Abstract
Ws/Ws rats have a small deletion of the c-kit gene, and are deficient in both mucosal-type mast cells (MMC) and connective tissue-type mast cells (CTMC). In the present study we investigated the role of intestinal MMC in the development of dextran sulphate sodium (DSS)-induced experimental colitis using Ws/Ws rats. Ws/Ws and control (+/+) rats were given a 3% DSS aqueous solution orally for 10 days, and the subsequent mucosal damage was evaluated macroscopically and histologically. The mucosal myeloperoxidase (MPO) activities and histamine levels were also measured. (i) DSS induced severe oedema and hyperaemia with sporadic erosions in the control (+/+) rats, but these changes were significantly attenuated in the Ws/Ws rats (P < 0.01). (ii) The microscopic mucosal damage score was lower in the Ws/Ws rats than in the control (+/+) rats (P = 0.06). (iii) There were no significant differences in mucosal MPO activity between the Ws/Ws and control (+/+) rats (P = 0.46). (iv) The mucosal histamine levels in the colon were significantly reduced in the Ws/Ws rats compared with the control (+/+) rats (P < 0.05). (v) Significant positive correlations were observed between mucosal histamine levels and the degree of mucosal oedema (calculated as colonic wet weight/protein content) (r = 0.778, P < 0.01), and between histamine levels and the macroscopic damage (r = 0.623, P < 0.05), respectively. (vi) DSS induced a local recruitment of MMC in the colonic mucosa of Ws/Ws rats, and mucosal damage gradually increased in accordance with this MMC recruitment. These results indicate that MMC play an important role in the development of DSS colitis.
Keywords: inflammatory bowel disease, c-kit gene, mucosal mast cell
INTRODUCTION
Mast cell activation leads to the release of large quantities of chemical mediators such as histamine [1,2], leukotrienes [3] and platelet-activating factor (PAF) [4], which then play an important role in acute and chronic tissue injury. The pathogenesis of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD), remains unknown. However, recent studies have suggested that mast cells may play a role in the pathogenesis of IBD [5,6]. For example, it has been reported that mast cell infiltration increases in the colonic mucosa of active IBD patients [7], and that mucosal histamine levels are significantly elevated in UC patients [8]. Furthermore, the therapeutic agents used for the treatment of IBD patients can also inhibit the release of chemical mediators from intestinal mucosal-type mast cells (MMC) [9].
The oral administration of dextran sulphate sodium (DSS) induces experimental colitis in animal models, and these colonic lesions are similar to those observed in UC patients [10].
Therefore, the purpose of the present study was to elucidate the role of mast cell activation in the development of DSS-induced experimental colitis in rats. The role of mast cell activation was evaluated in mast cell-deficient Ws/Ws (white spotting in the skin) rats. Ws/Ws rats have a 12-base deletion in the tyrosine kinase domain of the c-kit cDNA, and are genetically deficient in both connective tissue-type (CTMC) and MMC [11]. These rats are useful for studying the role of mast cells in the pathogenesis of IBD.
MATERIALS AND METHODS
Animals
The origin of Ws/Ws rats has been described in detail elsewhere [12,13]. Ws/Ws rats with a large white spot and coat colour dilution were maintained by the mating of heterozygous Ws/+ rats at SCL, Inc. (Shizuoka, Japan). These matings generate Ws/Ws, Ws/+ and (+/+) rats. We used specific pathogen-free male Ws/Ws and (+/+) rats (n = 6), weighing 200–250 g, in this experiment. The rats were maintained under standard laboratory conditions with controlled temperature (20–22°C), humidity (50–60%) and a light–dark cycle (12 h:12 h). The experimental protocol was approved by the Animal Care and Use Committee of Shiga University of Medical Science.
Development of DSS colitis
The rats were fed standard rat chow CE-2 (Nippon Clea Inc., Tokyo, Japan), and were provided with distilled water containing 3% (w/v) DSS (mol. wt 5000 D, total sulphur 15.0–20.0%; Wako Pure Chemical Industries Ltd, Osaka, Japan) ad libitum for 10 days. The DSS intake was recorded daily, and rat body weight was also monitored daily during the experimental period.
