Abstract
We investigated both in vitro and ex vivo the role of mature osteoblasts (OB) and bone marrow stromal cells (BMSC) in RA and OA by analysing the expression of the following IL-6-type cytokines: IL-11, leukaemia inhibitory factor (LIF), oncostatin M (OSM) and IL-6. OB and BMSC were isolated from femora of RA, OA and post-traumatic (PT) patients, cultured in vitro in the presence or absence of IL-1β and tumour necrosis factor-alpha (TNF-α), and assessed for the production and mRNA expression of IL-6-type cytokines. Trabecular bone biopsies were obtained from the inner portions of femoral heads and used for cytokine in situ immunostaining. Cultured OB and BMSC from different patients constitutively secreted IL-11 and IL-6 but not OSM. LIF was secreted only by BMSC, at very low levels. Interestingly, IL-11 basal production was significantly higher in BMSC than in OB in all three groups tested. IL-1β and TNF-α strongly stimulated IL-6-type cytokine release (except for OSM) by both OB and BMSC. OSM was expressed only at mRNA levels in all groups studied. Cytokine immunostaining on bone biopsies confirmed the data obtained on cultured cells: IL-11, IL-6 and LIF proteins were detected both in mesenchymal (BMSC and OB) and mononuclear cells; OSM was found only in mononuclear cells. These data demonstrate that IL-6-type cytokines are constitutively expressed in the bone compartment in RA, OA and PT patients and can be secreted by bone cells at different stages of differentiation (BMSC and OB). This suggests that these cytokines may be involved in the mechanisms of bone remodelling in OA and RA.
Keywords: osteoblasts, stromal cells, IL-11, leukaemia inhibitory factor, oncostatin M
INTRODUCTION
The evolution of OA and RA is characterized by processes of bone remodelling: in OA, subchondral bone stiffness increases, while erosions and osteonecrosis are typical features of RA bone [1–3]. Bone marrow stromal cells (BMSC) and osteoblasts (OB) may exert important functions in OA and RA bone since they can contribute directly or indirectly both to the remodelling processes and to cartilage alterations [4–6]. BMSC and OB belong to the same cell lineage and share phenotypic properties [7,8] including haematopoietic supporting capacity, phenotypic markers such as alkaline phosphatase and collagen type I [9,10], and the secretion of cytokines (including IL-6 and IL-11).
The so-called IL-6-type cytokines, IL-6, IL-11, leukaemia inhibitory factor (LIF), and oncostatin M (OSM) [11,12], have complex effects both on bone metabolism [13–15]—by stimulating mesenchymal progenitor differentiation towards the osteoblastic lineage [16]—and on the processes that contribute to the maintenance of normal bone homeostasis [17]. IL-6, which is an important regulator of haematopoiesis, can be produced by various cell types, including OB and BMSC [18,19]. This cytokine has contradictory effects on bone resorption and OB functions [20,21]: it has a specific function in bone metabolism, increasing the recruitment of osteoclasts and favouring bone resorption processes [9,10], but also plays a central role in the development of collagen-induced arthritis [22]. IL-11 exerts potent anti-inflammatory effects by inhibiting translocation of nuclear factor-κB (NF-κB) [23], and stimulates bone resorption by enhancing osteoclast formation [24] and OB-mediated osteoid degradation [25]. Unlike other cytokines, IL-11 is not produced by T lymphocytes or monocytes, suggesting that it is probably a specific product of the mesenchymal cell lineage, which includes BMSC and OB. LIF is a potent regulator of bone formation and resorption [26]. It is an osteoclast stimulating factor, its actions being mediated by OB, which are known to express LIF receptors [27]. Over-expression of LIF results in the accumulation of excess OB in the marrow and new bone formation [28]. In vitro studies have shown that LIF is involved in the regulation of OB differentiation and proliferation [29]. OSM is a potent inducer of IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) in human endothelial cell lines [30,31], and is capable of activating OB and inhibiting bone resorption [32].
In order to evaluate and compare the contributions of BMSC and OB to the expression of these IL-6-type cytokines in OA and RA bone compartments, we analysed steady-state and activated levels of IL-11, LIF, OSM and IL-6 in these cell types and in bone biopsies from OA and RA patients.
