Abstract
The effect of a fish oil diet on virus-specific cytotoxicity and lymphocyte proliferation was investigated. Mice were fed fish oil (17 g fish oil and 3 g sunflower/100 g) or beef tallow (17 g tallow and 3 g sunflower/100 g) diets for 14 days before intranasal challenge with influenza virus. At day 5 after infection, lung virus-specific T lymphocyte, but not macrophage or natural killer (NK) cell, cytotoxicity was significantly lower in mice fed fish oil, while bronchial lymph node cell proliferation to virus was significantly higher. In mice fed fish oil, spleen cell proliferation to virus was also significantly higher following immunization. The results showed that, despite improved lymphocyte proliferation, fish oil impairs primary virus-specific T lymphocyte cytotoxicity. This impairment may explain the delayed virus clearance that we have previously reported in infected mice fed the fish oil diet.
Keywords: fish oil, influenza, cytotoxicity, proliferation
INTRODUCTION
Dietary long chain (n-3) polyunsaturated fatty acids derived from fish oil have been shown to have beneficial effects on inflammatory and autoimmune disorders [1–4], and cancer [5], suggesting that fish oil has anti-inflammatory and immunomodulatory activities. For instance, fish oil has been reported to reduce the production of mediators such as eicosanoids and cytokines which contribute to inflammation and pathological changes in atherosclerosis, autoimmunity and infectious disease [6,7]. Because dietary fish oil has anti-inflammatory activities and the capacity to increase severity of infectious disease [8], concern exists that fish oil supplementation may have potentially adverse effects in individuals with greater susceptibility to infection, such as infants, the elderly and the immunocompromised.
In animal models of infection, fish oil diets reduce clearance of bacteria and decrease survival during infection [9–11]. We have previously reported higher virus loads and delayed clearance following influenza virus infection in naive mice fed a diet rich in fish oil [12]. The delayed virus clearance was associated with impaired lung immunoglobulin IgA antibody and interferon-gamma (IFN-γ) responses. IFN-γ has been implicated in the clearance of viral infection by modulating cytotoxicity and proliferation [13]. Several studies have shown that fish oil reduces spleen T cell and peritoneal macrophage cytotoxicity against virus-infected tumour target cells [14,15] and tumour cells [16], respectively. Natural killer (NK) cell cytotoxicity is also reduced in mice fed fish oil [17]. In the present study, the effect of dietary fish oil on cellular immunity to influenza virus infection in mice was examined in terms of bronchial lymph node cell proliferation to virus and lung cell-mediated cytotoxicity against virus-infected target cells. Our results showed that despite increased T cell proliferation, fish oil impaired primary virus-specific T cell cytotoxicity but had no effect on macrophage or NK cell cytotoxicity.
MATERIALS AND METHODS
Diets and animals
Specific pathogen-free male six-week-old BALB/c mice were randomly assigned to diets containing fish oil (17 g fish oil and 3 g sunflower/100 g) or beef tallow blend (17 g tallow and 3 g sunflower/100 g). The beef tallow diet contained 0.355 g/kg all-rac-α-tocopherol, while the fish oil diet contained 0.534 g/kg [12]. The fatty acid composition of the diets has been previously reported [12]. The mice were housed individually and fed 5 g food/day. Animal weights and food consumption were recorded at weekly intervals and immediately prior to killing.
Influenza virus infection
Mice on experimental diets for 14 days were infected with log105 plaque-forming units (PFU) of A/Queensland/6/72 (H3N2) influenza virus [12]. Lung macrophage, NK cell and virus-specific T cell cytotoxicity was determined at day 5 after infection. Bronchial lymph node cells were isolated for T cell proliferation assay at day 5 and day 12. The effect of the diets on lung pathology was determined at days 2, 5 and 7.
Intraperitoneal immunization
Mice were fed fish oil or beef tallow diets for 14 days (n = 10/diet group). At day 7 the mice were intraperitoneally immunized with live influenza virus vaccine (log105.82 PFU of A/Queensland virus in sterile PBS, 200 μl). At day 14, virus-specific serum IgG and virus-induced splenocyte proliferation were determined.