Measurement of wet colonic weight and macroscopic evaluation of colitis
After 10 days of DSS administration, the rats were fasted overnight and anaesthetized with an i.p. injection of pentobarbital sodium (40 mg/kg). After the rats were killed by cervical dislocation, the body weight was measured and then a laparotomy was performed. The large intestine from the anus to the caecocolonic junction was removed, irrigated with chilled saline, cut along the anti-mesenteric border and then the wet weight was measured. To evaluate any macroscopic changes, the mucosa was photographed and the damaged mucosal area (colour change to purple and red, structural change with irregular folds, oedema and erosion) per total colonic surface was determined using an image analysis apparatus (NIH image version 4.0/Macintosh).
Histological examination
A specimen (10 × 10 mm) was removed at 1 cm distance from the anal margin, fixed in Carnoy's fixative for 3 h, and then embedded in paraffin. The histological samples were cut into 5 μm thick sections, and were stained with haematoxylin and eosin (H–E) after paraffin removal. The mucosal damage score was determined according to a previously described method [14]. The following three parameters were used: surface epithelial loss, crypt destruction and inflammatory cell infiltration into the mucosa. A score of 0–4 was then assigned to each parameter: 0 = no change; 1 = localized and mild; 2 = localized and moderate; 3 = extensive and moderate; and 4 = extensive and severe. The sum of the scores from all three parameters was defined as the mucosal damage score for each animal. In addition, toluidine blue staining (pH 2.5) was also performed. For the immunohistochemical detection of MMC, the sections were incubated with a polyclonal rabbit anti-rat mast cell protease II (RMCP II; Moredun, Edinburgh, UK) antibody, followed by reaction with a biotin-conjugated goat anti-rabbit IgG and a peroxidase–streptavidin complex system (Dako, Glostrup, Denmark), as described previously [15,16]. RMCP II is regarded as a specific marker of rat MMC [17].
Mucosal myeloperoxidase activity and mucosal protein content
Intestinal myeloperoxidase (MPO) activity, a biochemical maker of MPO+ granulocytes, was measured using a standard assay as described previously [18]. Briefly, a mucosal scraping from the remnant colon was placed in 1 ml of a hexadecyltrimethylammonium bromide solution (0.5%, w/w). The solution was then homogenized and sonicated, and the resulting homogenate was subjected to three rapid cycles of freezing and thawing. The samples were centrifuged in a microfuge to remove any insoluble material (14 000 rev/min, for 20 min), and the supernatants were used for the measurement of MPO activity with 0.0005% hydrogen peroxide as the substrate. The protein content of the supernatant was also measured according to the method of Lowry [19].
Measurement of intestinal mucosal histamine
The concentration of mucosal histamine was measured by high performance liquid chromatography (HPLC) using a minor modification of the previously described method [20]. Briefly, 800 μl of 10% aqueous TCA solution was added to 400 μl of the supernatant prepared as described above. After mechanical shaking and centrifugation, the supernatant was filtered through a membrane filter (pore size 0.45 μm) and injected into the HPLC system. The histamine in these samples was separated with three reverse phase columns in series (Cosmosil 5C18-AR; Nacalai Tesque Inc., Kyoto, Japan). The temperature was maintained at 40°C throughout this experiment. The histamine was then detected using a RF-535 fluorescence detector (Shimadzu, Kyoto, Japan) at excitation and emission wavelengths of 330 nm and 430 nm, respectively. The HPLC mobile phase was a mixture of 50 mm sodium borate buffer (pH 11) and acetonitrile (75:25, v/v), containing 2 mmo-phthalaldehyde (OPA) and 2 mm N-acetyl-l-cysteine (NAC), which was shielded from exposure to sunlight and was delivered isocratically at a flow rate of 0.5 ml/min.