PATIENTS AND METHODS
Patients
Patients with RA and OA were selected according to American College of Rheumatology criteria [33,34]. Femoral head and bone marrow samples were obtained after informed consent and the study was approved by our hospital's institutional review board. Femoral heads were obtained from 13 OA (65 ± 10 years old (mean ± s.d.); disease duration 6 ± 5 years), 10 RA (64 ± 14 years; disease duration 11 ± 7 years) and nine post-traumatic ((PT) after fall; 67 ± 9 years old) patients undergoing selective total hip replacement. Bone marrow was obtained from 14 OA (63 ± 8 years; disease duration 5 ± 6 years), eight RA (63 ± 10 years; disease duration 12 ± 6 years) and eight PT (68 ± 7 years old) patients undergoing selective total joint replacement of the hip. Both femoral head and bone marrow were obtained simultaneously from three OA, two RA and two PT patients.
Human OB isolation
OB were obtained using a modification of the methods described by Robey & Termine [35]. Briefly, bone chips were obtained from trabecular bone from the inner portion of the head (Fig. 1) and minced in a sterile glass tube (Pierce Reacti-vials; Rockford, IL) containing serum-less 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM)/Ham's F12K no calcium (Life Technologies Ltd, Paisley, UK) supplemented with 100 U/ml penicillin, 25 μg/ml ascorbic acid (Sigma, St Louis, MO) and 2 mm calcium (Sigma). The minced bone chips were digested using 1 mg/ml of collagenase P (Boehringer Mannheim, Indianapolis, IN) at 37°C for 2 h with rotation, washed and placed in 35-mm dishes (approximately 0.05 ml condensed chips/dish) in the same medium containing 10% heat-inactivated fetal bovine serum (FBS; Life Technologies Ltd) without calcium (complete medium). Bone chips were fed twice a week with complete medium. After 2 weeks they were removed and OB were allowed to grow until confluent.
Fig. 1.
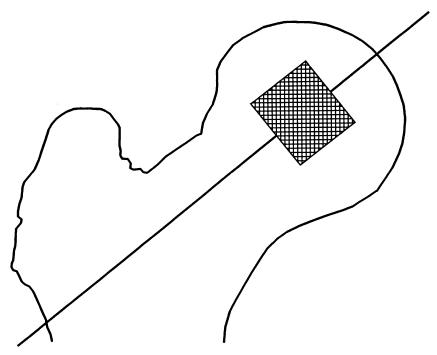
Diagram of femoral head depicting the zone selected for collecting samples for osteoblast isolation.
As already reported by our group [36], cultured cells expressed an OB phenotype, including alkaline phosphatase and osteocalcin-positive staining. Moreover, using immunohistochemical procedures it was ascertained that contamination of OB by haematopoietic cells was < 0.5% (data not shown).
BMSC isolation
Bone marrow was aspirated during hip surgery and collected in 15-ml tubes containing RPMI 1640 (Life Technologies Ltd) plus heparin (20 U/ml). Briefly, BMSC were isolated using Ficoll–Hypaque density gradient (d = 1.077g/ml; Pharmacia Biotech, Uppsala, Sweden) [37]. Cells were washed twice, resuspended in RPMI 1640 (Life Technologies Ltd) with 20% fetal calf serum (FCS; Life Technologies Ltd) and seeded at a concentration of 20 × 106 cells/T150 flask. After 1 week, non-adherent cells were removed and the adherent BMSC expanded in vitro. BMSC differed from OB in their higher alkaline phosphatase and reduced osteocalcin-positive staining. Immunohistochemical procedures were used to verify the presence of haematopoietic cells, which were < 0.1% (data not shown).
Cytokine production
OB and BMSC (2 × 104) were seeded in eight-well chamber slides (Nunc Inc., Napierville, IL) in the following conditions: untreated, activated with IL-1β (10 ng/ml) (specific activity 107U/mg) or tumour necrosis factor-alpha (TNF-α; 100 U/ml) (specific activity 108 U/mg) (Boehringer Mannheim) alone. The cytokine concentrations and incubation times were selected on the basis of results obtained in previous dose-dependence and kinetics experiments performed to assess the optimal conditions for detecting IL-6-type cytokine production. After 72 h, supernatants were collected and stored at −20°C. Slides were treated with FCS at room temperature for 5 min, dried and stored at −80°C until used for immunostaining. Cytokine production was evaluated by ELISA (R&D Systems, Minneapolis, MN and Genzyme, Cambridge, MA): IL-11 (sensitivity > 8 pg/ml), LIF (sensitivity > 4 pg/ml), OSM (sensitivity > 2.1 pg/ml) and IL-6 (sensitivity > 0.7 pg/ml) were tested for each experimental condition.
