Abstract
CSF-1 and GM-CSF have been implicated in the pathogenesis of rheumatoid arthritis. We report the effects of CSF-1 and GM-CSF in the development of an acute methylated bovine serum albumin (mBSA)-induced murine arthritis model. Examination of histopathological features revealed that the systemic administration of CSF-1 or GM-CSF following mBSA administration into the knee resulted in the exacerbation of arthritis. This included synovial hyperplasia and joint inflammation, most evident at 7 and 14 days post-mBSA administration, and the appearance of erosive pannus tissue. The exacerbation by CSF-1 and GM-CSF was not sustained but declined in incidence and severity by 21 days post-mBSA administration, similar to the effects of IL-1β in this model, reported here and previously. Macrophages expressing Mac-2 and F4/80 were a prominent feature of the pathology observed, particularly the infiltration of Mac-2+ macrophages seen in all mice administered CSF-1, GM-CSF or IL-1β. Present in inflamed knees was a locally dividing population of cells which included Mac-2+ and F4/80+ macrophages. These studies demonstrate that CSF-1 and GM-CSF can exacerbate and prolong the histopathology of acute inflammatory arthritis and lend support to monocytes/macrophages being a driving influence in the pathogenesis of inflammatory arthritis.
Keywords: rheumatoid arthritis, inflammatory mediators, cytokines, in vivo animal models, macrophages
INTRODUCTION
The colony-stimulating factors (CSFs) are a family of polypeptide growth factors critical to the development of haematopoietic cells, including those of the monocyte/macrophage lineage [1]. There is mounting evidence that CSF-1 and granulocyte-macrophage (GM)-CSF may play a role in the pathogenesis of rheumatoid arthritis (RA). For example, CSF-1 and GM-CSF have been detected in the synovial fluids of RA patients [2,3], while GM-CSF therapy to patients with Felty's syndrome can result in a flare-up of RA [4]. Data both from in vitro studies [5] and work with animal models [6–8] also support a role for CSFs in the pathogenesis of RA.
In RA lesions, macrophages have been identified as pivotal cells [9] as they are known to be a major source of growth factors and mediators of tissue destruction such as IL-1, tumour necrosis factor-alpha (TNF-α) and the CSFs [10], as well as chemokines such as macrophage inflammatory protein-1α (MIP-1α) and IL-8 [11]. Indeed, most cytokines detected in RA joints are macrophage-derived [12]. It has been postulated that a local network of CSFs [13] may exist which serves to orchestrate the complex cellular interactions and resulting tissue damage in arthritic lesions.
Animal models of arthritis have been used extensively to study the importance of various components of the immune system, including inflammatory mediators, in the pathogenesis of RA [14]. In a rapid onset monoarticular arthritis model with histopathological features similar to that seen in RA, Staite et al. [15] reported that subcutaneous injection of IL-1 exacerbated the arthritis in knees of mice injected intra-articularly with methylated bovine serum albumin (mBSA). A striking feature of this mBSA-induced arthritis model is the rapid synovial hyperplasia, making it particularly suitable for studies into this aspect of RA. For the study of experimental arthritis, there are some distinct advantages in employing the mBSA model over other traditional models such as collagen-induced arthritis, most notable being its acute onset and restriction to a defined joint.
The aim of the present study was to assess the effect of exogenous administration of CSF-1 and GM-CSF in the development of this acute mBSA-induced murine model of arthritis. We found that CSF-1 and GM-CSF both resulted in rapid exacerbation of the pathological features of mBSA-induced arthritis, but that these effects were acute and subsided within 21 days. The results of this study lend further support to the concept that monocytes/macrophages and the colony-stimulating factors CSF-1 and GM-CSF may play an important role in inflammatory arthritides such as RA.
MATERIALS AND METHODS
Cytokines
The following cytokines were obtained as gifts: recombinant human CSF-1 (specific activity 7 × 107 U/mg; Chiron, Emeryville, CA); recombinant murine GM-CSF (specific activity 3 × 107 U/mg) and recombinant human IL-1β (specific activity 5 × 108U/mg), both from AMGEN Inc. (Thousand Oaks, CA). Endotoxin levels were routinely monitored by limulus lysate tests (CSL, Parkville, Australia) with the minimum detectable level being 0.01 ng/ml.
Mice and induction of arthritis
C57Bl/6 male 8–10-week-old mice were used for these studies. The procedure used for the induction of arthritis was based on that of Staite et al. [15]. Under anaesthesia, a single intra-articular (i.a.) injection of 6 μl (10–20 mg/ml) of mBSA (Sigma Chemical Co., St Louis, MO) was made into the right knee on day 0 of the experiment, followed by daily subcutaneous (s.c.) injections of 20 μl of either saline, CSF-1 (7.6 μg), GM-CSF (15 μg) or IL-1β (250 ng) into the ipsilateral footpad on days 0–2. Groups of mice were killed at 4, 7, 14 or 21 days following mBSA administration. Contralateral control knee joints received vehicle (saline).
