Abstract
We have previously observed that aged lupus-prone (NZB/NZW)Fl (BWF1) mice when infected with Plasmodium chabaudi show an improvement in their clinical lupus-like symptoms. In order to study the mechanisms involved in the long-lasting protective effect of the P. chabaudi infection in lupus-prone mice we analysed specific aspects of the cellular response, namely the profiles of cytokine mRNA expression and cytokine secretion levels in old BWF1 mice, in comparison with uninfected age-matched BWF1 mice and infected or uninfected BALB/c mice. Two months after infection, cells from BWF1 mice were stimulated with concanavalin A (Con A) and demonstrated a recovery of T cell responsiveness that reached the levels obtained with BALB/c cells. Old BWF1 mice showed high levels of interferon-gamma (IFN-γ) and IL-5 production and correspondingly low levels of IL-2 and IL-4 secretion before infection with P. chabaudi. Infection did not modify the IFN-γ levels of BWF1 T cells, whereas it considerably increased the secretion of the Th2-related cytokines IL-4, IL-5 and IL-10. In addition, only BWF1T cells showed increased mRNA expression of tumour necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β). This counter-regulatory cytokine network of infected BWF1 mice may be involved in the improvement of their lupus symptoms. The results of our investigations using the complex model of P. chabaudi infection can be extended and, by using more restricted approaches, it may be possible to explain the multiple regulatory defects of lupus-prone mice.
Keywords: lupus/(NZB/NZW)F1 mice, cytokines, Plasmodium chabaudi, Th cells
INTRODUCTION
The (NZB/NZW)F1 (BWF1) mouse is a murine model of systemic lupus erythematosus (SLE). The evolution of the disease is characterized by an abnormal polyclonal B cell activation [1] with a high production of various autoantibodies, including those directed against DNA and other nuclear antigens [2], and against cytoskeleton proteins [3]. Elevated circulating immune complexes lead to fatal glomerulonephritis in old mice [2]. There are reports of a lower incidence of human autoimmune diseases in some regions with endemic malaria [4]. Greenwood et al. described a higher survival rate in young lupus-prone mice infected with Plasmodiumberghei yoelii [5]. Previous observations in our laboratory established that young BWF1 mice infected with P. chabaudi and treated with chloroquine displayed a retarded development of their autoimmune disease or delayed onset of the clinical symptoms of lupus [6]. Furthermore, old BWF1 mice when infected with P. chabaudi at the onset of clinical signs of lupus and subsequently treated with chloroquine developed temporary remission of the symptoms [6]. Plasmodium chabaudi infection in normal mice induces the production of natural autoantibodies, probably with immunoregulatory properties [6]. It was observed that the injection of immunoglobulins isolated from P. chabaudi-infected BALB/c mice produced similar protective effects as the infection itself in BWF1 mice. In addition to these humoral mechanisms leading to long-lasting protection in BWF1 mice, it appears that regulation at the T cell level is also involved, a presumption based on the reduction of Vβ14 T cells found after immunoglobulin treatment [6].
It is known that an imbalance in cytokine synthesis is frequently associated with the development of autoimmune pathology. We have observed T cell cytokine abnormalities in BWF1 mice, such as high interferon-gamma (IFN-γ) mRNA expression and impairment in the secretion of IL-2, IL-4 and IL-10 during ageing [7]. This altered cytokine production in aged BWF1 mice is associated with the loss of T cell regulatory properties during times of autoantibody production [7]. On the other hand, murine malaria infection can influence the differentiation of Th cells contributing to the outcome of blood-stage infections as well as to adaptive immunity [8,9]. Anti-malarial immunity has been correlated to the shift of CD4 cells from Th1 to Th2 induced by malaria infection, involving cytokine production [10,11].
Since malaria was observed to induce better survival in BWF1 mice, we felt that the thorough analysis of T cell and cytokine behaviour throughout the course of infection could be helpful in understanding the remission of symptoms and improved survival rate. The aim of this study was to examine possible alterations in the expression of various cytokine genes and in cytokine secretion, comparing old BWF1 infected with P. chabaudi with non-infected mice. Additionally, these changes were compared with those obtained with infected or uninfected BALB/c mice.
MATERIALS AND METHODS
Mice
Female NZB and BALB/c mice were obtained from the animal colony of the Pasteur Institute and male NZW from the Centre de Sélection et d'Elevage des Animaux de Laboratoire (CNRS, Orleans, France). (NZB/NZW)F1 hybrid mice were bred in our animal facilities.