Statistical analysis
The data were expressed as means ± s.e.m. The variance was analysed by the F-test. Subsequently, Student's t-test for unpaired values was performed to compare the means of the normally distributed data. Mann–Whitney U-test was also performed to compare the means of non-parametric or abnormally distributed data. P < 0.05 was considered statistically significant.
RESULTS
Wet colonic weight and damaged area
In the control (+/+) rats, diarrhoea occurred on days 2–3, and macroscopic bloody stools appeared on days 6–7. However, in the Ws/Ws rats, the diarrhoea and bloody stools did not occur during the experimental period. None of the rats in either group died during the experimental period. The DSS intake in the Ws/Ws rats was comparable to that of the control (+/+) rats (0.14 ± 0.02 ml/day per kg versus 0.12 ± 0.004 ml/day per kg, respectively). Significant differences in body weight gains between Ws/Ws rats and control (+/+) rats were observed during the experimental period (Table 1). The Ws/Ws rats remained healthy, and their body weight increased slightly. However, the body weight in the control (+/+) rats decreased gradually. The wet colonic weight per unit body weight was significantly lower in the Ws/Ws rats compared with control (+/+) rats, indicating that oedematous changes were prevented in the Ws/Ws rats (Table 1). Macroscopic examination of the colon revealed that hyperaemia, erosions and occasional tiny blood coagula occurred mainly in the rectum in both control (+/+) and Ws/Ws rats. However, this mucosal damage extended significantly further towards the oral side in the control (+/+) rats than in the Ws/Ws rats (Fig. 1).
Table 1.
The body weight gain, colonic wet weight per unit body weight, macroscopic colonic damaged area per entire colonic area and mucosal damage score
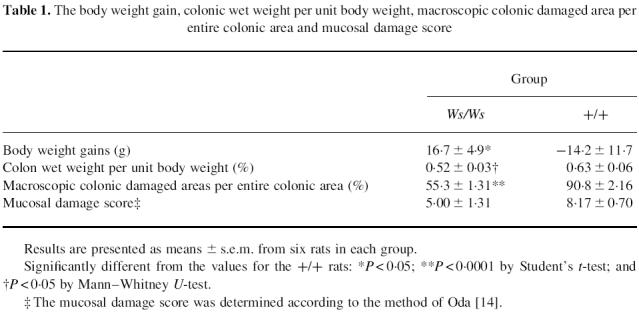
Fig. 1.
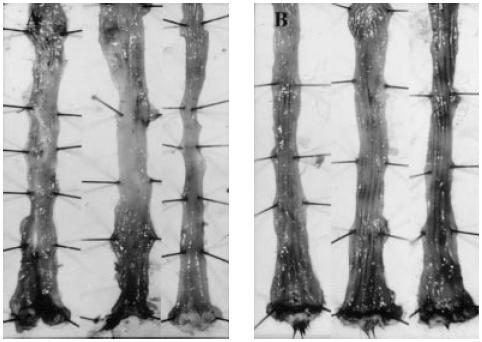
Dextran sulphate sodium (DSS)-induced colitis in Ws/Ws and control (+/+) rats. The rats were treated with a 3% DSS solution for 10 days. (A) Ws/Ws rats. (B) Control rats. The mucosal damage in the control rats extended further towards the oral side than the Ws/Ws rats.
Histological findings
H–E staining revealed a marked infiltration of inflammatory cells into the mucosa and submucosa in both control (+/+) and Ws/Ws rats. Crypt loss and surface epithelial loss were also evident (Fig. 2). The mucosal damage, as quantified by the scoring system, is summarized in Table 1. Mucosal damage scores in the Ws/Ws rats were lower than in control (+/+) rats, but these differences were not statistically significant (P = 0.06, Table 1). The oedematous changes in the mucosa and submucosa of control (+/+) rats were more severe than those observed in the Ws/Ws rats (Fig. 1). In the control (+/+) and Ws/Ws rats, toluidine blue staining revealed some mast cells and many macrophages containing DSS particles with metachromasia in the mucosa and submucosa (Fig. 3). RMCP II-immunopositive cells were scattered in the mucosa of the control (+/+) rats. In the Ws/Ws rats, RMCP II-immunopositive cells were also noted, but the number was lower (Fig. 4). In addition, sporadic globule leucocytes, which have been characterized as mast cells infiltrating into the epithelial layer, were also observed in both group of rats [21].