Bromodeoxyuridine labelling in vivo
In some experiments mice were given an i.p. injection with 300 μl (8 mg/ml in saline) bromodeoxyuridine (BrdU; Sigma) 2 h before they were killed. For other experiments, BrdU (1.0 mg/ml) was added to the drinking water for a period of 24 h prior to sacrifice.
Histological assessment of arthritis
Knees were removed, fixed in 10% neutral-buffered formalin for 48 h, decalcified (solution containing 0.05 m CH3COOH, 0.5 m HCl, 10% (v/v) chloroform, 70% (v/v) ethanol) for 48 h and processed to paraffin. Tissue sections were cut at 4 μm onto aminoalkylsilane-coated slides (Sigma) and stained for routine histology with haematoxylin and eosin (H–E) or used for immunohistochemistry as described below. For the histological examination of tissue sections stained with H–E, five defined pathological features were graded from 0 (normal) to 3 (severe) in a blinded manner. Soft tissue inflammation, assessed in the infrapatellar fat pads, joint capsule and the area adjacent to the periosteal sheath, was graded according to the extent of cellular infiltration and angiogenesis. Synovitis (synovial hyperplasia) was defined as hyperplasia of the synovium but did not include pannus formation. Pannus was defined as hypertrophic synovial tissue forming a tight junction with the articular surface. Joint space exudate was identified as leucocytes scattered discretely or in aggregates in the joint space. Evaluation of cartilage and bone damage was based on loss of cartilage matrix, disruption and loss of cartilage surface, and the extent and depth of the subchondral bone erosion. Mice were classified as arthritic when inflammation (score ≥ 1) was observed in association with either synovitis (score ≥ 2) or bone or cartilage damage (score ≥ 1).
Immunohistochemical studies
The MoAbs M3/38 (anti-Mac-2) and F4/80 were obtained from the American Type Culture Collection (Rockville, MD). Hybridoma culture supernatants were used for immunohistochemical staining of joint tissue sections and examined using the alkaline phosphatase technique. Briefly, deparaffinized tissue sections were denatured in 2 m HCl for 30 min at 37°C, washed and treated with trypsin (1 mg/ml) for 20 min at 37°C prior to immunostaining. Sections were incubated with anti-Mac-2 or anti-F4/80 for 60 min at room temperature, followed by washing in PBS and incubation with a biotinylated rabbit anti-rat immunoglobulin (Dakopatts, Glostrup, Denmark) for 60 min at room temperature. Slides were again washed before incubation with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (Dakopatts) for 60 min at room temperature. Immunoreactive cells were visualized using the 5-bromo-4-chloro-indolyl phosphate/nitroblue tetrazolium substrate detection kit (BCIP/NBT; Zymed Labs, San Francisco, CA).
For the visualization of BrdU-labelled cells, joint tissue sections were treated as described above before incubation with a mouse anti-BrdU MoAb (Boehringer, Mannheim, Germany) overnight at 4°C. Tissue sections were then washed thoroughly, incubated with peroxidase-conjugated sheep anti-mouse IgG (Serotec, Oxford, UK) for 60 min at room temperature, washed and reacted with diaminobenzidine (DAB; Sigma) as the chromogen. In some cases staining for Mac-2 and BrdU in tissue sections was performed simultaneously. A number of additional control slides (e.g. one of the primary antibodies omitted/replaced with irrelevant antibody) were also included.
Following a 24-h in vivo BrdU label, cells immunoreactive for BrdU were enumerated in the synovial lining of joint sections by examining five high power fields (using a × 100 oil immersion objective) in two areas of each tissue section (> 300 cells per sample). All nucleated cells were enumerated and the percentage of BrdU+ cells (labelling index) was determined.
Statistical analysis
Results were analysed using appropriate statistical tests as indicated. Values are expressed as means ± s.e.m. For each test P < 0.05 was considered statistically significant.
RESULTS
Enhancement of mBSA-induced arthritis following administration of CSF-1, GM-CSF or IL-1β
Knee joints were collected at different times following i.a. mBSA administration with or without s.c. injections of cytokines, and the severity of arthritis assessed histologically (see Materials and Methods). Arthritis incidence reached a maximum at day 7 after mBSA administration, with 80–100% of mice administered CSF-1, GM-CSF or IL-1β classified arthritic, compared with saline controls where no mice were deemed to be arthritic (Table 1). The severity of arthritis was also maximal at day 7, with the mean total arthritic score for each group given CSF-1, GM-CSF or IL-1β significantly increased above that for the mBSA/saline controls (Table 1). Subsequently, a gradual decline in the incidence and severity of arthritis was observed over days 14–21 for all mice (data not shown). Contralateral control joints injected with saline in lieu of mBSA appeared normal for all groups.