Parasite
Plasmodium chabaudi(clone F) was maintained by weekly passage in 3-month-old BALB/c mice. Female BWF1 or BALB/c mice (7 months old) were infected by i.p. injection of 108 parasitized erythrocytes as previously described [6,12]. To prevent death, mice were always given i.p. injections of chloroquine (40 mg/kg) in 0.2 ml saline on days 3 and 4 after parasite inoculation [6,12].
Antibodies
The following MoAbs were used throughout this study: anti-Thy1.2 (JlJ48), anti-CD4 (172-4), anti-CD8 (3.1.55); for cytokine-specific ELISA, unlabelled rat MoAb directed against murine cytokines: anti-IFN-γ (R46A2), anti-IL-2 (JES-61A12), anti-IL-4 (BVD4-lDl I), anti-IL-5 (TRFK5), anti-IL-10 (JES-52A5), and biotinylated rat MoAb such as anti-IL-2 (JES6-5H4), anti-IL-4 (BVD6-2462), anti-IL-5 (TRFK4) or anti-IL-10 (SXC1), anti-IFN-γ (AN-18.17.24, a kind gift from Dr Claude Leclerc, Institut Pasteur). All MoAb clones were obtained from the DNAX Research Institute for Molecular & Cellular Biology (Palo Alto, CA) and were prepared in the Unité d'lmmunoparasitologie. The specificity of these MoAbs has been described elsewhere [13–15].
Cell suspensions and proliferation assays
Cell suspensions were prepared from spleens and erythrocytes were removed by hypotonic shock. Purified splenic T cells were obtained, as described previously [7], by two cycles of negative panning using goat anti-mouse immunoglobulin, and were > 97% pure as scored by fluorescence staining in a FACSscan flow cytometer (Becton Dickinson, San José, CA). All procedures were carried out in a complete medium (RPMI 1640 medium containing 1 mm pyruvate, 5 U/ml penicillin, 50 μg/ml streptomycin, and 10% fetal calf serum (FCS; Gibco BRL, Grand Island, NY)). For the proliferation assay, spleen cells (105 cells/0.2 ml) were incubated in flat-bottomed 96-well plates in the absence or presence of mitogens at 37°C. Proliferation was measured by 3H-thymidine (TdR) incorporation after 72 h (concanavalin A (Con A), 5 μg/ml) or 96 h (lipopolysaccharide (LPS), 50 μg/ml) of stimulation in culture. 3H-TdR (Amersham, Aylesbury, UK), 0.5 μCi/well, was added 16 h prior to harvesting and the amount of radioactivity incorporated was determined in a β-counter.
Immunoglobulin isotype-specific ELISA
Serum samples collected from control and infected mice were evaluated for the presence of immunoglobulin isotypes using isotype-specific sandwich ELISA [7]. Unlabelled goat anti-mouse IgM, IgGl, IgG2a, IgG2b, IgG3 and IgA were used for coating; these same antibodies, β-galactosidase-conjugated, were used for detection, using mouse myeloma immunoglobulin as the standard. All were purchased from Southern Biotechnology Associates Inc. (Birmingham, AL). Enzyme activity was developed with o-nitrophenyl-β-d-galactopyranoside (ONPG; Sigma, St Louis, MO).
Cytokine-specific ELISA
The supernatants of 4 × 106 spleen cells incubated with 5 μg/ml of Con A were harvested after 24, 48, 72 or 96 h of culture, centrifuged and stored at − 20°C until tested. The presence of cytokines in the supernatants was assessed by sandwich ELISA using anti-cytokine MoAb, either unlabelled, for coating, or biotinylated, for detection, as described previously [7]. Cytokine contents were calculated by reference to standard curves constructed using known concentrations of murine rIFN-γ (PharMingen, San Diego, CA) or lymphokines derived from supernatants of the D10 Th2 cell line, the P815 mastocytoma cell line transfected with the IL-2 gene, the LTI-4 lymphoma transfected with the IL-4 gene or the J558 B cell hybridoma transfected with the IL-10 gene [7,15]. The detection limits of IFN-γ, IL-10, IL-5, IL-2 and IL-4 were 49 pg/ml, 0.24 ng/ml, 97.5 pg/ml, 49 pg/ml and 49 pg/ml, respectively.