Cytokine reverse-transcriptase polymerase chain reaction analysis
Cytokine mRNA expression was evaluated in OB and BMSC from RA, OA and PT patients. After 19 h incubation at 37°C, cultured cells were washed twice with PBS–diethylpyrocarbonate (DEPC; Sigma), spun down and then stored at −80°C as dry pellets. Total RNA was extracted from cell pellets (0.35–1 × 106 cells) using the single-step guanidinium thiocyanate-phenol-chloroform method (RNAzol B; Biotecx Labs, Houston, TX). Total RNA (100 ng per sample) was reverse transcribed using Moloney murine leukaemia virus reverse transcriptase (Perkin Elmer, Norwalk, CT) and oligo dT priming according to the manufacturer's instructions, at 42°C for 30 min. Amplification with specific primers was performed in a Gene Amp PCR System 9600 thermocycler (Perkin Elmer) for 35 cycles with a 15-s/95°C denaturation and 30-s/60°C annealing–extension profile. The parallel amplification of cDNA for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed to check the cDNA quality prior to use in any further study. Sequence specificity of the primers used for the amplification reactions was ascertained by a computer-assisted search of updated versions of GenBank (the expected product size and GenBank accession number are given in parentheses); forward and reverse primers were always from different exons so that possible signals from genomic DNA contamination could be distinguished from cDNA: IL-11 sense, 5′-CTG GTC GTG CTG AGC CTG TGG-3′, IL-11 anti-sense, 5′-GGG TCT TCA GGG AAG AGC CAC-3′ (340-bp product) (M81890); LIF sense, 5′-GTC AAC GCC ACC TGT GCC ATA-3′, LIF anti-sense, 5′-GGT GTC AGG GCC GTA GGT CAC-3′ (427-bp product) (M63420); OSM sense, 5′-AGA GTA CCG CGT GCT CCT T-3′, OSM anti-sense, 5′-AGC TTG CGC TGA AAA GCA T-3′ (468-bp product) (M26966); IL-6 sense, 5′-ATG AAC TCC TTC TCC ACA AGC GC-3′, IL-6 anti-sense, 5′-GAA GAG CCC TCA GGC TGG ACT G-3′ (628-bp product) (Y00081); GAPDH sense, 5′-CTG CCG TCT AGA AAA ACC-3′, GAPDH anti-sense, 5′-CCA AAT TCG TTG TCA TAC C-3′ (219-bp product) (J04038). Amplified products were electrophoresed on a 2% agarose gel stained with ethidium bromide: the size of the product ensured that the amplification was specific for each primer pair, as shown on the gel relative to a known size standard (123 bp DNA ladder; Life Technologies Ltd). To assess further the specificity of reverse-transcriptase polymerase chain reaction (RT-PCR) reactions, positive and negative controls were performed for each cytokine, using RNA from cells known either to express or not to express the cytokine tested. In particular, as a positive control for IL-6, LIF and OSM, RNA was used from the phorbol myristate acetate (PMA)-activated monocytic U937 cell line (American Type Culture Collection, Rockville, MD); for IL-11 we adopted RNA from a human skin fibroblastic cell line (Human and animal cell line repository; Dr R. Musanti, Laboratorio di Colture Cellulari, Milan, Italy) (data not shown). As a negative control in each experiment, RNA obtained from the rat thyroid cell line FRTL5 was used [38].
Cytokine immunolocalization on trabecular bone biopsy
Trabecular bone biopsies obtained from the inner portion of the femoral head of OA, RA and PT patients were immediately fixed in a freshly prepared 9:1 mixture of B5 solution (mercuric chloride-containing fixative)/40% formaldehyde at room temperature for 2 h. Biopsies were then passed in ethanol 70°C at room temperature for 30 min, decalcified at room temperature for 3 h and stored in ethanol at 70°C until dehydrated and embedded in paraffin. Embedded-samples were cut and then deparaffinized and rehydrated. Slides were preincubated with PBS containing 0.25% bovine serum albumin (BSA), 0.1% NaN3, 0.1% saponin and 5% normal rabbit serum (Dako, Glostrup, Denmark) at room temperature for 30 min, and then incubated with polyclonal anti-human IL-6 (R&D), anti-human LIF (R&D), or anti-human IL-11 (R&D) diluted in TBS containing 0.25% BSA, 0.1% NaN3, 0.1% saponin and 1.5% normal rabbit serum, at 4°C overnight. Slides were washed twice with TBS 0.04 m pH 7.6 containing 0.1% saponin and then sequentially incubated with rabbit anti-goat-biotinylated (Pierce) 1:100 and streptavidin–AP-conjugated (Boehringer Mannheim) 1:200 at room temperature for 1 h each. Developments were performed using new fuchsin kit (Dako). To assess OSM expression, slides were preincubated with both methanol:H2O2 (97:3) and PBS containing 3% BSA at room temperature for 30 min, respectively. Samples were then incubated with monoclonal anti human OSM (R&D) diluted 1:20 with PBS containing 0.25% BSA, 0.1% NaN3 at room temperature for 60 min. Slides were washed twice with TBS 0.04 m pH 7.6 and then sequentially incubated with goat anti-mouse-biotinylated and streptavidin–horseradish peroxidase-conjugated (both BioGenex, San Ramon, CA) at room temperature for 30 min, respectively. A peroxidase reaction using diaminobenzidine (Sigma) as substrate was performed. Negative staining control experiments were performed either omitting the primary antibody (for all the antibodies tested) or using a control isotype-matched antibody (for OSM). Specificity controls were performed using irrelevant polyclonal or monoclonal antibodies.