Table 1.
Incidence and histological assessment of arthritis 7 days following i.a. administration of methylated bovine serum albumin (mBSA) and s.c. injection of cytokine
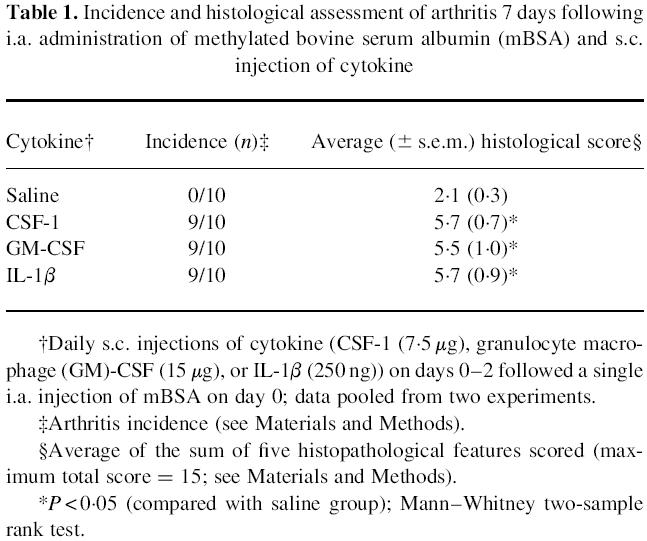
The prominent pathological feature which most clearly reflected severity of arthritis was the degree of synovitis (see Fig. 1). Synovitis was detected in all groups at day 4 following mBSA administration, but there was significant (P < 0.05) elevation in the level of synovitis on day 7 in mice that received CSF-1, GM-CSF or IL-1β compared with the mBSA/saline control group (Fig. 1). By day 14 there was still increased synovitis in mice that received IL-1β (Fig. 1).
Fig. 1.
Histopathological scores for synovial hyperplasia in methylated bovine serum albumin (mBSA)-injected knees. Mice were given an i.a. injection of mBSA on day 0 of the experiment together with three daily s.c. injections (days 0–2) of either saline (control), CSF-1 (7.5 μg), granulocyte macrophage (GM)-CSF (15 μg) or IL-1β (250 ng). The severity of synovial hyperplasia was scored on a scale of 0 (normal) to 3 (severe) (see Materials and Methods) following sacrifice on day 4, 7, 14 or 21 of the experiment. Values are presented as mean scores (± s.e.m.) obtained from groups of 10 mice. *P < 0.05 compared with the mBSA/saline control group; Mann–Whitney two-sample rank test.
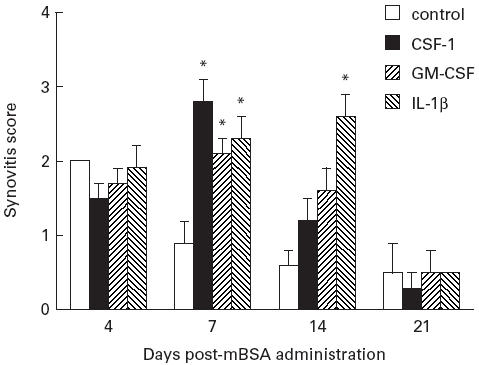
At day 7, in non-arthritic knees where mice received mBSA/saline there was little evidence of joint inflammation and damage (Fig. 2A). However in arthritic joints at day 7, inflammatory cells, predominantly macrophage-like cells and neutrophils, were seen in the synovium (synovial lining and stroma) and joint space (Fig. 2B,C), while monocytes and lymphocytes were also commonly seen. Rather striking was the appearance and ‘swarming’ of inflammatory cells around some of the blood vessels of the inflamed synovium, particularly the large vessels (Fig. 2D). In some arthritic knees the hyperplastic and inflamed synovium, comprising macrophages, fibroblasts and neutrophils, was seen to extend over the articular cartilage surface and occasionally this pannus tissue was observed to ‘tunnel’ into focal regions of cartilage (Fig. 2D). Several mice treated with CSF-1 or GM-CSF also showed evidence of cartilage and bone damage in the mBSA-injected knees. Overall, severity of the histopathological features observed in mice administered CSF-1 or GM-CSF was comparable to IL-1β (Table 1).
Fig. 2.