Lung T cell cytotoxicity
Lungs were finely minced with a scalpel and digested with collagenase type I (0.2 mg/ml; Worthington Biochemical Corp., Freehold, NJ) in AIM V medium (Life Technologies, Grand Island, NY) at 37°C for 30 min. The digested tissue was pressed through a wire mesh filter with a plastic syringe plunger and then passed through a 10-ml syringe packed with glass wool. After centrifugation, erythrocytes in the cell pellet were lysed in a solution containing NH4Cl 8.3 g, NaCO3 1 g and EDTA 0.037 g/l in deionized water, pH 7.35. Cell viability was 98% by trypan blue dye exclusion test. Mouse plasmacytoma P815 target cells (American Type Culture Collection (ATCC), Rockville, MD) were labelled with Na251CrO4 (3.7 MBq/2 × 106 cells; Amersham Int., Aylesbury, UK) for 1 h at 37°C in a 5% CO2 atmosphere. After washing and centrifugation the cells were infected with influenza virus (240 haemagglutinin units (HA) of A/Queensland/1 × 106 cells) by incubating for 1 h. Virus-infected cells were recovered by centrifugation and washed twice in PBS before use.
Lung cells at 10 × 106 cells/ml in complete RPMI 1640 medium were incubated with virus-infected target cells at effector:target (E:T) ratios of 100:1 and 50:1. After incubation for 6 h at 37°C in a 5% CO2 atmosphere, Cr release was measured in the supernatant using a LKB 1282 Compugamma CS gamma counter (LKB Wallac, Turku, Finland). Spontaneous 51Cr release was determined by incubating labelled P815 target cells without lung cells. The maximum (total) ct/min was determined after water lysis of target cells. The mean percentage of influenza virus-infected target cells killed by cytotoxic T cells was calculated using the formula: (mean ct/min (test) − mean spontaneous ct/min)/(mean maximum ct/min − mean spontaneous ct/min) × 100.
Lung macrophage virus-specific cytotoxicity assay
One hundred microlitre aliquots of lung cells at 5 × 106 cells/ml in complete RPMI 1640 medium were added in triplicate to wells of a 96-well flat-bottomed microtitre plate and then incubated for 2 h at 37°C in a 5% CO2 atmosphere [18]. After removal of non-adherent cells by washing three times in RPMI medium, the adherent cells were incubated with virus-infected P815 target cells at lung cell to target ratios of 100:1 and 40:1. Non-infected P815 cells were added to additional wells to determine non-specific macrophage cytotoxicity. After incubation and gamma counting the mean percentage of target cells killed by macrophages was calculated using the above formula.
Lung NK cell cytotoxicity assay
One hundred microlitre aliquots of lung cells at 5 × 106 cells/ml in complete RPMI 1640 medium were added in triplicate to V-bottomed microtitre wells loaded with Cr-labelled NK-sensitive mouse lymphoma YAC-1 target cells (ATCC) at 50:1 and 25:1 (E:T) ratios [19]. Spontaneous 51Cr release was determined by incubating target cells without lymphocytes. NK cytotoxicity was determined after incubation and gamma counting as described above.
T cell proliferation
Lymph node cells and spleen cells were isolated as previously described [12]. Cells (5 × 106/ml) were suspended in RPMI 1640 medium supplemented with fetal calf serum (FCS; 100 ml/l), HEPES buffer 0.02 m, NaHCO31.5 g/l, penicillin/streptomycin 50 mg/l, 2-mercaptoethanol (2-ME; 5 × 10−5m) and l-glutamine 1 mg/l (Trace Biosciences, Sydney, Australia). Cultures in triplicate were stimulated with inactivated influenza virus (1 HA/ml for spleen cells, 5 or 10 HA/ml for lymph node cells) or 10 μg/ml concanavalin A (Con A; Boehringer Mannheim, Mannheim, Germany) for spleen cells in flat-bottomed microtitre wells (5 × 105 cells/well) for 96 h or 48 h, respectively, at 37°C in a humid atmosphere of 5% CO2 and 95% air. Virus used in proliferation assays was inactivated by exposure to γ irradiation (60Co, 1.2 × 104 Gy). The cultures were pulsed with 3H-thymidine for the final 6 h before harvesting and counting using a TopCount scintillation counter (Canberra-Packard, Five Dock, Australia). Results were reported as the stimulation index (SI). An aliquot of the lymphocyte suspension in Hanks' balanced salt solution (HBSS) was stored at −20°C for fatty acid analysis.
Serum IgG antibody
Virus-specific IgG antibody in serum was determined by ELISA as previously described [12].