The slides were randomized and scored by two observers (G.L., A.F.) who were blinded to patient samples and clinical data. The mean percentage of positive mesenchymal (i.e. OB, BMSC, pericytes, endothelial cells) and mononuclear cells in 10 different fields was collected for each sample.
Statistical analysis
anova Kruscal–Wallis and Mann–Whitney tests were used to compare cytokine production by OB and BMSC from RA, OA and PT patients and to evaluate differences in OB and BMSC populations among OA, RA and PT. Wilcoxon's paired test was used to analyse the differences between cytokine basal production versus TNF-α- and/or IL-1β-activated production.
RESULTS
IL-6-type cytokine production
IL-11
As shown in Table 1, OB and BMSC from all groups tested produced IL-11 constitutively. In particular, the basal IL-11 production of the BMSC was higher than that of the OB in each ofthe three different groups of patients (OA, P < 0.0004; RA, P < 0.0047; PT, P < 0.0003; Table 1). Although both IL-1β and TNF-α up-regulated IL-11 production, IL-1β was a stronger inducer. We compared IL-11 production between activated OB and BMSC: in RA patients both IL-1β- and TNF-α-activated BMSC produced significantly higher levels of IL-11 than the correspondingly activated OB (P < 0.0047, Table 1); in PT patients the TNF-α-activated BMSC produced significantly higher amounts of IL-11 than the TNF-α-activated OB (P < 0.0003, Table 1). We also compared IL-11 production among BMSC isolated from OA, RA and PT patients: IL-1β-activated BMSC from RA patients produced significantly higher levels of IL-11 than those from PT patients (P < 0.009, Table 1). Finally, we compared IL-11 production among OB isolated from OA, RA and PT patients: unstimulated OB from OA patients produced significantly higher amounts of IL-11 than those from PT patients (P < 0.004, Table 1).
Table 1.
Cytokine production in vitro (pg/ml) of bone marrow stromal cells (BMSC) and osteoblasts (OB) isolated from RA, OA and post-traumatic (PT) patients, evaluated in basal conditions and after activation with IL-1β and tumour necrosis factor-alpha (TNF-α)
LIF
In all groups of patients analysed, BMSC but not OB constitutively released detectable levels of LIF in the culture supernatant. As with IL-11, IL-1β induced a higher LIF production than TNF-α, both in BMSC and OB (Table 1). Significantly higher amounts of LIF were produced by TNF-α-activated OB from RA patients tan by those from PT patients (P < 0.012, Table 1).
OSM
OB and BMSC did not release detectable amounts of OSM either in basal conditions or after activation with IL-1β or TNF-α.
IL-6
OB and BMSC constitutively produced IL-6 in all three groups of patients. As shown in Table 1, IL-1β always stimulated IL-6 production more significantly than TNF-α (Wilcoxon test, P < 0.05). Depending on the pathology, significant differences in IL-6 up-regulation were observed between OB and BMSC: in OA patients, following IL-1β activation OB released significantly more IL-6 than BMSC (P < 0.014, anova Kruscal–Wallis tests); in PT patients, after TNF-α activation BMSC produced significantly more IL-6 than OB (P < 0.006, anova Kruscal–Wallis tests).
Interestingly, when we studied OB and/or BMSC with respect to each individual patient, we found the same trends of cytokine production as in the mean data of each group of patients analysed. Figure 2 shows IL-6, IL-11 and LIF production in basal and activated conditions by BMSC and OB obtained from OA, RA and PT (one patient each).
Fig. 2.
IL-11, leukaemia inhibitory factor (LIF) and IL-6 production by bone marrow stromal cells (BMSC) and osteoblasts (OB) isolated from the same OA, RA and post-traumatic (PT) patients in basal conditions (□) and after IL-1β (▪) and tumour necrosis factor-alpha (TNF-α) (hatched) activation as described in Patients and Methods.