Fig. 2.
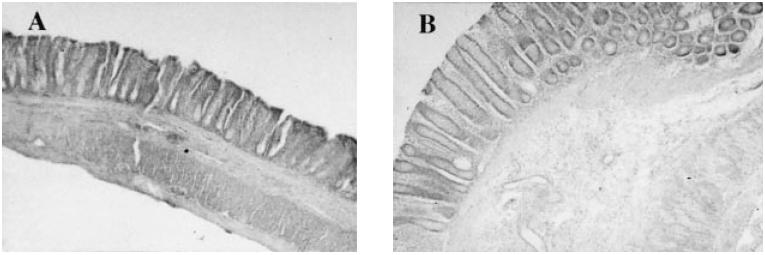
Microscopic findings of dextran sulphate sodium (DSS)-induced colitis in the Ws/Ws and control (+/+) rat. Rectal specimens taken at 1 cm from the anal margin were preserved in Carnoy's fixative, sectioned, and stained with H–E. (A) Ws/Ws rat, and (B) control (+/+) rat (× 100).
Fig. 3.
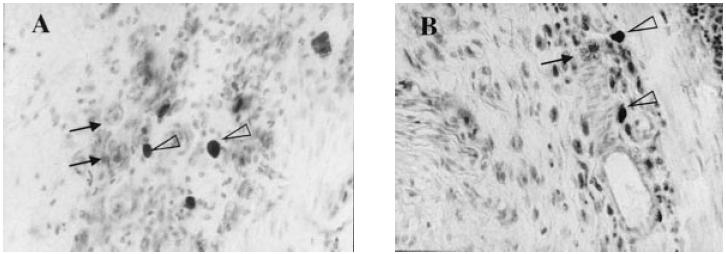
Microscopic findings of dextran sulphate sodium (DSS)-induced colitis in the Ws/Ws and control (+/+) rat. Toluidine blue staining was performed as described in Materials and Methods. (A) Ws/Ws rat. (B) Control (+%+) rat. Some mast cells (open triangle) and macrophages (arrow) containing ingested DSS particles, with metachromasia, were detected in the mucosa and submucosa (× 400).
Fig. 4.
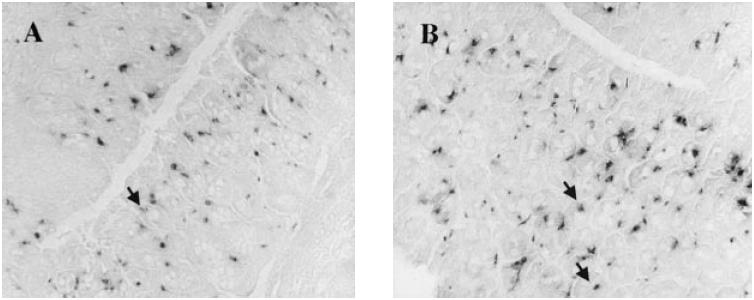
Rat mast cell protease II (RMCP II)-immunopositive cells in the mucosa. (A) Ws/Ws rat. (B) Control (+/+) rat. The RMCP II-immunopositive cells were scattered in the mucosa of Ws/Ws and (+/+) rats. Sporadic globule leucocytes, which are regarded as mast cells, infiltrating the epithelial layer, were also observed (arrow) (× 200).
