reticulum (SR)] that represents a finite reserve, and the extracellular milieu, which is effectively infinite. The quantitative importance of each of these sources in excitation contraction coupling is the inverse of their size. The SR contributes ≈75% of the Ca2+ for each contraction (variable with animal species) with the remainder entering from the extracellular environment (1). The maintenance of the intracellular Ca2+ store, suitably filled with Ca2+, is a prerequisite for excitation contraction coupling, and is achieved by the appropriate competition between Ca2+ transport systems of the SR and plasma membrane (1). In many other cell types the intracellular Ca2+ store also performs an important function. Ca2+ release enables the cell to effectively translate extracellular signals into functional responses. To guard against Ca2+ store depletion and loss of this capability, cells have evolved a mechanism that allows constant replenishment of the Ca2+ store. Store operated Ca2+ entry initiated by retrograde signaling pathways have been described in a wide variety of cell types, including smooth muscle cells and nonmuscle cells (2–4). The exact nature of the retrograde signal remains a matter of some debate. One example, however, is a protein phosphatase in human platelets that is responsive to the Ca2+ content of platelet stores and modifies the phosphorylation status of targets in response to store depletion (5). A second example may involve the mammalian homologue of the serine/threonine kinase IRE1 (6). Inhibition of Ca2+ uptake by intracellular Ca2+ stores of Chinese hamster ovary cells results in the increased expression of the molecular chaperone grp78 (6), an effect that is exaggerated by expression of the yeast kinase IRE1. By analogy, the Ca2+ concentration within the lumen of the SR of cardiac muscle may control kinase/phosphatase enzymes and thereby influence the rate of Ca2+ accumulation by the store. This could serve to safeguard the SR from progressive Ca2+-loss if the Ca2+-transport systems of the plasma membrane and SR were not balanced appropriately. Phospholamban represents a plausible target in this hypothesis; normally an inhibitor of SR Ca2+ pump activity, phosphorylation of phospholamban in response to Ca2+ store depletion would be expected to accelerate Ca2+-uptake by the SR (7) and facilitate store refilling. In the present study, experimental evidence is provided in support of this suggestion. First, freshly isolated cardiac myocytes (from the rat) responded to SR Ca2+ depletion by phosphorylating phospholamban on Ser-16. Second, a protein kinase that copurifies with the SR is active at low [Ca2+] (≤3 μM), but inhibited by high [Ca2+] (≥30 μM). This activity represents a potential candidate mediating the myocyte response to Ca2+ store depletion. Finally, we have demonstrated that the site of this regulation by Ca2+ is from the lumenal aspect of the SR membrane ([Ca2+]L). Manipulating lumenal Ca2+ alters phospholamban phosphorylation in a manner consistent with our hypothesis, i.e., at steady-state loading, which represents [Ca2+]L of 35 μM phospholamban phosphorylation is inhibited. Collapse of the Ca2+ gradient and a return to low [Ca2+]L (3 μM) results in a concomitant increase in phospholamban phosphorylation.
EXPERIMENTAL PROCEDURES
Materials.
Cardiac SR vesicles were prepared as described in Li et al. (8), phospholamban antibodies as described in Drago and Colyer (9). The catalytic subunit of protein kinase A was purified from bovine heart according to Peters et al. (10). The synthetic peptide PLl919Y (RSAIRRASTIEY) was purchased from Neosystem (Strasbourg, France) and used as received (≈80% pure).
Ca2+ Store Depletion in Cardiac Myocytes.
Ventricular myocytes were isolated from adult Wistar rats (≈250 g as described in ref. 11), loaded with Fura-2AM (Molecular Probes, 5 μM for 15 min at room temperature), and superfused with physiological saline solution (11) in a perfusion chamber mounted on an inverted microscope at room temperature (≈22°C). The cells were electrically stimulated (0.5 Hz). Myocytes were illuminated at 340 nm and 380 nm, alternately, and the emission at 510 nm from a single cell was recorded and subsequently expressed as the ratio of that generated by 340/380 excitation. The Ca2+-content of the SR was assessed by challenge with 10 mM caffeine (12). Once control data had been captured from individual cells, the suspension was exposed to sarcoplasmic/endoplasmic reticulum Ca2+-ATPase inhibitors [thapsigargin (Tg, 2.5 μM), 2,5-di-(t-butyl)-1,4-hydroquinone (t-BHQ, 30 μM), and cyclopiazonic acid (CPA, 50 μM)] for 15 min. The SR Ca2+ content was assessed by challenge with 10 mM caffeine after 15 min exposure to drug.
Phospholamban Phosphorylation in Response to Store Depletion.