Cytokine gene expression
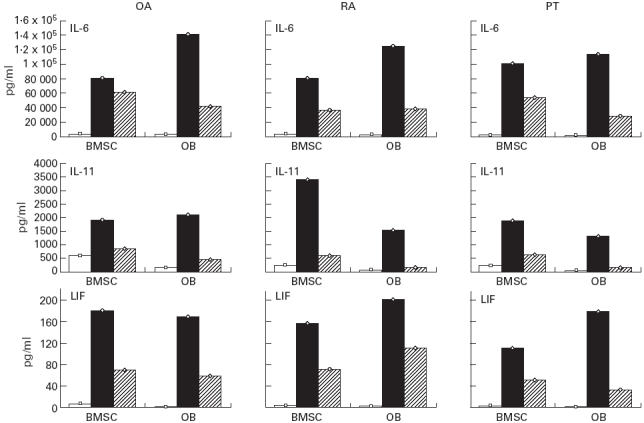
Using RT-PCR, we examined cytokine mRNAs from OB and BMSC of OA, RA and PT in order to determine whether our findings on protein production could be confirmed at the RNA level. Figure 3 shows the representative results from unstimulated BMSC and OB of one OA, one RA and one PT patient: RT-PCR demonstrated a constitutive mRNA expression for all the cytokines tested, including OSM, in all three groups analysed. The synthesis of OSM, which could not be detected as a protein, may therefore be controlled, at least in part, at the post-transcriptional level.
Fig. 3.
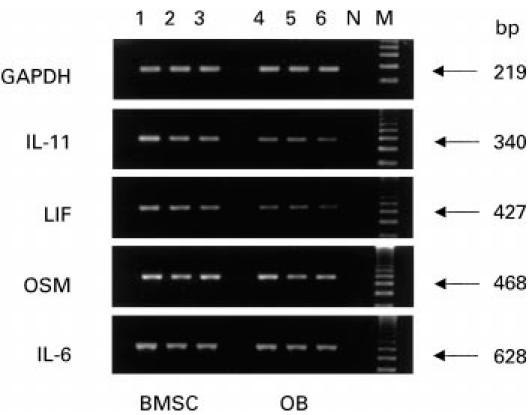
mRNA expression of IL-11, leukaemia inhibitory factor (LIF), oncostatin M (OSM) and IL-6 in unstimulated bone marrow stromal cells (BMSC) and osteoblasts (OB) isolated from one OA, one RA and one post-traumatic (PT) patient, as described in Patients and Methods. Lanes 1, 4, BMSC and OB from an OA patient; lanes 2, 5, BMSC and OB from an RA patient; lanes 3, 6, BMSC and OB from a PT patient; N, negative control (FRTL5 cell line); M, molecular weight marker (123-bp ladder). GAPDH was used as a quality control.
Cytokine immunolocalization on trabecular bone biopsy
Cytokine immunostaining on trabecular bone biopsies confirmed the data obtained on cultured cells. As shown in Fig. 4, IL-11, IL-6 and LIF were positive both in mesenchymal cells (i.e OB, BMSC, pericytes and endothelial cells), located mainly along trabeculae and vessels, and in mononuclear cells, localized in the bone marrow. Interestingly, OA biopsies showed a higher number of positive mesenchymal and mononuclear cells for IL-11 than RA and PT (P < 0.003, anova Kruscall–Wallis tests). OSM was expressed only by mononuclear cells.
Fig. 4.
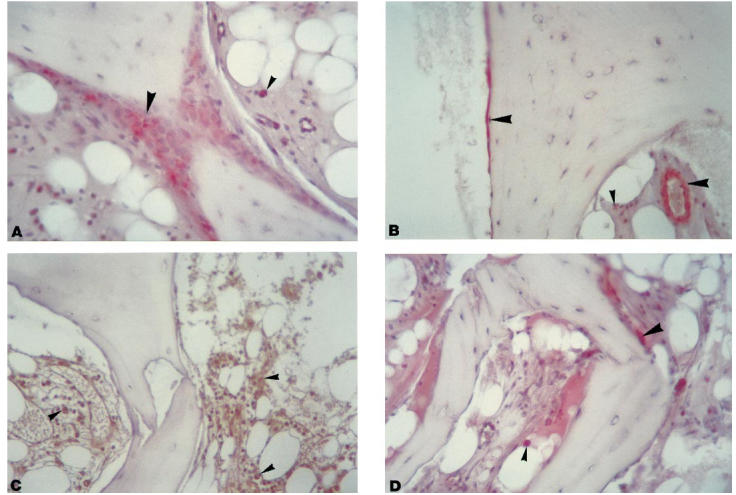
IL-11 (A), leukaemia inhibitory factor (LIF) (B), oncostatin M (OSM) (C) and IL-6 (D) immunostaining of an OA bone biopsy representative of all groups of patients analysed as described in Patients and Methods. (Mag. × 250.) IL-11, LIF and IL-6 were developed using new fucsin (red positive cells); peroxidase reaction (brown positive cells) was used for OSM. Mesenchymal and mononuclear cells are indicated by long arrowheads and short arrowheads, respectively.