RNA isolation, reverse transcription and semiquantitative polymerase chain reaction
Total RNA isolation of pools of freshly isolated splenic T cells were obtained from BWF1 and BALB/c mice, either uninfected or 2 months after P. chabaudi infection. cDNA was synthesized from RNA samples using Moloney leukaemia virus reverse transcriptase (Gibco) in the presence of oligo (dT) (Pharmacia Fine Chemicals, Uppsala, Sweden) [16]. Analysis of IL-2, IL-4, IL-10, IFN-γ, tumour necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β) gene expression and specificity of the primers and probes used has been described in detail elsewhere [15,17]. Conditions for polymerase chain reaction (PCR) amplifications of the housekeeping enzyme, hipoxanthine phosphoribosyltransferase (HPRT), dot blot and hybridization with a specific HPRT internal probe labelled with 32P-γ-ATP to confirm the HPRT level adjustments were as described previously [7,15,17]. The cDNA samples were titrated and standardized to 850 or 1450 pg equivalents of the HPRT housekeeping gene, to correct for differential mRNA expression between the samples. Autoradiograms were analysed either in a MASTERSCan (BIONIS-CSPI, Richebourg, France) or by direct counting of the membranes using a PhosphoImager (Molecular Dynamics, Sunnyvale, CA). After HPRT level adjustment, cDNA samples were amplified for lymphokine mRNA using specific pairs of primers. PCR products were dot blotted and hybridized with lymphokine-specific 32P-γ-ATP-labelled internal probes. mRNA pg equivalents were calculated for each sample after quantification of these final dot blots from the linear parts of the standard curves obtained with the Th2 clone, D10.G4.1 (anti-conalbumin, I-Ak) and Th1 clone, HDK1 (anti-keyhole limpet haemocyanin, I-Ad).
Statistical analysis
Student's t-test was applied to determine the statistical significance of differences observed.
RESULTS
T cell proliferative response of lupus-prone BWF1 mice infected with P. chabaudi
As described previously, when BWF1 mice were infected with P. chabaudi at 7 months of age, that is at the onset of clinical symptoms, 87% of the mice survived for 12 months and 12% for 14 months [6]. The majority of the animals from the uninfected group started to die at 7.5 months and none remained alive at 10 months. The chloroquine treatment alone (2 × 1.2 mg/mice) had no effect on the survival of mice [6].
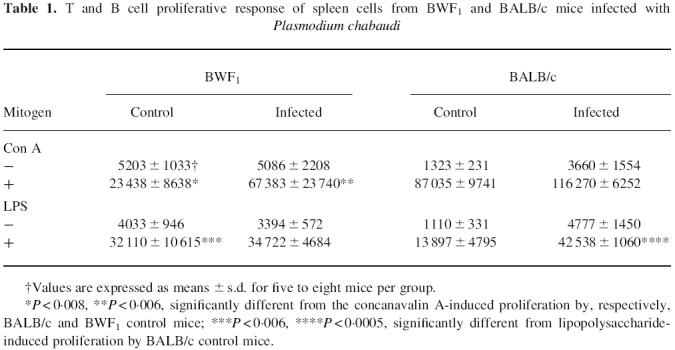
At 2 months after infection with P. chabaudi, when the parasitaemia had become undetectable, we evaluated the proliferative responses of spleen cells from BWF1 and BALB/c mice to Con A and LPS. As shown in Table 1, spleen cells from uninfected BWF1 mice were significantly less responsive to Con A (P < 0.008) than those from uninfected BALB/c mice. However, after infection, BWF1 spleen cells were able to mount a significant response to Con A (P < 0.006) compared with the control BWF1 cells. The already high LPS response from control BWF1 splenocytes was not modified with infection, whereas it was markedly increased (P < 0.0005) in infected BALB/c compared with the uninfected control (Table 1).
Table 1.
T and B cell proliferative response of spleen cells from BWF1 and BALB/c mice infected with Plasmodium chabaudi
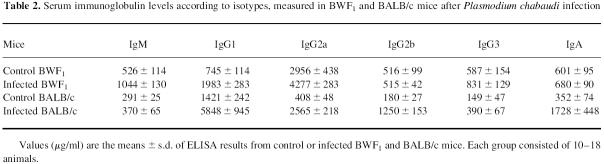
Serum immunoglobulin levels measured after P. chabaudi infection
The ability of BWF1 cells to proliferate in vitro after LPS stimulation, shown in Table 1, probably reflects the potential of B cells to secrete high immunoglobulin levels in vivo. Table 2 shows that uninfected BWF1 mice secreted more immunoglobulin than BALB/c mice, except for IgG1. Among the IgG isotypes, uninfected BWF1 mice displayed IgG2a levels seven times higher than BALB/c mice. The infection of BWF1 mice led to a moderate increase (× 2) in IgM and IgGl levels, while the other isotypes were slightly raised or remained unchanged (Table 2). In contrast, the infection of BALB/c mice induced an increase in all IgG isotype levels, especially IgG2a (× 6), IgG2b (× 6), and IgA levels (× 5), whereas the IgM level was not modified (Table 2).