Rat ventricular myocytes were isolated as described in ref. 11 and resuspended at a density of ≈105 cells/ml in 5 mM Hepes (pH 7.3), 113 mM NaCl, 1 mM Na2HPO4, 1 mM MgSO4, 5 mM KCl, 10 mM glucose, 20 mM Na acetate, 5 units/ml insulin, 0.75 mM CaCl2. A single sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2 inhibitor (2.5 μM Tg, 30 μM t-BHQ, or 50 μM CPA) was added to individual cell suspensions, and dimethylsulfoxide (0.5% by volume) was added to control suspensions. The cells were agitated and electrically stimulated (0.5 Hz, 80 V) for 5 min. The reaction was stopped by the addition of Laemmli sample buffer (13) and the site-specific phosphorylation of phospholamban (on Ser-16 and Thr-17) was determined by Western blot analysis (9) by using 10,000 cells per observation.
In Vitro Phosphorylation of Phospholamban.
Phosphorylation was performed at 30°C in the absence and presence of 200 nM c-protein kinase A in 50 mM histidine (pH 7.0), 5 mM MgSO4, 6.25 mM NaF, 1 mM vanadate, 1 mM EGTA, 2 μM A23187, 0.5 mg/ml cardiac SR, 0.1 mM [γ32P]-ATP (≈100 cpm/pmol), and CaCl2 to achieve free Ca2+ concentrations of 3, 30, 300, and 3,000 μM. The phosphorylation reaction was terminated by the addition of Laemmli sample buffer (13) and SR proteins (10 μg) separated by SDS/PAGE (13).
Peptide Phosphorylation and Dephosphorylation.
Phosphorylation of the synthetic peptide RSAIRRASTIEY.amide (PL919Y, 14 μM) was performed in 50 mM histidine (pH 7.0), 5 mM MgSO4, 0.25 μM Tg, 2 μM A23187, 0.2 mg/ml cardiac SR, and 0.1 mM [γ32P]-ATP (≈100 cpm/pmol) with either 3 μM or 3 mM CaCl2. The reaction was terminated by spotting onto P81 phosphocellulose paper (Whatman) and washing in 1% phosphoric acid as described (14). Once dry, the papers were scintillation counted in Emulsifier-safe (Canberra–Packard). Dephosphorylation of PL919Y (140 μM) was examined following the phosphorylation of peptide at low Ca2+ as described above with the assistance of 50 nM c-protein kinase A. Phosphorylation was terminated by the addition of 1 μM PKI(5–22 amide) inhibitor peptide (15) (effective against both enzymes at this concentration), plus 3 μM or 3 mM CaCl2. Dephosphorylation samples were collected and processed as described above.
Measurement of [Ca2+]L.
Cardiac SR vesicles were removed from preparation buffer by centrifugation at 4,000 rpm for 2 min. The pellet was resuspended in buffer A (sucrose 150 mM/PEG 5% (wt/vol)/KCl 50 mM/Hepes 10 mM, pH 7.0). Vesicles were then incubated with 10 μM Magfura-5 AM (Molecular Probes) for 120 min at 4°C. Loaded vesicles were harvested by centrifugation (as above) and washed twice in buffer A before being resuspended in buffer A at a final concentration of 2 mg/ml. An excitation scan from 250–450 nm was used to determine success of dye loading (see Fig. 4). Fluorescent measurements were performed on a Perkin–Elmer LS50B luminescent spectrophotometer, using a filter wheel to alternate between the two excitation wavelengths of 340 and 380 nm. Emission was measured at 510 nm. Ca2+ uptake assay composition was routinely 1.5 mls; buffer B (75 mM sucrose/2.5% PEG (wt/vol)/25 mM KCl/5 mM Hepes, pH 7.0) and Magfura-5 loaded cardiac SR vesicles 67 μg/ml. Calcium uptake was initiated by addition of Mg⋅ATP (1.5 mM), assays were performed routinely at 30°C.
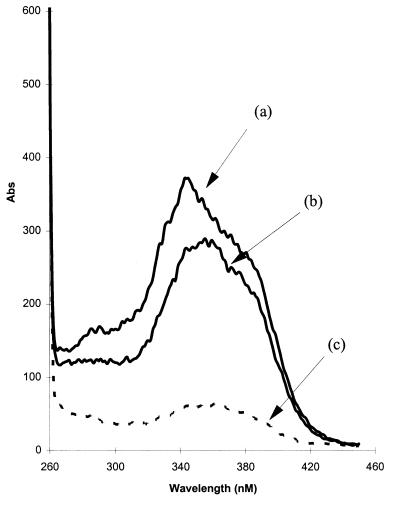
Figure 4.