Lung pathology
Whole lungs were removed from mice killed at day 2 (n = 3/diet group), day 5 (n = 4) and day 7 (n = 3) after infection and fixed in neutral buffered 4% formalin solution (Sigma-Aldrich Pty Ltd, Castle Hill, Australia). Paraffin sections were stained with haematoxylin and eosin. Lung sections assigned random numbers were examined under × 400 magnification and the number of macrophages per field was counted with the aid of a graticule. Macrophages were identified on the basis of morphology. The macrophage count for each mouse was derived from the mean of counts in three separate fields.
Analysis of serum α-tocopherol
Mouse serum was analysed to determine α-tocopherol concentration as described previously [20]. Briefly, serum was mixed with methanol containing 0.12 mg/ml α-tocopherol acetate as an internal standard. After addition of hexane, the samples were centrifuged (1700 g, 5 min) and the hexane phase transferred to a glass tube and evaporated. The dried samples were reconstituted with methanol (100 μl) and analysed on a GBC high pressure liquid chromatograph (GBC Scientific Equipment Pty Ltd, Dandenong, Australia) fitted with a Waters 4-μm Novapak C18 column (100 × 8 mm; Waters Australia Pty Ltd, Rydalmere, Australia). Samples were injected, eluted with a mobile phase of 99.75/0.25% methanol/water at 1.5 ml/min and α-tocopherol was detected using a UV/visible detector at a wavelength of 290 nm for 15 min. The α-tocopherol concentration in each sample was calculated by reference to the relative responses of an authentic α-tocopherol standard and the internal standard.
Bronchial lymph node cell fatty acid composition
Aliquots of bronchial lymph node cells (1 ml) were freeze-dried prior to the addition of methanol and toluene (4:1 v/v, 2 ml) [21]. The samples, suspended in methanol and toluene, were directly transesterified by the addition of acetyl chloride (200 μl) while vortex mixing and then heated for 1 h at 100°C. The fatty acid composition was then determined by gas chromatography as previously described [12].
Statistical analysis
Statistical significance was evaluated using Student's t, the Mann–Whitney and Kruskal–Wallis tests. All calculations were performed using a statistical software program (Abacus Concepts, Berkley, CA; StatView Version 4.5). Differences were considered significant at P < 0.05. Data are presented as mean ± s.e.m.
RESULTS
Weight and food consumption