Table 2.
Serum immunoglobulin levels according to isotypes, measured in BWF1 and BALB/c mice after Plasmodium chabaudi infection
Profiles of cytokine secretion in BWF1 and BALB/c mice infected with P. chabaudi
Lupus-prone BWF1 mice secreted higher amounts of IFN-γ after Con A stimulation than BALB/c mice. The P. chabaudi infection did not modify the level of secretion in either strain of mice (Fig. 1a). In contrast, BWF1 mice produced lower amounts of IL-2 compared with BALB/c mice and the infection significantly increased the IL-2 secretion in both strains (Fig. 1b).
Fig. 1.
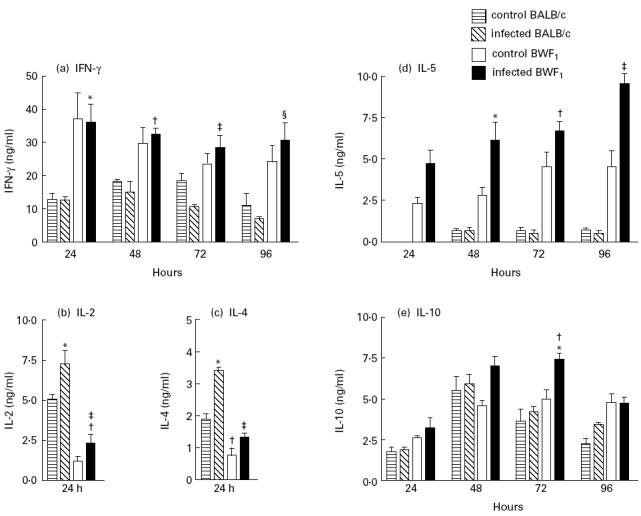
Cytokine secretions by spleen cells from BWF1 and BALB/c mice infected with Plasmodium chabaudi. Supernatants from concanavalin A-stimulated spleen cells were tested by ELISA for the presence of IFN-γ (a), IL-2 (b) IL-4 (c), IL-5 (d) and IL-10 (e). Results are expressed as the mean ± s.e.m. from six to eight supernatants per time point. (a) *P ≤ 0.015; †P ≤ 0.004; ‡P ≤ 0.002; §P ≤ 0.01, significantly different from infected BALB/c. (b) *P ≤ 0.002, significantly different from control BALB/c; ‡P ≤ 0.05, †P ≤ 0.0004, significantly different from, respectively, control BWF1 and BALB/c. (c) *P ≤ 0.02, ‡P ≤ 0.008, significantly different from their respective uninfected controls; †P ≤ 0.0003, significantly different from control BALB/c. (d) *P ≤ 0.03, †P ≤ 0.016, ‡P ≤ 0.008, significantly different from control BWF1. (e) *P ≤ 0.004, †P ≤ 0.006, significantly different from, respectively, control and infected BALB/c.
The secretion of Th2-related cytokines, IL-4, IL-5 and IL-10, is shown in Fig. 1c,d,e, respectively. BWF1 mice secreted lower amounts of IL-4 compared with BALB/c mice, and P. chabaudi infection led to increased secretion in both strains of mice (Fig. 1c). High amounts of IL-5 secretion were already observed in uninfected BWF1 mice and these increased progressively up to 96 h after Con A stimulation, and infection with P. chabaudi enhanced this pattern (Fig. 1d). In contrast, BALB/c mice infected or uninfected secreted low levels of IL-5. Both mouse strains secreted considerable amounts of IL-10 which only increased significantly 72 h after Con A stimulation in infected BWF1 mice (Fig. 1e).
T cell lymphokine mRNA expression after P. chabaudi infection
To determine the pattern of lymphokine gene expression, freshly purified splenic T cell preparations from control and infected mice were assessed using semiquantitative PCR [7,15,17].