Excitation wavelength scans indicate substantial lumenal de-esterification of Magfura-5 AM in canine cardiac SR vesicles. Vesicles were incubated with Magfura-5 AM (10 μM) for 120 min at 4°C. Each assay consists of 100 μg of protein in 1.5 ml of assay buffer (150 mM sucrose/50 mM KCl/5% PEG/10 mM Hepes, pH 7.0). Assays were performed at 30°C in a stirred cuvette. Traces describe excitation scans from 260–450 nm measuring emission at 510 nm; (a) Control; (b) addition of 20 μM Mn2+ (to quench external Magfura-5); (c) Addition of ionomycin (5 μM) plus Mn2+ (50 μM). The ion selectivity of ionomycin necessitates an increase in [Mn2+] to observe rapid quenching of lumenal Magfura-5.
Phosphorylation of Phospholamban During Ca2+ Uptake and Release.
Ca2+ uptake into SR vesicles was performed in parallel with the preceding section by addition of 1.5 mM Mg⋅ATP to a suspension of vesicles (67 μg/ml) in buffer B at 30°C. Samples were removed at various times and quenched in Laemmli sample buffer (13). The phosphorylation status of phospholamban was examined by SDS/PAGE and Western blot analysis as described above.
RESULTS AND DISCUSSION
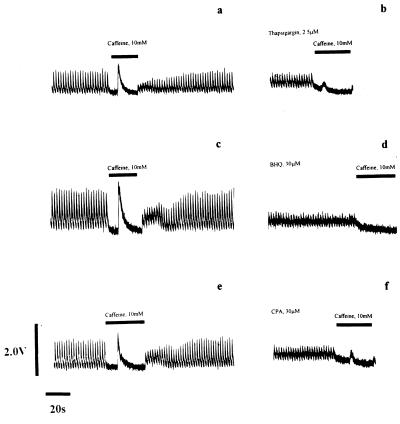
Interested in the signaling events triggered by Ca2+ store depletion in many cells, we sought to investigate the possibility of an analogous process occurring in cardiac muscle. Our proposal was that manipulation of the phosphorylation status of phospholamban would be an appropriate response to the depletion of Ca2+ from the SR. We would anticipate an increase in the phosphorylation of phospholamban, because this would promote store refilling through increased Ca2+ ATPase activity. To evaluate this hypothesis we first investigated the response of a population of freshly isolated rat cardiac myocytes to Ca2+ store depletion. The SR was depleted of Ca2+ by treating cells with three structurally dissimilar Ca2+ ATPase inhibitors, Tg, t-BHQ, and CPA. Fig. 1 illustrates the effectiveness of this strategy: Ca2+ transients from individual rat ventricular myocytes (evoked by electrical stimulation) were of uniform amplitude and short duration (Fig. 1 a, c, and e). The Ca2+ content of the SR was determined by exposure to 10 mM caffeine (12, 16). This maneuver opens the Ca2+ release channels in the SR (17) and mobilizes stored Ca2+. The Ca2+ transient evoked by caffeine was larger than that arising from electrical stimulation, indicative of a large amount of Ca2+ mobilized from the SR. The effects of caffeine were fully reversible. Removal of caffeine solutions and restoration of electrical stimulation was seen to refill the SR back to steady state Ca2+ transient levels. Exposure of the myocytes to each of the three SR Ca2+ pump inhibitors Tg (18) (2.5 μM, Fig. 1b), t-BHQ (19) (30 μM, Fig. 1d), or CPA (20) (50 μM, Fig. 1f) decreased the amplitude and increased the duration of the Ca2+ transient, consistent with inhibition of uptake by the SR. This was accompanied by a small increase in diastolic [Ca2+]. Under these conditions caffeine mobilizes little or no calcium after 15-min exposure to these drugs (Fig. 1 b, d, and f), confirming depletion of SR Ca2+ stores. According to our hypothesis a coincident increase in phospholamban phosphorylation would be anticipated.
Figure 1.
Depletion of Ca2+ from the SR of rat ventricular myocytes by exposure to Ca2+-pump inhibitors. The intracellular [Ca2+] of individual rat ventricular myocytes (11) was measured by Fura-2 fluorescence ratio before (a, c, and e) and during (b, d, and f) exposure to Ca2+-pump inhibitors. Electrical stimulation of control cells in physiological saline solution generated Ca2+-transients of regular amplitude and short duration. The cessation of electrical stimulation and application of 10 mM caffeine (▪) provoked release of Ca2+ from the SR detected by a large rise in the Fura-2 fluorescence ratio (a, c, and e). Removal of caffeine and resumption of electrical stimulation facilitated refilling of the SR and recovery of the peak amplitude of the Ca2+-transient to control levels. Exposure of the cells to a Ca2+-pump inhibitor for 15 min [b, 2.5 μM Tg; d, 30 μM t-BHQ (BHQ); f, 50 μM CPA] caused loss of Ca2+ from the SR that resulted in a reduction in the Ca2+-transient size observed upon electrical stimulation, and ablation or massive attenuation of the Ca2+-transient induced by caffeine challenge (▪). A small rise in diastolic Ca2+ accompanied exposure to these drugs. Each row of the figure shows Fura-2 fluorescence before and after 15-min exposure to the SR Ca2+-pump inhibitor in the same cell.