Two groups of mice were fed fish oil (n = 10) or beef tallow (n = 10) for 14 days before infection with influenza virus. The body weight and food consumption were measured before and after challenge at days 5 and 12. Mice fed fish oil prior to infection with influenza virus had lower body weight prior to challenge (P < 0.05) and were consuming, but not significantly so, less than those fed beef tallow (Table 1). At day 5 after infection the mice fed fish oil had lower body weights and food consumption (P < 0.05). By day 12 after infection, there were no significant differences in body weight, although the mice fed fish oil were eating more food (P < 0.05). In mice immunized intraperitoneally with influenza virus, there was no significant difference in body weight or food consumption before or after immunization (data not shown).
Table 1.
Food consumption and body weight before and after challenge with influenza virus in mice fed beef tallow or fish oil*
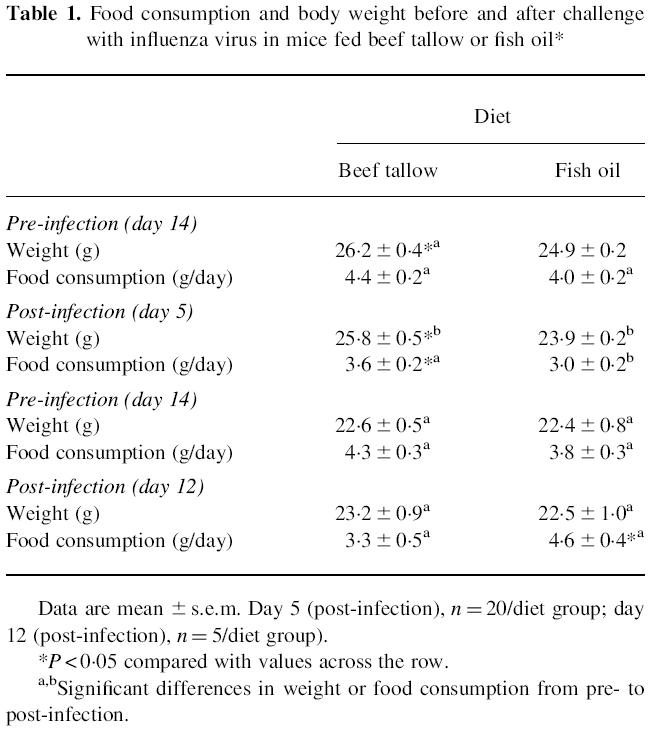
Cell cytotoxicity
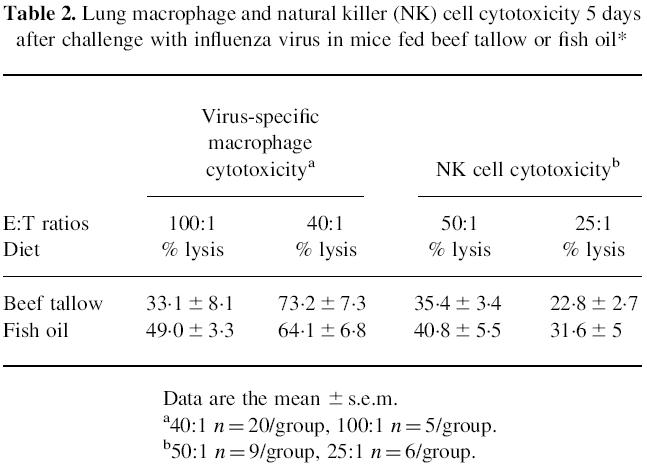
Five days after infection, lung virus-specific cytotoxic T cell activity against infected target cells was significantly higher in mice fed beef tallow than in those fed fish oil (P < 0.05) (Fig. 1). No cytotoxicity was detected against uninfected target cells in either diet group. There were no significant differences between diet groups in virus-specific lung macrophage cytotoxicity or lung NK cell cytotoxicity irrespective of (E:T) ratios (Table 2). The lower macrophage cytotoxicity against virus-infected target cells at high E:T ratios (100:1) may reflect the interference of lung macrophages in adhering to the plastic microwells, due to greater numbers of contaminating adherent lung epithelial and fibroblast cells at high concentrations of lung cells.
Fig. 1.
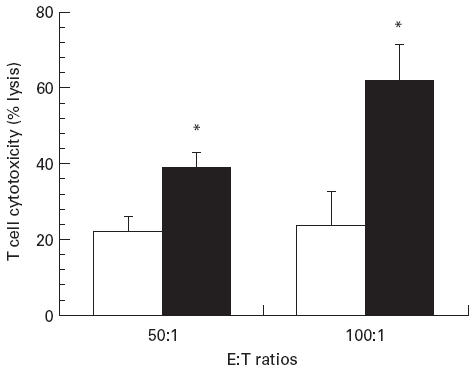
Influenza virus-specific lung cytotoxic T cell activity in mice. The mice were fed diets containing fish oil (□) or beef tallow (▪) (20 g/100 g) for 14 days before intranasal challenge with A/Queensland influenza virus. Cytotoxic T cell activity was determined at 50:1 and 100:1 E:T ratios with influenza virus-infected P815 target cells. Values are mean specific lysis activity ± s.e.m. (n = 5/diet group) at day 5. *Significantly higher cytotoxic activity (P < 0.05).
Table 2.
Lung macrophage and natural killer (NK) cell cytotoxicity 5 days after challenge with influenza virus in mice fed beef tallow or fish oil*
T cell proliferation
At day 5 after influenza infection, the highest bronchial lymph node cell proliferative response to virus was in the fish oil-fed group (P < 0.01) (Fig. 2). At day 12, there were no significant differences between diet groups in infected and non-infected mice. Within the fish oil group there were no significant differences over time. Within the beef tallow-fed group, T cell response from infected mice stimulated with 10 HA/ml was significantly lower at day 5 than at day 12 (P < 0.05). At day 12, mice fed beef tallow had a higher proliferative response than non-infected controls (P < 0.05).
Fig. 2.