DISCUSSION
In the evolution of RA and OA, the destruction or increased stiffness of the bone is influenced by enhanced osteoclastic activity, joint loading, the presence of healing microfractures, inflammatory cells, cytokines and enzymes. In the present study we analysed and compared the expression of IL-6-type cytokines in the bone compartment of RA, OA and PT patients. The study was performed both on bone biopsies and on isolated OB and BMSC.
Our study demonstrates for the first time a constitutive production of IL-11 in the bone compartment of OA, RA and PT patients. In all three groups BMSC constitutively produced significantly higher levels of IL-11 than OB. This indicates that IL-11 production by committed cells (i.e. OB) can be modulated on the basis of the specific functions (e.g. maintaining bone homeostasis) they acquire in the target tissue. Moreover, unstimulated OB from OA patients released significantly higher amounts of IL-11 than those from PT patients. This finding, along with the observation that IL-11 was most highly expressed in the biopsies of OA patients, suggests that this cytokine may play a specific role in OA. This could be related to IL-11's regulatory action on collagen metabolism [25], which is known to be increased within OA femoral heads [6]. In the synovial and serum compartments, some authors have reported a higher basal production of IL-11 in RA patients [39]. Our data on the bone indicate a higher basal production in OA patients. This finding confirms the importance of evaluating the bone compartment, as well as the synovia and cartilage, since bone cells could contribute to modulation of cartilage homeostasis, to pannus formation, and to destruction or remodelling of bone tissue. Finally, the observation that, when stimulated with IL-1β or TNF-α, BMSC from RA and PT patients produce significantly more IL-11 than OB provides a further demonstration that committed cells respond differently to proinflammatory cytokines.
As regards LIF, we found that detectable (albeit small) levels of this cytokine were constitutively released only by BMSC. LIF stimulates bone resorption and increases the number of osteoclasts in a prostaglandin-dependent manner [28], but also exerts anabolic effects on bone by promoting both DNA and protein synthesis [29,30]. These two different actions seem to be due to the biphasic dose–response of LIF, which has already been demonstrated in vitro, with bone resorption dominating at high concentrations and bone formation at lower doses [29]. Our finding that the proinflammatory cytokines IL-1β and TNF-α significantly induce LIF production by OB and BMSC, as was observed both in the culture supernatants and in the bone biopsies (Fig. 4), suggests that LIF may play an important role in the mechanisms of bone remodelling.
OSM was expressed at the mRNA level by both OB and BMSC, but was not produced as a protein by these cells. Nevertheless, we detected OSM production by mononuclear cells in bone biopsies (Fig. 4). It is known that OSM activates OB and inhibits bone resorption [32], promotes collagen fragment release when combined with proinflammatory cytokines [40] and stimulates collagenase-3 expression in OB [41]. Our finding suggests that OSM may play an important role not only in bone remodelling but also in the modulation of important factors (e.g. chemokines and metalloproteinases) responsible for the inflammatory processes in RA patients and for the degrading of type I collagen in OA patients.
As to IL-6, our data confirm that this cytokine is constitutively produced by both OB and BMSC [19,42]. IL-1β was more efficient in stimulating IL-6 production than TNF-α, as has already been reported for isolated chondrocytes and synovial cells [43,44]. This cytokine is involved in the regulation of inflammatory responses, and production of IL-6 allows OB and BMSC to communicate with other cells during their homeostatic functions and in response to injury. The role of IL-6 in bone may not be primarily autocrine, since human OB show IL-6 receptors but do not respond to IL-6 [45]. These considerations suggest that IL-6 may show a more classic endocrine involvement (as an inducer of the acute-phase response) than the other IL-6-type cytokines investigated by us.
In conclusion, the present data confirm that OB and BMSC isolated from OA and RA patients produce cytokines that are involved both in the local regulation of bone and as autocrine/paracrine regulators of OB and BMSC function and differentiation. These findings confirm the importance of studying different compartments in OA and RA in order to obtain a more complete picture of these pathologies.