The effect of store depletion on the phosphorylation of Ser-16 of phospholamban was measured by using polyclonal antibodies specific for this phosphorylation site (9). These antibodies are wholly specific for phospholamban phosphorylated at this site and facilitate immunodetection of the phosphoprotein in whole-cell extracts. Rat cardiac myocytes exhibit low basal phosphorylation of phospholamban on Ser-16; this was increased dramatically on exposure of the cells to the β-adrenergic agonist, isoprenaline (Fig. 2, Con and Iso). A similar dramatic increase in the phosphorylation of phospholamban was provoked by depletion of the SR Ca2+ stores by 5 min exposure to each of the three Ca2+ pump inhibitors [Fig. 2, Tg (2.5 μM), BHQ (30 μM), CPA (50 μM)]. The identical effect of the three Ca2+ pump inhibitors suggest that the phosphorylation of phospholamban was provoked by their common action, i.e., Ca2+ pump inhibition and the subsequent depletion of Ca2+ stores. These data were reproduced in five other experiments with rat ventricular myocytes and a sixth with ferret ventricular myocytes (although not all drugs were used in all experiments). No change in Thr-17 phosphorylation of phospholamban was observed as a result of store depletion (data not shown). The small rise in diastolic Ca2+ produced by Ca2+ pump inhibitors (Fig. 1) was not responsible for the increasing Ser16 phosphorylation (in experiments of Fig. 2), because a similar elevation of cytoplasmic Ca2+, achieved by raising extracellular [Ca2+] (21), was without effect on phospholamban phosphorylation (data not shown). Thus, depletion of the Ca2+ store provides the signal to increase the phosphorylation of phospholamban. This in turn would promote refilling of the Ca2+ store through stimulation of Ca2+ pump activity (7).
Figure 2.
Stimulation of phospholamban phosphorylation on Ser-16 by depletion of Ca2+ from the SR of cardiac myocytes. The site-specific phosphorylation of phospholamban was measured by using antibodies specific to the phosphoprotein (9) following exposure of rat ventricular myocytes to Ca2+-pump inhibitors or the β-agonist, isoprenaline. Rat ventricular myocytes (1.1 × 105/ml, ref. 21) were exposed to 0.5% dimethylsulfoxide (control, Con), 2.5 μM Tg (TG), 30 μM t-BHQ (BHQ), 50 μM CPA, or 200 nM isoprenaline (Iso) for 5 min. Total cell protein (10,000 cells) was resolved by SDS/PAGE (13) and transferred to poly(vinylidene difluoride) membrane (9). The phosphorylation of phospholamban on Ser-16 was determined by using a phosphorylation site-specific antibody (9), developed by using an enhanced chemiluminescent substrate, and the region of the autoradiograph containing phospholamban is shown. Similar results have been obtained in six experiments with rat and ferret myocytes.
The time course of Ca2+ loss from the SR (illustrated by a progressive reduction in the amplitude of the Ca2+ transient) upon exposure to sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2 inhibitors was very similar to that of the increase in Ser-16 phosphorylation. Negretti et al. (12) demonstrated that Tg (2.5 μM) reduced the caffeine-induced Ca2+ mobilization of Ca2+ from the SR of rat ventricular myocytes (which is believed to equate to SR load) by ≈50% after 2 min. In our experiments Tg (2.5 μM) induced phosphorylation of phospholamban was first evident at 3 min (data not shown). Thus the signaling process that conveys the lumenal Ca2+ status to phospholamban is activated upon partial loss of Ca2+ from the SR, and may be an important control point to balance the Ca2+-sequestration activity of the SR with those of the sarcolemma.
Identification of a State of Filling Kinase (SOF Kinase).