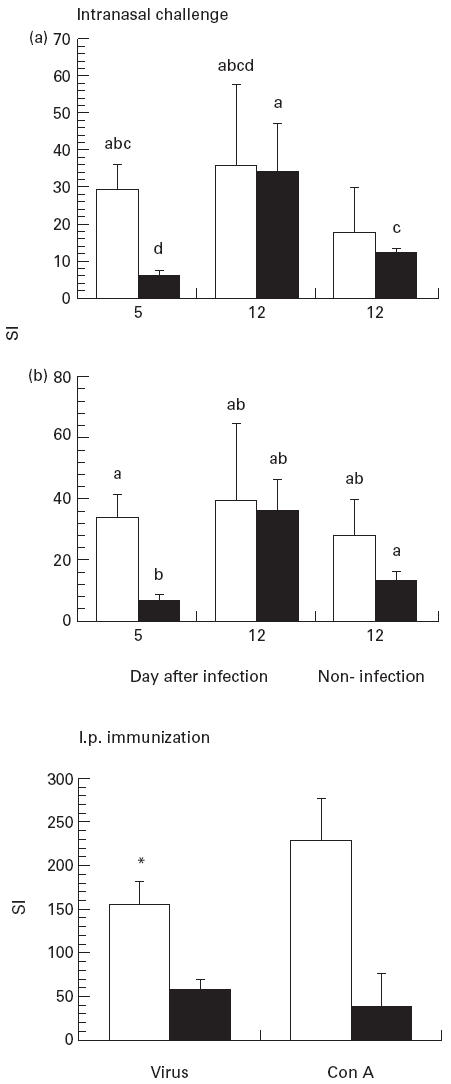
Proliferative response to influenza virus in mice infected or immunized with influenza. Mice were fed diets containing fish oil or beef tallow. Mice were killed at day 5 or day 12 after challenge with A/Queensland influenza virus. Mice immunized intraperitoneally were killed at day 14. Values are mean stimulation index (SI) ± s.e.m. (n = 20/group at day 5, n = 5/group at day 12). Bars not sharing common superscript letters are significantly different (P < 0.05). SI is calculated from (ct/min stimulated cells)/(ct/min control cells). Top panel, BLN stimulated with 10 haemagglutinin units (HA) influenza virus/ml (A) or 5 HA influenza virus/ml (B). Bottom panel, spleen cells stimulated with virus (10 HA/ml) or concanavalin A (Con A; 5 μg/ml). Data are mean ± s.e.m. (n = 10/diet group). *P < 0.05 compared with values from beef tallow diet group.
In mice immunized by the i.p. route with influenza virus, spleen T cell proliferation from the fish oil group to influenza virus was significantly higher than the beef tallow group (P < 0.02), while there was no significant difference in the response to Con A (P < 0.07) (Fig. 2). No significant differences in influenza virus IgG antibody were detected between dietary groups (data not shown).
Lung pathology
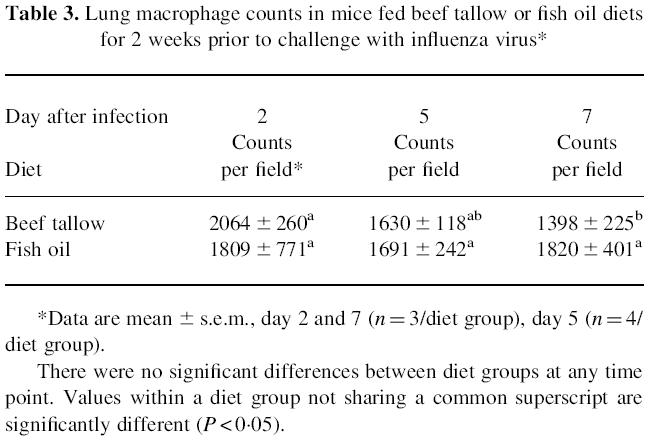
The alveolar septa displayed marked thickening due to the infiltration of large numbers of macrophages in infected mice, particularly those fed fish oil (not shown). Between diet groups, the differences in macrophage number per field were not statistically significant (Table 3). However, macrophage numbers were significantly lower at day 7 relative to day 2 in mice fed beef tallow, but not in those fed fish oil (P < 0.05).
Table 3.
Lung macrophage counts in mice fed beef tallow or fish oil diets for 2 weeks prior to challenge with influenza virus*
Serum α-tocopherol
The serum α-tocopherol concentration at day 5 after infection did not differ significantly between diet groups (μmol/l± s.e.m., beef tallow 8.9 ± 0.9, fish oil 8.2 ± 1.5, n = 10/diet group).