Mucosal MPO activity, mucosal histamine level and mucosal protein content
The intestinal mucosal MPO activities in the Ws/Ws rats were comparable to those of control (+/+) rats (Table 2). On the other hand, the mucosal histamine levels in the Ws/Ws rats were significantly lower than those in control (+/+) rats (Table 2). The ratio of the wet weight to the protein content was used to evaluate the oedematous changes in the colonic tissue samples. A relative decrease in mucosal oedema was observed in Ws/Ws rats compared with control (+/+) rats, but there were no significant differences (P = 0.09, Table 2). A positive correlation was observed between histamine levels and degree of mucosal oedema in Ws/Ws rats (Fig. 5). A positive correlation was also found between mucosal histamine levels and the area of macroscopic damage in the colon (Fig. 5).
Table 2.
Colonic mucosal myeloperoxidase (MPO) activity, histamine level and degree of oedema
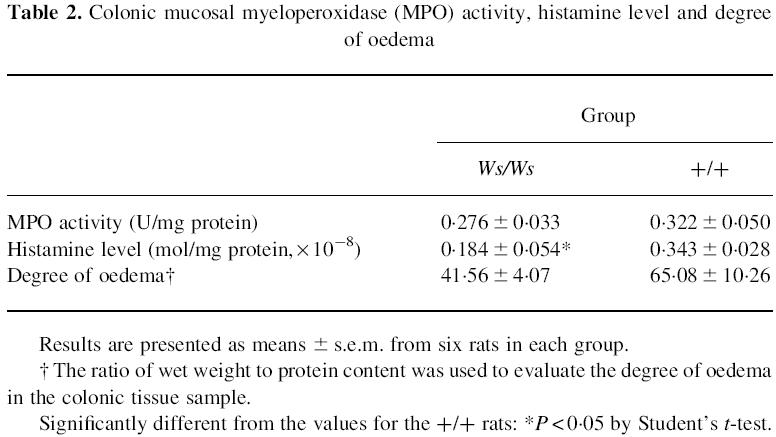
Fig. 5.
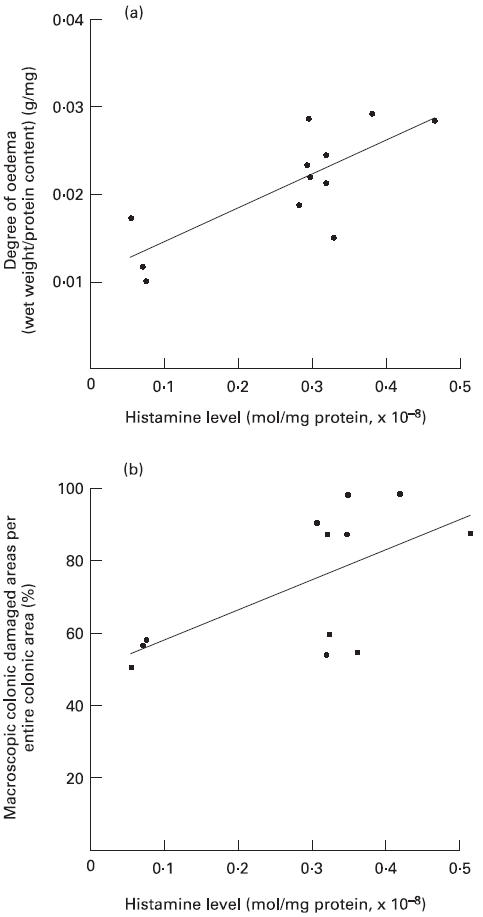
Relationship between mucosal histamine levels and mucosal damage markers. (a) Correlation between mucosal histamine levels and the degree of mucosal oedema (the ratio of the wet weight to the protein content in the colonic mucosa). The line represents the regression line for data from both Ws/Ws and control rats (Y = 0.038X − 0.011, R = 0.778, P < 0.01). (b) Correlation between mucosal histamine levels and the ratio of the macroscopically damaged area to the entire colonic surface (Y = 0.9074X + 0.4916, R = 0.623, P < 0.05).