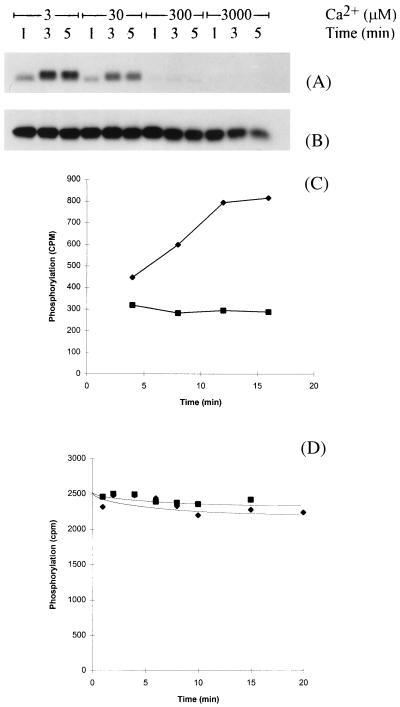
The next step in this investigation was to identify enzymes involved in mediating the phosphorylation of phospholamban in response to Ca2+ store depletion. In the first series of experiments we sought to determine whether the candidate enzymes were associated with cardiac SR vesicles. Phosphorylation of phospholamban, catalyzed by enzymes associated with SR vesicles was investigated as a function of [Ca2+]. SR vesicles are generally of right side out orientation (22), therefore a Ca2+ ionophore (A23187) was included in these experiments to facilitate manipulation of lumenal as well as cytosolic [Ca2+]. The phosphorylation of phospholamban was observed at low [Ca2+] (3 μM) upon addition of ATP, but was inhibited progressively as the [Ca2+] rose (30–3,000 μM) (Fig. 3A). Under these conditions phosphorylation of phospholamban was on Ser-16 exclusively (data not shown) as had been the case in vivo (Fig. 2). Other SR proteins phosphorylated in these experiments were unaffected by titration of Ca2+ (data not shown). Thus the phosphorylation of phospholamban by enzyme(s) associated with the SR was inhibited by high [Ca2+]. The concentration dependence of this phenomenon is inappropriate for cytoplasmic Ca2+, but within the range of measured [Ca2+]L found in the literature, which vary from 12 μM to 2 mM (23–26).
Figure 3.
Inhibition of phospholamban phosphorylation at high Ca2+. The phosphorylation of phospholamban in SR vesicles prepared from canine heart (8) was catalyzed by an endogenous kinase (A) or 200-nM cAMP-dependent protein kinase (B) at various free Ca2+ concentrations in the presence of A23187 (2 μM) for the times indicated. Phosphoproteins (10 μg) were separated by SDS/15% PAGE (13) and an autoradiograph of the region of the gel containing phospholamban displayed. These data were reproduced in six separate experiments by using different SR preparations. The phosphorylation (C) and dephosphorylation (D) of an exogenous synthetic peptide substrate derived from the sequence of phospholamban (9RSAIRRASTIE19Y, single letter codes) was performed at either 3 μM (⧫) or 3 mM (▪) CaCl2 in the presence of A23187 (2 μM) by enzymes associated with the SR vesicles (see Materials and Methods). Data shown are typical of four independent experiments.
Two separate mechanisms could account for the reduced phosphorylation of phospholamban at high [Ca2+]. Either the inhibition of a protein kinase or the activation of a phosphatase could underlie these observations. The phosphorylation of a synthetic peptide derived from the primary sequence of phospholamban (residues 9–19 plus a carboxy terminal tyrosine amide, refs. 9 and 27) was used to identify which of these enzymes in cardiac SR was Ca2+ sensitive. A Ca2+ ionophore (A23187) was included in the assay to ensure manipulation of both lumenal and extravesicular [Ca2+]. The peptide was phosphorylated by an SR kinase at Ser-16; and the rate of phosphorylation was inhibited by high [Ca2+] (3 mM; Fig. 3C). In contrast, dephosphorylation of this phosphopeptide (which had previously been phosphorylated by protein kinase A) by the SR phosphatase was slow and Ca2+ insensitive (Fig. 3D). These data identify a kinase as the Ca2+ responsive element of this process. The site of phosphorylation of phospholamban was identified as Ser-16 (using a combination of phosphoamino acid analysis and phosphorylation site specific antibodies; ref. 9), which is noteworthy because this site can be phosphorylated by cyclic AMP-dependent kinase (27). However, Ser-16 phosphorylation of phospholamban by protein kinase A was Ca2+ insensitive (Fig. 3B). The kinase cannot be Cam kinase II, because this enzyme phosphorylates phospholamban on Thr-17 exclusively. Neither is it protein kinase C; this enzyme’s preferred site of phosphorylation is Ser-10. Furthermore, protein kinase C is unaffected by protein kinase I and microsystin, both of which inhibit SOF kinase. Thus a kinase that is distinct from PKA and PKC is associated with cardiac SR and represents a potential candidate mediating the response to Ca2+ store depletion in cardiac myocytes. The Ca2+ profile of the kinase would tend to preclude regulation by cytosolic Ca2+, because even during a Ca2+ transient, the [Ca2+] would barely reach concentrations required to cause its inhibition. However, because both lumenal and cytosolic Ca2+ were manipulated in the experiment of Fig. 3, it is possible that the inhibitory effects of high Ca2+ were achieved from the lumenal aspect of the SR. To investigate the site of regulation further, we investigated the correlation between lumenal [Ca2+] and phospholamban phosphorylation. Intravesicular Ca2+ was measured by using the Ca2+ indicator Magfura-5. Incubation of the membrane permeable form (acetoxymethylester) with cardiac SR vesicles resulted in significant deesterification of the dye. Thus loading of vesicles with the Ca2+ responsive form was clearly shown by a strong fluorescence signal in the excitation spectrum 250–450 nm (Fig. 4A). Addition of Mn2+ (20 μM) caused quenching of the extracellular signal (Fig. 4B). This was repeated in all experiments. Permeabilizing the vesicular membrane by introducing ionomycin in the presence of Mn2+ resulted in almost complete quench (Fig. 4C), indicating that the majority (≈80%) of the fluorescent signal originated from within the membrane vesicles, i.e., from dye trapped in the lumen.