Bronchial lymph node cell fatty acid composition
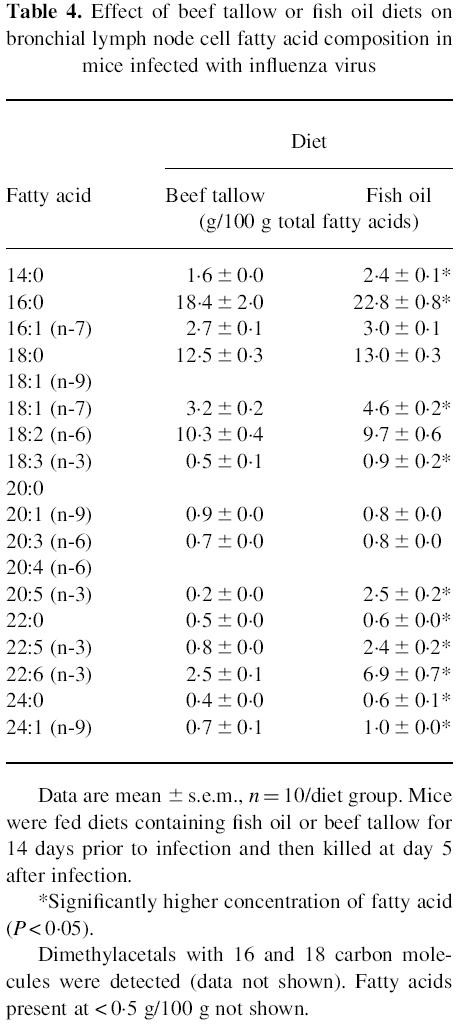
The incorporation of fatty acids into the bronchial lymph node cells of the influenza-infected mice after 19 days on diet reflected the dietary intake (Table 4). The fish oil-fed mice had significantly more myristic (tetradecanoic), palmitic (hexadecanoic), vaccenic (Δ11 octadecanoic), α-linolenic (Δ9, 12, 15 octadecatrienoic), eicosapentaenoic, docosapentaenoic and docosahexaenoic acid, while the beef tallow group had significantly more oleic (octadecenoic) and arachidonic (eicosatetraenoic) acid (P < 0.05).
Table 4.
Effect of beef tallow or fish oil diets on bronchial lymph node cell fatty acid composition in mice infected with influenza virus
DISCUSSION
In the present study, we have demonstrated that mice fed fish oil but not beef tallow have increased T cell proliferation and lower virus-specific lung T cell cytotoxicity following influenza virus infection. In addition, mice fed fish oil but not beef tallow have significantly greater incorporation of long chain n-3 fatty acids into bronchial lymph node cells. While weight loss and reduced food consumption were observed in mice fed fish oil at day 5, it was not evident at day 12 following influenza virus infection.
In our previous studies, we have shown that in mice fed fish or beef tallow the virus was cleared from the lung by day 7. This suggests that the weight loss and reduced food consumption at day 5 seen in the present study were probably due to the effects of an acute inflammatory response to virus infection which would be expected to be resolved by day 12. However, there is evidence to suggest that the decreased growth rate in mice fed fish oil may be due to the formation of lipid peroxides [22], although the diet contained more α-tocopherol than beef tallow diet.
The present study showed that mice fed fish oil have contrasting cell-mediated immunity to influenza virus infection. There was no significant difference in the number of alveolar macrophages between the diet groups, suggesting that fish oil had no effect on the recruitment of macrophages to the lung following influenza virus infection. While the numbers remained elevated in the fish oil group, there was a stepwise decline in the number of macrophages with time in the beef tallow group, suggesting that a sustained accumulation of macrophages in the lung of mice fed fish oil may be a response to delayed virus clearance [12]. Both macrophage and NK cell cytotoxicity were not significantly influenced by the diets, suggesting that that lung macrophage and NK cells are less susceptible to modulation by fish oil than those reported in the spleen.
The lower cytotoxic T cell response to influenza virus in mice fed fish oil is consistent with the reported study of vaccinia virus [14]. Whether this is due to a reduction in body weight or a direct effect of fish oil on cytotoxic T cells is unclear. Reduction in cytolytic activity is likely to be associated with impaired production of lung IFN-γ that occurs following influenza infection in mice fed the fish oil diet [12]. Since IFN-γ is a mediator of T cell cytotoxicity and is secreted by CD8+ cytotoxic T cells [13], down-regulation of its production in the lungs of mice fed a fish oil diet may lead to a defective T cell cytotoxic response to virus infection.