DISCUSSION
Animal models of UC can be induced by the oral administration of sulphated polysaccharides such as DSS. It has been reported that models of DSS-induced colitis exhibit some of the clinical and histological features of UC patients. For example, several studies have reported that similar to UC patients, DSS initially induced colonic lesions in the distal colon, which spread to the whole colon [10,22–24]. In this model, the development of colitis is dependent on the molecular weight and sulphation of the DSS, in addition to the dosage and the duration of administration [22]. However, the precise mechanisms responsible for DSS-induced colitis remain unclear. With respect to the pathogenic factors in the development of DSS-induced colitis, previous reports have postulated the importance of various factors such as local immunological disturbance [25], the activation of mucosal macrophages [26], effects related to the strong negative charge of DSS [27], the obliteration of the crypt lumina [28], and changes in the intestinal microflora [29]. However, the role of intestinal MMC in the development of DSS colitis has not been fully evaluated.
To define the role of mast cells in the development of experimental colitis, two studies have reported on mast cell-deficient mice. One study reported that mast cells were not involved in the colitis induced by intrarectal administration of trinitrobenzene sulphonic acid (TNBS) in mast cell-deficient W/WV mice [18]. The other experiment revealed that oral DSS administration induced colitis in both mast cell-deficient W/WV and control (+/+) mice in a similar fashion [30]. In this study, we attempted to elucidate the potential role of mast cell activation in the pathogenesis of DSS-induced colitis in rats. To induce the colitis, we administered DSS to Ws/Ws and control (+/+) rats for 10 days in accordance with the procedures described in several previous reports [14,18,22,24]. In addition, because DSS-induced colitis occurs at the distal colon in both Ws/Ws and control (+/+) rats, we evaluated and compared the most severe lesions at the rectum. Mast cells have been reported to be closely associated with the development of tissue oedema and hyperaemia. In this process, the histamine released by mast cells plays an important role. In the present study, the development of colitis was significantly attenuated in the Ws/Ws rats. Moreover, a positive correlation between the development of colitis and mucosal histamine levels was observed. This indicates that the MMC contributed to the development of DSS-induced colitis at least via histamine release, although mast cell activation is accompanied by the release of other chemical mediators such as leukotrienes and/or PAF [31]. These results are also compatible with the previous report that ketotifen, an inhibitor of the release of chemical mediators from mast cells, prevented the development of DSS colitis [14].
We previously reported that there were few mast cells in the intact intestinal mucosa of Ws/Ws rats [32]. Interestingly, DSS administration over 10 days induced the recruitment of MMC in the colonic mucosa of Ws/Ws rats. This finding is consistent with the previous study from Arizono and co-workers, who reported that Nippostrongylus brasiliensis infection induces the appearance of intestinal MMC in Ws/Ws rats [33]. Alizadeh and co-workers also reported that a small number of MMC appear in the small intestine of W/WV mice after infection with Trichinella spiralis [34]. Since we detected a statistical correlation between the mucosal histamine levels and the macroscopic damaged area, it is feasible that the recruited MMC might have contributed to the process of mucosal damage in the Ws/Ws rats. In another report, significant increases in IL-3 levels were reported in the mesenteric lymph nodes of mice with DSS-induced colitis [35]. In general, IL-3 as well as the stem cell factor c-kitligand have been established as potent growth factors for mast cells. These observations suggest that DSS may be able to induce intestinal MMC recruitment through the stimulation of IL-3 generation. On the other hand, in previous studies of mast cell-deficient animals, there have been no descriptions of MMC recruitment in the colonic mucosa. Therefore, it is possible that the discrepancy between the present study and previous studies of mast cell-deficient animals is due to differences in MMC recruitment during the experimental period. It may be important to investigate the participation of recruited MMC in the development of experimental colitis in mast cell-deficient animals.
In conclusion, the development of DSS-induced colitis was attenuated in Ws/Ws rats. These results strongly suggest that mast cells play an important role in the development of DSS-induced colitis. Moreover, the sequential induction of MMC may affect tissue inflammation in Ws/Ws rats.