Acknowledgments
We thank Mrs Patrizia Rappini and Mrs Graziella Salmi for editorial assistance, Mr Luciano Pizzi and Mr Aurelio Valmori for technical assistance, and Mr Robin M. T. Cooke for helping to revise the manuscript. This work was supported by grants from Istituti Ortopedici Rizzoli, Bologna, Italy and MURST, Università degli Studi di Bologna, Italy.
REFERENCES
- 1.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of progression of joint space narrowing in osteoarthritis of the knee. Annu Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwata Y, Mort JS, Tateishi H, Lee ER. Macrophage cathepsin L, a factor in the erosion of subchondral bone in rheumatoid arthritis. Arthritis Rheum. 1997;40:499–509. doi: 10.1002/art.1780400316. [DOI] [PubMed] [Google Scholar]
- 3.Sharif M, George E, Dieppe PA. Correlation between synovial fluid markers of cartilage and bone turnover and scintigraphic scan abnormalities in osteoarthritis of the knee. Arthritis Rheum. 1995;38:78–81. doi: 10.1002/art.1780380112. [DOI] [PubMed] [Google Scholar]
- 4.Westacott CI, Webb GR, Warnock MG, Sims JV, Elson CJ. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum. 1997;40:1282–91. doi: 10.1002/1529-0131(199707)40:7<1282::AID-ART13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Hilal G, Martel-Pelletier J, Pelletier JP, Ranger P, Lajeunesse D. Osteoblastic-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro. Arthritis Rheum. 1998;41:891–9. doi: 10.1002/1529-0131(199805)41:5<891::AID-ART17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Mansell JP, Bailey AJ. Abnormal cancellous bone collagen metabolism in osteoarthritis. J Clin Invest. 1998;101:1596–603. doi: 10.1172/JCI867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ. Osteogenic stem cells in the bone marrow. In: Heersche JNM, Kanis JA, editors. Bone and mineral research/7. Amsterdam: Eslevier; 1990. pp. 243–71. [Google Scholar]
- 9.Oyajobi BO, Russell RGG. Bone remodelling, cytokines, and joint diesease. In: Kuettner K, editor. Articular cartilage and osteoarthritis. New York: Raven Press; 1992. pp. 333–48. [Google Scholar]
- 10.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodelling. Emerging insights into the pathophysiology of osteoporosis. N Eng J Med. 1995;332:305–11. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 11.Taga T, Kishimoto T. gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 12.Bellido T, Stahl N, Farruggella TJ, Borba V, Yancopoulos GD, Manolagas SC. Detection of receptors for interleukin-6, interleukin-11, leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor in bone marrow stromal/osteoblastic cells. J Clin Invest. 1996;97:431–7. doi: 10.1172/JCI118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manolagas SC. The role of IL-6 type cytokines and their receptors in bone. Ann N Y Acad Sci. 1998;840:194–204. doi: 10.1111/j.1749-6632.1998.tb09563.x. [DOI] [PubMed] [Google Scholar]
- 14.Badolato F, Oppenheim JJ. Role of cytokines, acute-phase proteins, and chemokines in the progression of rheumatoid arthritis. Semin Arthritis Rheum. 1996;26:526–38. doi: 10.1016/s0049-0172(96)80041-2. [DOI] [PubMed] [Google Scholar]
- 15.Westacott CI, Sharif M. Cytokines in osteoarthritis: mediators or markers of joint destruction? Semin Arthritis Rheum. 1996;25:254–72. doi: 10.1016/s0049-0172(96)80036-9. [DOI] [PubMed] [Google Scholar]
- 16.Taguchi Y, Yamamoto M, Yamate T, et al. Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc Assoc Am Physicians. 1998;110:559–74. [PubMed] [Google Scholar]
- 17.Gowen M. Cytokines and cellular interactions in the control of bone remodelling. In: Heersche JNM, Kanis JA, editors. Bone and mineral research/8. A regular survey of developments in the field of bone and mineral metabolism. Amsterdam: Elsevier; 1994. pp. 77–114. [Google Scholar]
- 18.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–84. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 19.Rifas L, Kenney JS, Marcelli M, et al. Production of interleukin-6 in human osteoblasts and human bone marrow stromal cells: evidence that induction by interleukin-1 and tumor necrosis factor-α is not regulated by ovarian steroids. Endocrinology. 1995;136:4056–67. doi: 10.1210/endo.136.9.7649114. [DOI] [PubMed] [Google Scholar]
- 20.Barton BE, Mayer R. IL-3 and IL-6 do not induce bone resorption in vitro. Cytokine. 1990;2:217–20. doi: 10.1016/1043-4666(90)90019-p. [DOI] [PubMed] [Google Scholar]