In contrast to the suppressive effect on lung cytotoxic T cell activity, the fish oil diet was associated with increased virus-induced lymphoproliferation of spleen and bronchial lymph node cells, especially at day 5 following infection. While the effect is consistent with the increased proliferative response of spleen cells to mitogen that we [23] and others [24,25] have reported in mice fed fish oil, the response to Con A stimulation was not statistically significant. In the present study, exposure to virus by i.p. immunization or intranasal challenge led to significantly higher eicosapentaenoic acid concentrations in spleen and bronchial lymph node cells from mice fed fish oil than those from mice fed beef tallow. The incorporation of n-3 fatty acids into tissues is associated with lower prostaglandin E2 production [16,22]. Prostaglandin E2 is known to suppress lymphocyte proliferation and the production of cytokines, including IL-2 and IFN-γ [26,27]. It is possible that the lower proliferative response of cells from mice fed beef tallow results from higher prostaglandin E2 levels.
Increased dietary vitamin E enhances the proliferative response of mouse lymphocytes [28]. The fish oil diet contained more α-tocopherol as a precaution against auto-oxidation. The difference in dietary α-tocopherol did not translate into differences in the serum, although lymphocyte α-tocopherol concentration was not measured in the present study. The stronger lymphoproliferation of spleen and bronchial lymph node cells from mice fed fish oil may relate to a difference in lymphocyte α-tocopherol. Future studies should consider the relationship between diet, lymphocyte and serum α-tocopherol and proliferation in the mouse model of influenza.
In conclusion, a diet containing fish oil resulted in a suppression of virus-specific lung T cell cytotoxicity and an increase in virus-specific proliferative responses in mice infected with influenza virus. Impaired T cell cytotoxicity may explain the delayed virus clearance that we have previously reported in mice fed the fish oil diet [12]. The present study has demonstrated that feeding a high fat diet rich in n-3 fatty acids may lead to impairment of acquired cellular immunity, but not innate immunity.
Acknowledgments
The authors thank Ma Cong, Helen Haynes and Jian Ping for their expert technical assistance and the Hunter Area Pathology Service for performing the serum α-tocopherol analysis. We also thank R. P. Scherer Holdings (Braeside, Australia) for donating the MaxEPA fish oil.
REFERENCES
- 1.Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Arthritis Rheum. 1990;33:810–20. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- 2.Stenson WF, Cort D, Rodgers J, et al. Dietary supplementation with fish oil in ulcerative colitis. Ann Intern Med. 1992;116:609–14. doi: 10.7326/0003-4819-116-8-609. [DOI] [PubMed] [Google Scholar]
- 3.Walton AJE, Snaith ML, Locniskar M, Cumberland AG, Morrow WJW, Isenberg DA. Dietary fish oil and the severity of symptoms in patients with systemic lupus erythematosus. Ann Rheum Dis. 1991;50:463–6. doi: 10.1136/ard.50.7.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dyerberg J, Bang HO, Stofferson E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–9. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 5.Wigmore SJ, Fearon KC, Maingay JP, Ross JA. Down-regulation of the acute-phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin-6. Clin Sci. 1997;92:215–21. doi: 10.1042/cs0920215. [DOI] [PubMed] [Google Scholar]
- 6.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA. Interleukin-1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes and related leukocyte cell lines. J Clin Invest. 1985;76:2003–11. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endres S, Eisenhut T, Sinha B. n-3 Polyunsaturated fatty acids in the regulation of human cytokine synthesis. Biochem Soc Trans. 1995;23:277–81. doi: 10.1042/bst0230277a. [DOI] [PubMed] [Google Scholar]
- 8.Bell SJ, Chavali S, Bistrian BR, Connolly CA, Utsunomiya T, Forse RA. Dietary fish oil and cytokine and eicosanoid production during human immunodeficiency virus infection. J Parent Enteral Nutr. 1996;20:43–9. doi: 10.1177/014860719602000143. [DOI] [PubMed] [Google Scholar]