Calibration of Magfura-5.
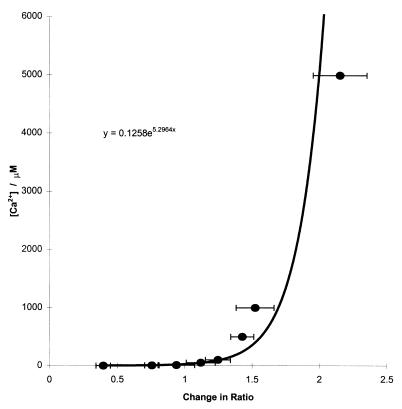
The response of Magfura-5 to [Ca2+] in our system was markedly nonlinear. The method of Loomis-Husselbee and Dawson (29) was used to calibrate the dye. A line of best fit to the response of the dye to a stepwise increase in free [Ca2+] was used to derive an empirical relationship between fluorescence and [Ca2+] (Fig. 5). This equation was then applied to raw fluorescence data to convert to [Ca2+]. Addition of Ca2+ (to a final concentration of 1 mM) to a standard uptake assay, indicated a lumenal [Ca2+] of 100 μM. This represents the extent of passive loading. However, introduction of ionomycin results in a measured [Ca2+] of 1 mM, correctly reflecting the free [Ca2+] of the assay. Therefore, application of the experimentally derived equation is an accurate method of determining [Ca2+].
Figure 5.
Calibration of Magfura-5 fluorescence with [Ca2+]. Magfura-5 loaded cardiac SR vesicles in a standard uptake assay were permeabilized by ionomycin (5 μM). Free [Ca2+] was manipulated by using EGTA.Ca2+ buffering. EGTA (50 μM) was included in each assay followed by the addition of varying concentrations of Ca2+ (determined by the computer program of ref. 31), depending on the required free [Ca2+] (1 μM–5 mM). Changes in the 340 nm/380 nm ratio were calculated from excitation wavelength scans for each [Ca2+] and plotted against free [Ca2+]. An equation was then derived from the line of best fit (Microsoft excel).
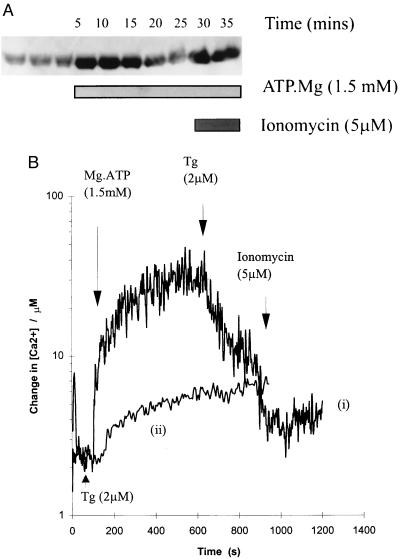
[Ca2+]L Following Active Ca2+ Transport.