- 21.Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–303. [PubMed] [Google Scholar]
- 22.Ohshima S, Saeki Y, Mima T, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–6. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trepicchio L, Wang L, Bozza M, Dorner AJ. IL-11 regulates macrophage effector function through the inhibition of nuclear factor-κB. J Immunol. 1997;159:5661–70. [PubMed] [Google Scholar]
- 24.Girasole G, Passeri G, Jilka RL, Manolagas SC. Interleukin-11: a new cytokine critical for osteoclast development. J Clin Invest. 1994;93:1516–24. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill PA, Tumber A, Papaioannou S, Meikle MC. The cellular actions of interleukin-11 on bone resorption in vitro. Endocrinology. 1998;139:1564–72. doi: 10.1210/endo.139.4.5946. [DOI] [PubMed] [Google Scholar]
- 26.Taupin JL, Pitard V, Dechanet J, Miossec V, Gualde N, Moreau JF. Leukemia inhibitory factor: part of a large ingathering family. Int Rev Immunol. 1998;16:397–426. doi: 10.3109/08830189809043003. [DOI] [PubMed] [Google Scholar]
- 27.Cornish J, Callon K, King A, Edgar S, Reid IR. The effect of leukemia inhibitory factor on bone in vivo. Endocrinology. 1993;132:1359–66. doi: 10.1210/endo.132.3.8440191. [DOI] [PubMed] [Google Scholar]
- 28.Reid IR, Lowe C, Cornish J, et al. Leukemia inhibitory factor: a novel bone-active cytokine. Endocrinology. 1990;126:1416–20. doi: 10.1210/endo-126-3-1416. [DOI] [PubMed] [Google Scholar]
- 29.Cornish J, Callon KE, Edgar SG, Reid IR. Leukemia inhibitory factor is mitogenic to osteoblasts. Bone. 1997;21:243–7. doi: 10.1016/s8756-3282(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 30.Bruce AG, Linsley PS, Rose TM, Oncostatin M. Prog Growth Factor Res. 1992;4:157–70. doi: 10.1016/0955-2235(92)90029-h. [DOI] [PubMed] [Google Scholar]
- 31.Brown TJ, Liu J, Brashem-Stein C, Shoyab M. Regulation of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor expression by oncostatin M. Blood. 1993;82:33–7. [PubMed] [Google Scholar]
- 32.Jay PR, Centrella M, Lorenzo J, Bruce AG, Horowitz MC. Oncostatin-M: a new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology. 1996;137:1151–8. doi: 10.1210/endo.137.4.8625883. [DOI] [PubMed] [Google Scholar]
- 33.Altman R, Asch E, Bloch D. Development of criteria for the classification of osteoarthritis of the knee. Arthritis Rheum. 1986;20:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 34.Arnett FC, Edworth SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 35.Gehron Robey P, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985;37:453–60. [PubMed] [Google Scholar]
- 36.Lisignoli G, Toneguzzi S, Pozzi C, et al. Proinflammatory cytokines and chemokine production and expression by human osteoblasts isolated from patients with rheumatoid arthritis and osteoarthritis. J Rheumatol. 1999;26:791–9. [PubMed] [Google Scholar]
- 37.Lisignoli G, Toneguzzi S, Pozzi C, et al. Chemokine expression by subchondral bone marrow stromal cells isolated from osteoarthritis and rheumatoid arthritis patients. Clin Exp Immunol. 1999;116:371–8. doi: 10.1046/j.1365-2249.1999.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambesi-Impiombato FS, Parks LA, Coon HG. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci USA. 1980;77:3455–9. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- 40.Cawston TE, Curry VA, Summers CA, et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998;41:1760–71. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Varghese S, Kyung Y, Canalis E. Leukemia inhibitory factor and oncostatin M stimulate collagenase-3 expression in osteoblasts. Am J Physiol. 1999;276:E465–E471. doi: 10.1152/ajpendo.1999.276.3.E465. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhary LR, Spelsberg TC, Riggs BL. Production of various cytokines by normal human osteoblast-like cells in response to interleukin-1β and tumor necrosis factor-α: lack of regulation by 17β-estradiol. Endocrinology. 1992;130:2528–34. doi: 10.1210/endo.130.5.1572280. [DOI] [PubMed] [Google Scholar]
- 43.Guerne P-A, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83:585–92. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerne P-A, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990;144:499–505. [PubMed] [Google Scholar]
- 45.Littlewood AJ, Aarden LA, Evans DB, Russell RGG, Gowen M. Human osteoblastlike cells do not respond to interleukin-6. J Bone Miner Res. 1991;6:141–8. doi: 10.1002/jbmr.5650060207. [DOI] [PubMed] [Google Scholar]