- 9.Chang HR, Dulloo AG, Vladoianu IR, et al. Fish oil decreases natural resistance to infection with Salmonella typhimurium. Metabolism. 1992;41:1–2. doi: 10.1016/0026-0495(92)90181-9. [DOI] [PubMed] [Google Scholar]
- 10.D'ambola JB, Aeberhard EE, Trang N, Gaffar S, Barrett CT, Sherman MP. Effect of dietary (n-3) and (n-6) fatty acids on in vivo pulmonary bacterial clearance by neonatal rabbits. J Nutr. 1991;121:1262–9. doi: 10.1093/jn/121.8.1262. [DOI] [PubMed] [Google Scholar]
- 11.Fritsche KL, Shahbazian LM, Feng C, Berg JN. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin Sci. 1997;92:95–101. doi: 10.1042/cs0920095. [DOI] [PubMed] [Google Scholar]
- 12.Byleveld PM, Pang GT, Clancy RL, Roberts DCK. Fish oil feeding delays influenza virus clearance and impairs production of interferon-γ and virus-specific immunoglobulin A in the lungs of mice. J Nutr. 1999;129:328–35. doi: 10.1093/jn/129.2.328. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay AJ, Ruby J, Ramshaw IA. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–7. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 14.Fritsche KL, Johnstone PV. Effect of dietary omega-3 fatty acids on cell-mediated cytotoxic activity in BALB/c mice. Nutr Res. 1990;10:577–88. [Google Scholar]
- 15.Black JM, Kinsella JE. Dietary n-3 fatty acids alter murine peritoneal macrophage cytotoxicity. Ann Nutr Metab. 1993;37:110–20. doi: 10.1159/000177758. [DOI] [PubMed] [Google Scholar]
- 16.Berger A, German JB, Chiang B-L, et al. Influence of feeding unsaturated fats on growth and immune status of mice. J Nutr. 1993;123:225–33. doi: 10.1093/jn/123.2.225. [DOI] [PubMed] [Google Scholar]
- 17.Meydani SN, Yogeeswaran G, Liu S, Baskar S, Meydani M. Fish oil and tocopherol induced changes in natural killer cell mediated cytotoxicity and PGE2 synthesis in young and old mice. J Nutr. 1988;118:1245–52. doi: 10.1093/jn/118.10.1245. [DOI] [PubMed] [Google Scholar]
- 18.Mak NK, Leung KN, Ada GL. The generation of ‘cytotoxic’ macrophages in mice during infection with influenza A or Sendai virus. Scand J Immunol. 1982;15:553–61. doi: 10.1111/j.1365-3083.1982.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 19.Kiessling R, Klein E, Wigzell H. ‘Natural’ killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 20.Thurnham DI, Smith E, Flora PS. Concurrent liquid-chromatographic assay of retinol, alpha-tocopherol, beta-carotene, alpha-carotene, lycopene, and beta-cryptoxanthin in plasma, with tocopherol acetate as internal standard. Clin Chem. 1988;34:377–81. [PubMed] [Google Scholar]
- 21.Rodríguez-Palmero M, Lopez-Sabater MC, Castellote-Bargallo AI, De La Torre-Boronat MC, Rivero-Urgell M. Comparison of two methods for the determination of fatty acid profiles in plasma and erythrocytes. J Chromatogr A. 1997;778:435–9. doi: 10.1016/s0021-9673(97)00554-2. [DOI] [PubMed] [Google Scholar]
- 22.Mounie J, Faye B, Magdalou J, Goudeonnet H, Truchot R, Siest G. Modulation of UDP glucorosyltransferase activity in rats by dietary lipids. J Nutr. 1986;116:2034–43. doi: 10.1093/jn/116.10.2034. [DOI] [PubMed] [Google Scholar]
- 23.Byleveld PM, Pang GT, Clancy RL, Roberts DCK. Virus specific and polyclonal responses following challenge with influenza in immunised mice fed fish oil, linseed oil or beef tallow. Nutr Res. 1999;19:1049–61. [Google Scholar]
- 24.Hosack Fowler K, Chapkin RS, McMurray DN. Effect of purified dietary n-3 ethyl esters on murine T lymphocyte function. J Immunol. 1993;151:5186–97. [PubMed] [Google Scholar]
- 25.Hardard'ottir I, Whelan J, Kinsella JE. Kinetics of tumour necrosis factor and prostaglandin production by murine resident peritoneal macrophages as affected by dietary n-3 polyunsaturated fatty acids. Immunol. 1992;76:572–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Simkin NJ, Jelinek DF, Lipsky PE. Inhibition of human B cell responsiveness by prostaglandin E2. J Immunol. 1987;138:1074–81. [PubMed] [Google Scholar]
- 27.Walker C, Kristensen F, Bettens F, DeWeck AL. Lymphokine regulation of activated (G1) lymphocytes. J Immunol. 1983;130:1770–3. [PubMed] [Google Scholar]
- 28.Tanaka J, Fujiwara H, Torisu M. Vitamin E and immune response. I. Enhancement of helper T-cell activity by dietary supplementation of vitamin E in mice. Immunol. 1979;38:727–35. [PMC free article] [PubMed] [Google Scholar]