BWF1 T cells expressed abnormally high amounts of constitutive IFN-γ messenger RNA compared with BALB/c cells (Fig. 2a). Infection with P. chabaudi did not alter this expression in BWF1 cells, whereas it induced an considerable increase in BALB/c cells (Fig. 2a). The expression of IL-2 mRNA was modified by parasite infection only in BWF1 T cells, increasing two-fold (Fig. 2b). IL-4 mRNA expression was slightly lower in BWF1 than in BALB/c T cells and, after infection, the level increased six times in BWF1 and 10 times in BALB/c T cells (Fig. 2c). However, the amount of IL-4 mRNA in infected BWF1 T cells remained low (30 pg/equivalents of HPRT) when compared with that of infected BALB/c T cells (135 pg/equivalents of HPRT). In contrast, IL-10 mRNA expression in BWF1 mice was higher than in BALB/c mice and increased considerably (× 4) after infection (Fig. 2d). A marked increase in TNF-α (Fig. 2e) and TGF-β (Fig. 2f) mRNA levels was detected in BWF1 T cells after infection, whereas the level of TGF-β was decreased in infected BALB/c T cells compared with uninfected control T cells. The search for IL-5 mRNA, carried out in all analysed T cell preparations, did not lead to the detection of this cytokine, even though the samples were standardized to 1450 pg/equivalents of HPRT gene expression.
Fig. 2.
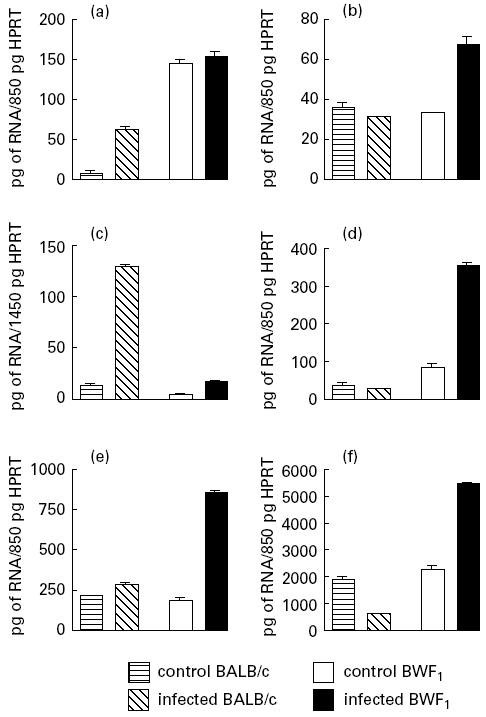
T cell cytokine gene expression in BWF1 and BALB/c mice infected with Plasmodium chabaudi. IFN-γ (a), IL-2 (b), IL-4 (c), IL-10 (d), tumour necrosis factor-alpha (TNF-α) (e) and transforming growth factor-beta (TGF-β) (f) gene expressions were assessed by semiquantitative polymerase chain reaction (PCR) analysis. RNA was extracted from 106 freshly purified splenic T cells from infected or control BWF1 and BALB/c mice. Different amounts of RNA were co-reverse transcribed in parallel with known concentrations of RNA obtained from the standard Th1 cell line (HDKI). All samples were amplified by PCR using specific primers for the housekeeping hipoxanthine phosphoribosyltransferase (HPRT) gene, followed by dot blot hybridization with an HPRT internal probe labelled with 32P-γ-ATP. All samples, adjusted to equivalent amounts of 850 pg or 1450 pg of the HPRT transcript (see Materials and Methods) were amplified with IFN-γ-, IL-2-, IL-4-, IL-10-, TNF-α- and TGF-β-specific primers, in parallel with cDNA from the standard Th1 or Th2 cell line. Semiquantitative measurements were obtained by Phosphoimager scanning of the membranes from which the ct/min per dot were obtained. Results are expressed as RNA pg equivalents for each lymphokine calculated from the respective Th1 and Th2 standard curves.
DISCUSSION
Plasmodium chabaudi infection in old BWF1 mice, induced at the onset of clinical symptoms (7 months old), considerably alters the profile of Th2-related cytokines and increases TGF-β and TNF-α mRNA expression, amongst others.