Histopathological manifestations of acute arthritis 7 days after administration of methylated bovine serum albumin (mBSA) and either CSF-1, granulocyte macrophage (GM)-CSF or IL-1β. (A) Relatively normal (non-arthritic) joint (frontal knee section) from mouse administered mBSA/saline showing normal joint architecture and no evidence of inflammation. (B) Representative frontal knee joint section from an mBSA/GM-CSF-injected mouse showing a thickened, inflamed synovium (arrows) and inflammatory cells (arrowheads) in the joint space. (C) Higher magnification of area in (B) showing detail of the inflammatory exudate, typically comprising monocytes/macrophages and neutrophils, some seen in contact with the articular cartilage and synovium. (D) Inflammatory cells localized around some of the larger blood vessels (arrows) of the inflamed synovium (in this instance taken from a mouse injected with mBSA/CSF-1). Note also invasive pannus tissue seen adjacent to the articular cartilage (arrowhead). J, Joint space; AC, articular cartilage. (H–E, original mag. × 200 (A), × 100 (B,D) and × 400 (C).)
Distribution of macrophages in arthritic and non-arthritic joints
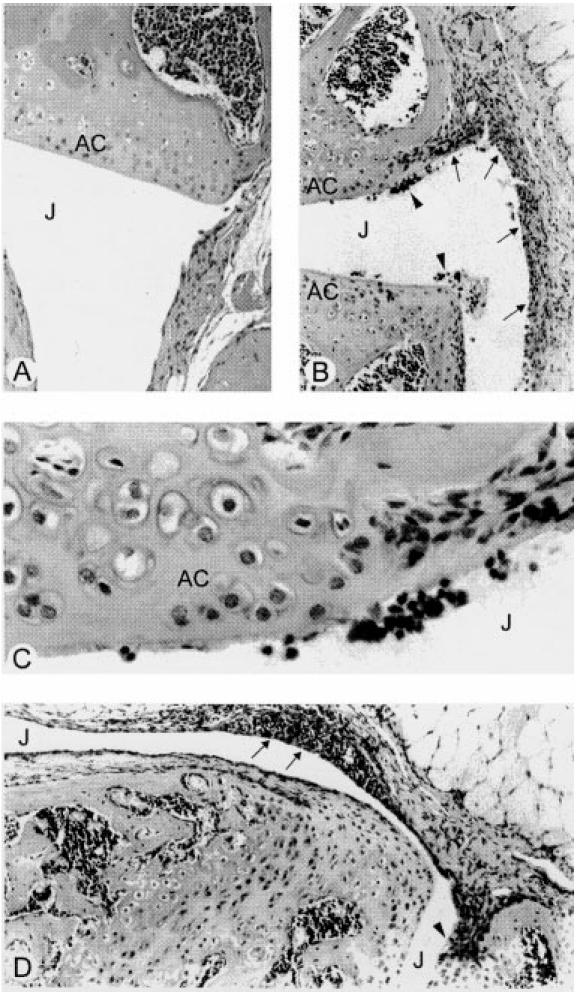
To confirm and characterize the presence and distribution of macrophages in arthritic joints, tissue sections from non-arthritic and arthritic knees at 7 days after mBSA injection, were stained using MoAbs against F4/80 (expressed by mature macrophages) and Mac-2 (expression on a subpopulation of macrophages associated with inflammation [16]). In normal knees, macrophage-like cells immunoreactive for F4/80 were scattered within the synovial lining layer (Fig. 3A). There were few cells stained with the anti-Mac-2 antibody in normal joints; staining was restricted to the chondrocytes in cartilage and small clusters of mononuclear cells in the bone marrow (Fig. 3B). However, in arthritic joints macrophages staining positive for F4/80 (Fig. 3C) or Mac-2 (Fig. 3D) were more prominent in the synovial lining, with cells intensely immunoreactive for Mac-2 localized around blood vessels in particular (Fig. 3E). A similar distribution and intensity of staining for F4/80 and Mac-2 were found in the inflamed knees of CSF-1-, GM-CSF- and IL-1β-treated mice.
Fig. 3.
Immunohistochemical detection of macrophages in inflamed joints. (A,B) In normal joints, cells immunoreactive for F4/80 (A) were seen scattered in the synovial tissue, while staining for Mac-2 (B) was sparse in the synovium and localized mainly in the bone marrow. (C–E) In inflamed joints, synovial hyperplasia (arrows) was associated with an increase in immunoreactivity for F4/80 (C) and Mac-2 (D) antigens. Mac-2+ cells were sometimes seen as accumulations around blood vessels (V) in the adjacent synovial stroma (E). J, Joint space; AC, articular cartilage. (Sections lightly counterstained with haematoxylin, original mag. × 100 (A–D) and × 400 (E).)