Calcium uptake into Magfura-5 loaded vesicles was initiated by the addition of Mg⋅ATP (1.5 mM). After a rapid increase in lumenal [Ca2+], a steady-state loading of ≈35 μM was achieved and maintained after 10 min (Fig. 6B). Tg released ≈70% of sequestered Ca2+ (Fig. 6B, trace i) and addition of Tg before ATP resulted in no significant uptake (Fig. 6B, trace ii), confirming the majority of Ca2+ uptake was into SR vesicles. Extravesicular [Ca2+] in this experiment would be ≈2.5 μM, a condition that supports full SR kinase activity in vitro. However, if the effect of Ca2+ is manifest from the SR lumen, then we would expect some inhibition of SOF kinase activity at this [Ca2+]L (≈35 μM; Fig. 3A). Cardiac SR vesicles not loaded with Magfura-5 were treated in the same way and used in the standard uptake assay. Calcium uptake was again initiated by the addition of Mg⋅ATP. Aliquots of assay medium (7.5 μg protein) were removed at several points before and after the addition of Mg⋅ATP and quenched by using Laemmli sample buffer (13). Fig. 6A describes the phosphorylation of phospholamban during the course of uptake. The level of phospholamban phosphorylation is low before the addition of Mg⋅ATP. On addition of Mg⋅ATP an increase in phosphorylation is observed, which is consistent with our hypothesis, because the vesicles at this point would contain a low [Ca2+]. As steady state was achieved (≈10 min of uptake, Fig. 6B, trace i) and maintained, a gradual decrease in phospholamban phosphorylation is observed (Fig. 6A, 20′, 25′). When [Ca2+]L is reduced to 2.5 μM following introduction of ionomycin an increase in phospholamban phosphorylation was observed (Fig. 6A, 30′, 35′) consistent with activation of the SR kinase (SOF kinase) in response to Ca2+ store depletion. Keeping [Ca2+]L low by adding ionomycin and Mg⋅ATP simultaneously results in SOF kinase activity and a high level of phospholamban phosphorylation throughout the time course of Ca2+ uptake (not shown). Thus, [Ca2+]L has been directly measured in intact vesicles and manipulations of [Ca2+]L have resulted in the predicted changes in the phosphorylation of phospholamban. Therefore, a direct causal link between [Ca2+]L and phospholamban phosphorylation has been established, indicating that SOF kinase is indeed regulated at the lumenal face of the SR membrane.
Figure 6.
Calcium uptake into canine cardiac SR vesicles; Control of phospholamban phosphorylation by lumenal [Ca2+]. The fluorescence change of lumenal Magfura-5 on addition of Mg⋅ATP was measured. Conversion of raw fluorescence data by application of the experimentally determined equation shows a steady-state loading of ≈35 μM Ca2+. (B, trace i) Addition of Mg.ATP (1.5 mM) at 100 s; addition of Tg (2 μM) at 900 s. (B, trace ii) Addition of Tg (2 μM) at 30 s; addition of Mg⋅ATP (1.5 mM) at 100 s. (A) In parallel, aliquots of assay medium (7.5 μg protein) were removed at several points before and after the addition of Mg⋅ATP and quenched by using Laemmli sample buffer (13). Proteins were separated by using SDS/PAGE and phospholamban phosphorylation on Ser-16 was determined by using Western blot analysis (see Materials and Methods).
In conclusion, cardiac myocytes increase phospholamban phosphorylation in response to store depletion. A phospholamban kinase associated with cardiac SR vesicles and sensitive to high Ca2+ has been identified. Furthermore, loading Magfura-5 into the lumen of these SR vesicles has enabled measurement of active Ca2+ transport, a [Ca2+]L of ≈35 μM has been observed. This figure is unlikely to reflect SR [Ca2+]L in vivo (see ref. 30), however, has allowed us to determine the site of SOF kinase regulation. Measurement of the time course of phospholamban phosphorylation during active Ca2+ transport indicated inhibition of SOF kinase activity when [Ca2+]L rose to 35 μM. This inhibition is consistent with both the Ca2+ profile of SOF kinase activity (Fig. 3) and the time taken to reach steady state Ca2+ uptake (≈10 min; Fig. 6B, trace i). This evidence strongly supports the presence of a local feedback mechanism in cardiac SR. Depletion of Ca2+ load activates SOF kinase, which in turn phosphorylates phospholamban. In its phosphorylated state phospholamban can no longer exert its inhibitory influence on sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 2, therefore an increase in Ca2+ pump activity promotes the refilling of the Ca2+ store.
Acknowledgments
We are grateful for the help and advice of Professor Clive H. Orchard throughout these studies and for the provision of myocytes. The financial support of the British Heart Foundation (PG/96125) and the Medical Research Council (G9428756MA) is gratefully acknowledged. J.C. is a British Heart Foundation lecturer (BS/6).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations; SR, sarcoplasmic reticulum; Tg, thapsigargin; t-BHQ, 2,5-di-(t-butyl)-1,4-hydroquinone; CPA, cyclopiazonic acid; [Ca2+]L, lumenal calcium concentration; SOF kinase, state of filling kinase.