REFERENCES
- 1.Boros M, Kaszaki J, Nagy S. Histamine release during intestinal ischemia–reperfusion: role of irons and hydrogen peroxide. Circ Shock. 1991;35:174–80. [PubMed] [Google Scholar]
- 2.Boros M, Kaszaki J, Nagy S. Oxygen free radical induced histamine release during intestinal ischemia and reperfusion. Eur Surg Res. 1989;21:297–304. doi: 10.1159/000129042. [DOI] [PubMed] [Google Scholar]
- 3.Lehr HA, Guhlmann A, Nolte D, et al. Leukotrienes as mediators in ischemia–reperfusion injury in a microcirculation model in the hamster. J Clin Invest. 1991;87:2036–41. doi: 10.1172/JCI115233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubes P, Ibbotson G, Russell JM, et al. Role of platelet-activating factor in ischemia/reperfusion induced leukocyte adherence. Am J Physiol. 1990;259:G300–5. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- 5.McAuley RL, Somers SC. Mast cells in nonspecific ulcerative colitis. Am J Dig Dis. 1961;6:233–6. [Google Scholar]
- 6.Dvorak AM, Monahan RA, Osage JE, Dickersin GR. Crohn's disease: transmission electron microscopic studies. Immunologic inflammatory response. Alteration of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980;11:606–19. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- 7.Hiatt RB, Katz L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962;37:541–5. [PubMed] [Google Scholar]
- 8.Baenkler HW, Lux GG, Günthner R, Kohlhäufl M, Matek W. Biopsy histamine in ulcerative colitis and Crohn's disease. Hepatogastroenterology. 1987;34:289–90. [PubMed] [Google Scholar]
- 9.Fox CC, Moore WC, Lichtenstein LM. Modulation of mediator release from human intestinal mast cells by sulfasalazine and 5-amino-salicylic acid. Dig Dis Sci. 1991;36:179–84. doi: 10.1007/BF01300753. [DOI] [PubMed] [Google Scholar]
- 10.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 11.Niwa Y, Kasugai T, Ohno K, et al. Anemia and mast cell depletion in mutant rats that are homozygous at ^White Spotting (Ws)¤ locus. Blood. 1991;78:1936–41. [PubMed] [Google Scholar]
- 12.Tsujimura T, Hirota T, Nomura S, et al. Characterization of Ws mutant allele of rats: a 12-base deletion in tyrosine kinase domain of c-kit gene. Blood. 1991;78:1942–6. [PubMed] [Google Scholar]
- 13.Tei H, Kasugai T, Tsujimura T, et al. Characterization of cultured mast cells derived from Ws/Ws mast cell-deficient rats with a small deletion at tyrosine kinase domain of c-kit. Blood. 1994;83:916–25. [PubMed] [Google Scholar]
- 14.Oda T. Role of mast cells in dextran sulfate sodium-induced experimental colitis in rats. J Kyoto Pref Univ Med. 1995;104:1069–82. [Google Scholar]
- 15.Kimura T, Andoh A, Fujiyama Y, et al. A blockade of complement activation prevents rapid intestinal ischaemia–reperfusion injury by modulating mucosal mast cell degranulation in rats. Clin Exp Immunol. 1998;111:484–90. doi: 10.1046/j.1365-2249.1998.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanwer S, Kubes P. Mast cells contribute to ischemia–reperfusion-induced granulocyte infiltration and intestinal dysfunction. Am J Physiol. 1994;267:G316–21. doi: 10.1152/ajpgi.1994.267.2.G316. [DOI] [PubMed] [Google Scholar]
- 17.Kido H, Fukusen N, Katsunuma N. Chymotrypsin and trypsin type serine proteases in rat mast cells: properties and functions. Arch Biochem Biophys. 1985;239:436–43. doi: 10.1016/0003-9861(85)90709-x. [DOI] [PubMed] [Google Scholar]