Local cell proliferation in arthritic joints
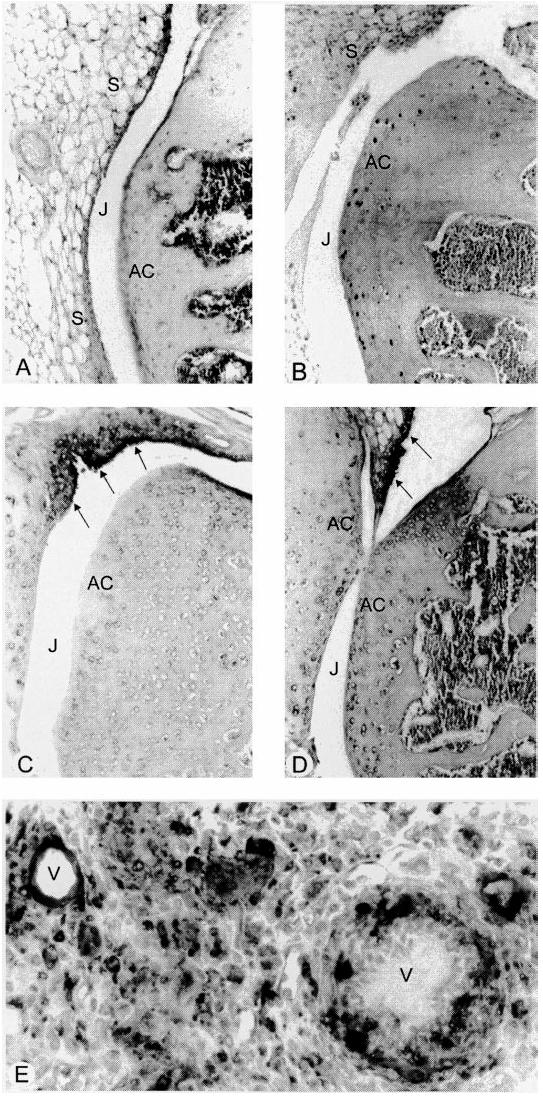
To assess the degree of local cell proliferation in the arthritic joints of mice that received mBSA ± cytokine, immunohistochemical analyses were performed on joint tissues collected following a 2-h BrdU pulse in vivo. In the non-arthritic joints of mBSA/saline-injected mice, only the occasional BrdU-labelled cell was seen in the synovial lining layer and the underlining stroma (not shown)—immunoreactive cells were more commonly observed throughout the bone marrow stroma. There was increased staining for BrdU in inflamed joints of mice injected intra-articularly with mBSA and subcutaneously with CSF-1, GM-CSF or IL-1β. BrdU+ cells were seen in the hyperplastic synovium and stroma (Fig. 4A) and occasionally in the joint space, and most of these cells resembled monocytes/macrophages or fibroblasts. The occasional endothelial cell or blood vessels in the synovial stroma stained positive for BrdU, while at focal sites of bone damage some of the cells seen invading bone and bone marrow were also BrdU+. Two-colour immunohistochemistry using anti-BrdU with anti-Mac-2 revealed a small subpopulation of proliferating (BrdU+) macrophages in arthritic joints (Fig. 4B). These proliferating macrophages were present in the lining and sublining layers of the synovium. Elsewhere, such as around blood vessels where cells immunoreactive for Mac-2 were common, no BrdU+Mac-2+ macrophages were seen.
Fig. 4.
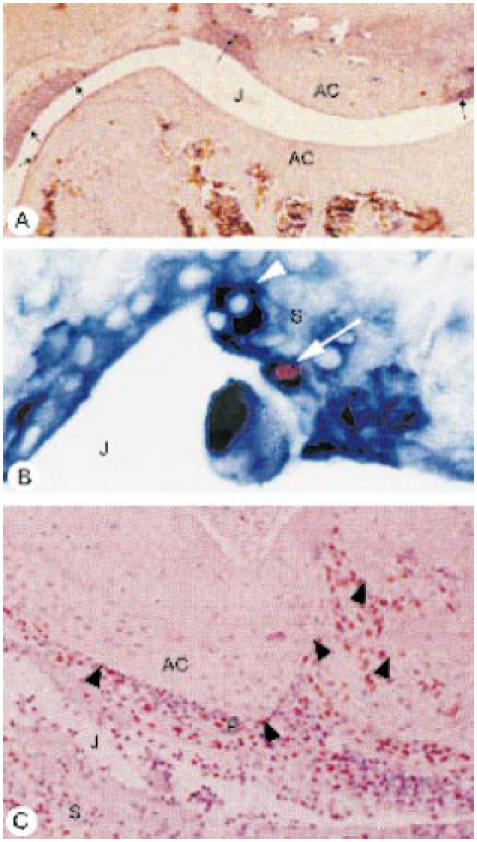
Immunodetection of bromodeoxyuridine (BrdU)-positive cells (red-brown stain) in inflamed joints of mice administered CSF-1 (A) and granulocyte macrophage (GM)-CSF (B,C), at 7 days post-methylated bovine serum albumin (mBSA) injection. (A) Local population of dividing cells revealed in an inflamed joint after a 2-h BrdU label, with BrdU+ cells (arrows) seen along the lining of the thickened synovium. (B) Dual staining using anti-BrdU (red-brown) and anti-Mac-2 (deep blue) of a macrophage (arrow) seen in the synovial lining presumably following recent cell division (2-h BrdU label), with a BrdU−Mac-2+ cell nearby (arrowhead). (C) Following a 24-h BrdU pulse, numerous BrdU+ cells detected in the joint space, inflamed synovium and invasive pannus tissue (P) overlying the articular cartilage (arrowheads). J, Joint space; AC, articular cartilage; S, synovium. (Tissue sections lightly counterstained with haematoxylin, original mag. × 40 (A), × 400 (B) and × 100 (C).)