References
- 1.Bers D M, Bassani J W M, Bassani R A. Cardiovasc Res. 1993;27:1772. doi: 10.1093/cvr/27.10.1772. [DOI] [PubMed] [Google Scholar]
- 2.Putney J W., Jr Adv Second Messenger Phosphoprotein Res. 1992;26:143–151. [PubMed] [Google Scholar]
- 3.Irvine R F. Adv Second Messenger Phosphoprotein Res. 1992;26:143–151. [PubMed] [Google Scholar]
- 4.Fasolato C, Innocenti B, Pozzan T. Trends Pharmacol Sci. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 5.Vostal J G, Jackson W L, Shulman N R. J Biol Chem. 1991;266:16911–16916. [PubMed] [Google Scholar]
- 6.Cao X, Zhou Y, Lee A S. J Biol Chem. 1995;270:494–502. doi: 10.1074/jbc.270.1.494. [DOI] [PubMed] [Google Scholar]
- 7.Colyer J, Wang J H. J Biol Chem. 1991;266:17486–17493. [PubMed] [Google Scholar]
- 8.Li C, Wang J H, Colyer J. Biochemistry. 1990;29:4535–4540. doi: 10.1021/bi00471a005. [DOI] [PubMed] [Google Scholar]
- 9.Drago G A, Colyer J. J Biol Chem. 1994;269:25073–25077. [PubMed] [Google Scholar]
- 10.Peters K A, Demaille J G, Fischer E H. Biochemistry. 1977;16:5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- 11.Frampton J E, Orchard C H, Boyett M R. J Physiol (London) 1992;453:385–400. doi: 10.1113/jphysiol.1992.sp019234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negretti N, O’Neill S C, Eisner D A. J Physiol (London) 1993;468:35–52. doi: 10.1113/jphysiol.1993.sp019758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Corbin J, Lincoln T M. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H-C, Kemp B E, Pearson R B, Smith A J, Misconi L, Van Patten S M, Walsh D A. J Biol Chem. 1986;261:989–992. [PubMed] [Google Scholar]
- 16.Bers D M. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer; 1993. pp. 100–109. [Google Scholar]
- 17.Rousseau E, Meissner G. Am J Physiol. 1989;256:H328–333. doi: 10.1152/ajpheart.1989.256.2.H328. [DOI] [PubMed] [Google Scholar]
- 18.Lytton J, Westlin M, Hanley M R. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- 19.Witcome M, Michalengeli F, Lee A G, East J M. FEBS Lett. 1992;304:109–113. doi: 10.1016/0014-5793(92)80599-c. [DOI] [PubMed] [Google Scholar]
- 20.Seidler N W, Jona Vegh M, Martonosi A. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- 21.Frampton J E, Orchard C H, Boyett M R. J Physiol (London) 1991;437:351–375. doi: 10.1113/jphysiol.1991.sp018600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamberlain B K, Levitsky D O, Fleischer S. J Biol Chem. 1983;258:6602–6609. [PubMed] [Google Scholar]
- 23.Chatton J-Y, Liu H, Stucki J W. FEBS Lett. 1995;368:165–168. doi: 10.1016/0014-5793(95)00632-j. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Goldman W F. Am J Physiol C. 1995;269:698–705. doi: 10.1152/ajpcell.1995.269.3.C698. [DOI] [PubMed] [Google Scholar]
- 25.Dawson A P, Rich G T, Loomis-Husselbee J W. Biochem J. 1995;310:371–374. doi: 10.1042/bj3100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bygrave F L, Benedetti A. Cell Calcium. 1996;19:547–551. doi: 10.1016/s0143-4160(96)90064-0. [DOI] [PubMed] [Google Scholar]
- 27.Fujii J, Ueno A, Kitano K, Tanake S, Kadoma M, Tada M. J Clin Invest. 1987;79:301–304. doi: 10.1172/JCI112799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmerman H K B, Collins J H, Theibert J L, Wegener A D, Jones L R. J Biol Chem. 1986;261:13333–13338. [PubMed] [Google Scholar]
- 29.Loomis-Husselbee J W, Dawson A P. Biochem J. 1993;289:861–866. doi: 10.1042/bj2890861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Steenbergen C, Levy L A, Vance J, London R E, Murphy E. J Biol Chem. 1996;271:7398–7403. [PubMed] [Google Scholar]
- 31.Gould G W, East J M, Froud R J, McWhirter J M, Stefanova H I, Lee A G. Biochem J. 1986;237:217–227. doi: 10.1042/bj2370217. [DOI] [PMC free article] [PubMed] [Google Scholar]