- 18.Chin KW, Barrett KE. Mast cells are not essential to inflammation in murine model of colitis. Dig Dis Sci. 1994;39:513–25. doi: 10.1007/BF02088336. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosenbrough NJ, Farr AL, Udenfriend S. Protain measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–76. [PubMed] [Google Scholar]
- 20.Saito K, Horie M, Nose N, et al. Determination of polyamines in foods by liquid chromatography with on-column fluorescence derivatization. Anal Sci. 1992;8:675–80. [Google Scholar]
- 21.Murray M, Miller HRP, Jarrett WFH. The globule leukocyte and its derivation from the subepithelial mast cell. Lab Invest. 1968;19:222–34. [Google Scholar]
- 22.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 23.Axelsson LG, Midtvedt T, Bylund-Fellenius AC. The role of intestinal bacteria, bacterial translocation and endotoxin in dextran sodium sulphate-induced colitis in the mouse. Microb Ecol Health Dis. 1996;9:225–37. [Google Scholar]
- 24.Kanauchi O, Nakamura T, Agata K, Mitsuyama K, Iwanaga T. Effects of germinated barley foodstuff on dextran sulfate sodium-induced colitis in rats. J Gastroenterol. 1998;33:179–88. doi: 10.1007/s005350050067. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi K, Asakura H, Hamada Y, et al. T lymphocyte subpopulations and immunoglobulin-containing cells in the colonic mucosa of ulcerative colitis; a morphometric and immunohistochemical study. J Clin Lab Immunol. 1988;25:63–8. [PubMed] [Google Scholar]
- 26.Abraham R, Fabian RJ, Golberg L, et al. Role of lysosomes in carrageenan-induced cecal ulceratation. Gastroenterology. 1976;67:1169–81. [PubMed] [Google Scholar]
- 27.Watt J, Marcus R. Experimental ulcerative disease of the colon. Meth Achiev Exp Pathol. 1975;7:56–71. [PubMed] [Google Scholar]
- 28.Iwanaga T, Hoshi O, Han H, et al. Morphological analysis of acute ulcerative colitis experimentally induced by dextran sulfate sodium in the guinea pig: some possible mechanisms of cecal ulceration. J Gastroenterol. 1994;29:430–8. doi: 10.1007/BF02361239. [DOI] [PubMed] [Google Scholar]
- 29.Onderdonk AB, Hermos JA, Bartlett JG. The role of the intestinal microflora in experimental colitis. Am J Clin Nutr. 1977;30:1819–25. doi: 10.1093/ajcn/30.11.1819. [DOI] [PubMed] [Google Scholar]
- 30.Minocha A, Thomas C, Omar R. Lack of crucial role of mast cells in pathogenesis of experimental colitis in mice. Dig Dis Sci. 1995;40:1757–62. doi: 10.1007/BF02212698. [DOI] [PubMed] [Google Scholar]
- 31.Wasserman SI. Mediators of immediate hypersensitivity. J Allergy Clin Immunol. 1983;72:101–15. doi: 10.1016/0091-6749(83)90512-2. [DOI] [PubMed] [Google Scholar]
- 32.Andoh A, Kimura T, Fukuda M, Araki Y, Fujiyama Y, Bamba T. Rapid intestinal ischaemia–reperfusion injury is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin Exp Immunol. 1999;116:90–3. doi: 10.1046/j.1365-2249.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arizono N, Kasugai T, Yamada M, et al. Infection of Nippostrongylus brasiliensis induces development of mucosal-type but not connective tissue-type mast cells in genetically mast cell-deficient Ws/Ws rats. Blood. 1993;81:2572–8. [PubMed] [Google Scholar]
- 34.Alizadeh H, Murrell KD. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient W/Wv mice. J Parasitol. 1984;70:767–73. [PubMed] [Google Scholar]
- 35.Dieleman LA, Ridwan BU, Tennyson GS, et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]