When BrdU was included in the drinking water over a 24-h period, immunostaining revealed an increase in BrdU+ cells in the synovia of joint sections. The frequency of BrdU+ cells was particularly striking in inflamed joints with more severe pathology (Fig. 4C). There was a slight increase in labelling index in mBSA-injected mice administered CSF-1 or GM-CSF (7.4 ± 3.3% and 7.1 ± 3.4%, respectively; n = 4 mice) compared with the mBSA/saline controls (3.9 ± 0.5%), but this was found to be non-significant. In the case of IL-1β there was a significant increase in the BrdU labelling index over 24 h in inflamed knees (9.7 ± 0.7%; P < 0.05; Student's t-test) compared with the mBSA/saline-injected controls.
DISCUSSION
In this study, we report that the colony-stimulating factors CSF-1 and GM-CSF exacerbate the histopathological features of inflammation seen in an acute mBSA-induced arthritis model. This arthritis model was first described by Staite et al. [15], who found that i.a. administration of mBSA, together with s.c. injections of IL-1, induces an acute monoarthritis in the mBSA-injected knee characterized by inflammation and associated tissue damage which resolves after 14–21 days. In the present study we confirm the effect of IL-1β and further show that CSF-1 and GM-CSF can also exacerbate and prolong the inflammatory response induced in the mBSA-injected knee. With respect to the histopathological changes and immunohistochemistry observed following the administration of CSF-1 or GM-CSF, the resultant inflammation and synovitis was similar to that seen in the IL-1β-treated mice. It seems that in this acute mBSA-induced model of arthritis there may be common elements whereby CSF-1, GM-CSF and IL-1β exert their effect in the inflamed joint. In support of this, preliminary work (Bischof and Campbell, unpublished data) in our laboratory using GM-CSF-deficient (GM-CSF−/−) mice indicates that a less severe form of the mBSA/IL-1β-induced arthritis develops in this mouse strain compared with normal (GM-CSF+/+) littermates.
A great body of evidence now implicates the involvement of numerous cytokines as inflammatory mediators in the pathogenesis of RA, with particular emphasis on IL-1 and TNF-α [11]. In the rheumatoid joint the monocyte/macrophage, as a major cell type and predominant source of proinflammatory cytokines, including IL-1 and TNF-α, is considered a pivotal player in the pathogenesis of RA [9,10]. CSF-1 and GM-CSF are important regulators of myelopoeisis and mediators of inflammation; they also represent key elements of a possible haematopoietic growth factor network active at sites of inflammation [13]. For example, in the inflamed joint CSF-1 and GM-CSF may drive the differentiation and activation of monocytes/macrophages, causing the release of inflammatory mediators and resulting in inflammation and local tissue damage.
In the present study, CSF-1 and GM-CSF may have been effective through local or systemic actions, or perhaps a combination of these. Both local and systemic effects have been demonstrated following the administration of CSFs in vivo. In animal models of arthritis the symptoms of disease have clearly been shown to be exacerbated by systemic CSF-1 ([6]; Campbell et al., submitted) or GM-CSF [7] administration. It has been demonstrated that CSF-1 and GM-CSF administration in vivo results in the mobilization of monocytes from the bone marrow [17,18], while GM-CSF also induces splenomegaly [18]. Local activation and proliferation of monocytes/macrophages may also occur within the joint following exogenous CSF administration [13]. In the acute mBSA-induced arthritis model mBSA is slowly cleared from the joint cavity [15], implicating the involvement of phagocytic cells and antigen-presenting cells in the joint. In the present study macrophages and neutrophils were the prominent cell types that appeared in the inflamed joints, where they most likely played a leading role in the inflammatory response to mBSA and the resultant joint pathology. The relevance of macrophages in particular should be emphasized, as they are potent producers of inflammatory cytokines (e.g. IL-1, TNF-α), chemokines (e.g. MIP-1α, IL-8) and proteolytic enzymes (e.g. collagenase, stromelysin) that directly impact on the appearance and functional activity of leucocytes and other cellular constituents (synovial fibroblasts, chondrocytes, endothelial cells) as well as tissue degradation in rheumatoid lesions [10,11].
Immunohistochemical studies confirmed the presence of cells with reactivity for the macrophage-specific cell surface antigens F4/80 and Mac-2. Of particular interest was the involvement of Mac-2+ macrophages with the histopathology of mBSA-induced arthritis. There is an established association of Mac-2 with inflammatory conditions; in mice, Mac-2 has been shown to be present on a subpopulation of thioglycollate-elicited macrophages in the inflamed peritoneum [16], while macrophages expressing Mac-2 have been found in arthritic joints [19], and in inflamed lesions of autoimmune-prone MRL lpr/lpr mice [20,21]. In the present study strong immunoreactivity for Mac-2 was seen around blood vessels in the inflamed joints, indicative of their recent arrival from the circulation. This characteristic Mac-2 staining was seen only in arthritic joints of mice administered CSF-1, GM-CSF or IL-1 and may reflect an important role for this Mac-2+ subpopulation of macrophages in the progression or persistence of inflammatory arthritis in this model. Our finding that chondrocytes (in normal and inflamed joints) expressed the Mac-2 antigen is perhaps not unusual given that human chondrocytes have been shown to express a number of macrophage-associated membrane molecules [22].
In immunohistochemical studies, in vivo BrdU incorporation showed a significant level of local cell proliferation in the joint (2-h pulse). This was supported by the presence of labelled cells seen deep in the subsynovium and distant to blood vessels, suggesting local proliferation rather than migration. To our knowledge, BrdU incorporation to study in vivo DNA synthesis in joint tissues has not previously been used. In the present study it was confirmed with two-colour immunohistochemistry that a small proportion of the dividing cells in inflamed joints were Mac-2+ (Fig. 4B) or F4/80+ (data not shown) macrophages. There appeared to be some trend to increased BrdU labelling in inflamed synovial tissues (24-h pulse). It should be noted that with BrdU incorporation following a 24-h pulse, many of the BrdU-labelled cells may have been recent immigrants and therefore cannot be considered representative of the locally dividing cell population.
In the present study there were no major differences observed in severity of the histopathology of the inflamed joints when comparing the effects of CSF-1, GM-CSF or IL-1β. There was both an influx of inflammatory cells which included macrophages (Mac-2+), and evidence of local cell proliferation which may have contributed to the increased cellularity within the inflamed joints. It is interesting to note that the increased BrdU labelling index in inflamed joints was significant only for mice administered IL-1β and not CSF-1 or GM-CSF. There may be a different mode of action of IL-1β compared with the CSFs in its effect on the proliferation of cells in the inflamed joint; the monocytes/macrophages may be the direct or indirect target of IL-1β. IL-1β is known to augment CSF-1 and GM-CSF production by joint tissue cells [23–26], or could also serve to activate macrophages and T lymphocytes locally. Conversely, GM-CSF is known to be a potent inducer of IL-1 secretion [27].
In conclusion, this study has shown that systemic administration of GM-CSF or CSF-1 exacerbates the histopathology observed in an acute murine model of mBSA-induced arthritis. With the swift appearance of the histopathological features of arthritis, this acute mBSA-induced arthritis model offers a distinct advantage over other well-worked models, such as collagen-induced arthritis. The mBSA-induced arthritis model may serve as a useful tool for studies on the role of monocytes/macrophages and the colony-stimulating factors in arthritis. This work serves to strengthen further the central role of monocytes/macrophages as a driving influence in the pathogenesis of inflammatory arthritis.
Acknowledgments
R.J.B. was the recipient of an Arthritis Foundation of Australia Fellowship. This work was supported in part by the NHMRC of Australia, and by AMGEN. We thank Jennifer Davis for assistance with the maintenance of the mice.
REFERENCES
- 1.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoieitic cells. Nature. 1989;339:27. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS, Xu W-D, Townsend K, et al. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988;168:1573–86. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu WD, Firestein GS, Taetle R, et al. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989;83:876–82. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazenberg BPC, van Leeuwen MA, van Rijswijk MH, et al. Correction of granulocytopenia in Felty's syndrome by granulocyte-macrophage colony-stimulating factor. Simultaneous induction of interleukin-6 release and flare-up of the arthritis. Blood. 1989;74:2769–70. [PubMed] [Google Scholar]
- 5.Hamilton JA, Butler DM, Stanton H. Cytokine interactions promoting DNA synthesis in human synovial fibroblasts. J Rheumatol. 1993;21:797–803. [PubMed] [Google Scholar]
- 6.Abd A-HA, Savage NW, Halliday WJ, et al. The role of macrophages in experimental arthritis induced by Streptococcus agalactiae sonicate: actions of macrophage colony-stimulating factor (CSF-1) and other macrophage-modulating agents. Lymphokine Cytokine Res. 1991;10:43–50. [PubMed] [Google Scholar]
- 7.Campbell IK, Bendele A, Smith DA, et al. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56:364–8. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell IK, Rich MJ, Bischof RJ, et al. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161:3639–44. [PubMed] [Google Scholar]
- 9.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–24. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 10.Burmester GR, Stuhlmüller B, Keyszer G, et al. Mononuclear phagocytes and rheumatoid synovium: mastermind or workhorse in arthritis? Arthritis Rheum. 1997;40:5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- 11.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 12.Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis. Arthritis Rheum. 1990;33:768–73. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton JA. Rheumatoid arthritis: opposing actions of haemopoietic growth factors and slow-acting anti-rheumatic drugs. Lancet. 1993;342:536–9. doi: 10.1016/0140-6736(93)91653-4. [DOI] [PubMed] [Google Scholar]
- 14.Koch AE, Kunkel SL, Strieter RM. Cytokines in rheumatoid arthritis. J Invest Med. 1995;43:28–38. [PubMed] [Google Scholar]
- 15.Staite ND, Richard KA, Aspar DG, et al. Induction of an acute erosive monarticular arthritis in mice by interleukin-1 and methylated bovine serum albumin. Arthritis Rheum. 1990;33:253–60. doi: 10.1002/art.1780330215. [DOI] [PubMed] [Google Scholar]
- 16.Ho M-K, Springer TA. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982;128:1221–8. [PubMed] [Google Scholar]
- 17.Hume DA, Pavli P, Donahue RE, et al. The effect of human recombinant macrophage colony-stimulating factor (CSF-1) on the murine mononuclear phagocyte system in vivo. J Immunol. 1987;141:3405–9. [PubMed] [Google Scholar]
- 18.Metcalf D, Begley CG, Williamson DJ, et al. Haemopoietic responses in mice injected with purified recombinant murine GM-CSF. Exp Haematol. 1987;15:1–9. [PubMed] [Google Scholar]
- 19.Sack U, Kuhn H, Ermann J, et al. Synovial tissue implants from patients with rheumatoid arthritis cause destruction in knee joints of SCID.bg mice. J Rheumatol. 1994;21:10–6. [PubMed] [Google Scholar]
- 20.Kanno H, Nose M, Itoh J, et al. Spontaneous development of pancreatitis in the MRL/Mp strain of mice in autoimmune mechanism. Clin Exp Immunol. 1992;89:68–73. doi: 10.1111/j.1365-2249.1992.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi Y, Ito MR, Mori S, et al. Role of macrophages in the development of arteritis in MRL strains of mice with a deficit in Fas-mediated apoptosis. Clin Exp Immunol. 1996;106:26–34. doi: 10.1046/j.1365-2249.1996.d01-814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summers KL, O'donnell JL, Marina SH, et al. Monocyte-macrophage antigen expression on chondrocytes. J Rheumatol. 1995;22:1326–34. [PubMed] [Google Scholar]
- 23.Leizer T, Cebon J, Layton JE, et al. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990;76:1989–96. [PubMed] [Google Scholar]
- 24.Campbell IK, Novak U, Cebon J, et al. Human articular cartilage and chondrocytes produce hemopoietic colony-stimulating factors in culture in response to IL-1. J Immunol. 1991;147:1238–46. [PubMed] [Google Scholar]
- 25.Campbell IK, Ianches G, Hamilton JA. Production of macrophage colony-stimulating factor (M-CSF) by human articular cartilage and chondrocytes. Modulation by interleukin-1 and tumour necrosis factor α. Biochim Biophys Acta. 1993;1182:57–63. doi: 10.1016/0925-4439(93)90153-r. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton JA, Filonzi EL, Ianches G. Regulation of macrophage colony-stimulating factor (M-CSF) production in cultured human synovial fibroblasts. Growth Factors. 1993;9:157–65. doi: 10.3109/08977199309010831. [DOI] [PubMed] [Google Scholar]
- 27.Morrissey PJ, Bressler L, Park LS, et al. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987;139:1113–9. [PubMed] [Google Scholar]