The decreased T cell proliferative response to Con A, already described in old BWF1 mice in comparison with their normal parental NZW mice [7] and also with BALB/c mice [18], was partially restored after infection, and this recovery may be due to an increase in IL-2 production, confirmed by our assays of protein secretion and mRNA expression. This indicates that the defect in IL-2 secretion observed in BWF1 mice presenting clinical signs of lupus is reversible, suggesting the absence of an intrinsic cell defect. In other words, BWF1 T cells previously unable to proliferate because of deficient IL-2 production are stimulated by the P. chabaudi infection.
The high IFN-γ mRNA constitutive levels observed in BWF1 mice remained stable after infection. Indeed, it has been postulated that IFN-γ worsens autoimmune disease, as demonstrated by the aggravation of glomerulonephritis in BWF1 mice after treatment with IFN-γ, aggravation which could be reversed by the administration of anti-IFN-γ antibody [19]. TGF-β mRNA was expressed at high levels in T cells from BWF1 mice as a consequence of infection, and the well-known anti-inflammatory properties of this cytokine could eventually be one of the factors promoting the improvement of clinical symptoms of lupus. In fact, TGF-β is recognized as a regulator of the inflammatory response that occurs in various types of autoimmune disease, especially those mediated by excessive Th1 responses, such as experimental autoimmune encephalomyelitis and experimental colitis [20,21]. Furthermore, TGF-β somatic gene therapy has been described to prevent autoimmune disease in the MRL/lpr/lpr murine model of SLE and in non-obese diabetic mice [22,23].
TNF-α, another important cytokine whose mRNA levels in T cells of aged BWF1 mice were observed to be enhanced by the P. chabaudi infection, is known to play an important role in lupus autoimmune disease. BWF1 mice are considered to be poor TNF-α producers due to a genetic abnormality derived from their NZW parent [24]. Replacement therapy with recombinant TNF-α [25] prolongs the survival of BWF1 mice, suggesting that the treatment supplements the low TNF-α production. A broad range of TNF-α activities has been identified, and one of them opposes the regulatory effect on class II MHC antigen expression induced by IFN-γ [26]. The enhanced expression of TNF-α mRNA induced by P. chabaudi infection suggests a beneficial effect to BWF1 mice somehow modulating the deleterious effect caused by IFN-γ. Ishida et al. [27] described an interesting interaction between TNF-α and IL-10, the production of which we also found to be enhanced after P. chabaudi infection. They associated the recovery of clinical symptoms with the continuous administration of anti-IL-10 antibodies to BWF1 mice, and this was apparently due to an up-regulation of endogenous TNF-α. In our model, the stimulation of IL-10 production in BWF1 after P. chabaudi infection did not worsen the disease and, in spite of its already described potent inhibitory action on IFN-γ secretion [28], did not interfere with this aspect either.
In contrast to BWF1 mice, the only disturbances triggered by P. chabaudi infection in the cytokine profile of BALB/c mice were a dramatic increase in IL-4 synthesis and a moderate increase in production of IFN-γ. Usually, in the malaria murine infection studied in non-autoimmune strains, CD4+ T cells play a major role in protective immunity against the blood stage, leading to a shift from Th1 to Th2 cell subsets that occurs during the period of final clearance of the parasite load [10,11]. The protective effect of Th2 cells is exerted by the enhancement and accelerated production of specific IgG1 antibodies [10], an isotype dependent on IL-4 secretion [29]. Apart from the peculiarities of each mouse strain, the changes observed in the cytokine production of infected BALB/c mice corresponded with the recovery period of the murine malaria infection.
One of the characteristics of BWF1 mice is a polyclonal B cell hyperactivity that leads to high levels of autoantibodies [2,3] and immunoglobulins. It is worth noting that BWF1 mice produce high levels of IgG2a, a situation which differs from normal BALB/c mice that present IgG1 as the predominant IgG subclass. This can be explained by the high IFN-γ production observed in BWF1 mice, which has been reported to induce a switch to IgG2a production [29]. In addition, the decreased IgG1 levels of BWF1 mice, an isotype dependent on IL-4, was enhanced by infection, which brings IgG1 as well as IL-4 secretions near to the normal levels of control BALB/c mice.
The B cell hyper-responsiveness to T cell-derived stimuli has been described as an intrinsic defect characteristic of BWF1 mice [30]. Interestingly, BWF1 mice were able to secrete a high level of IL-5 that was enhanced with P. chabaudi infection. It is known that large numbers of cells belonging to the Ly1 B cell subset (B1 cells) are found in BWF1 mice, as in their parental NZB strain [31]. Most members of B1 cells express IL-5 receptors [32] and IL-5, in turn, can stimulate autoantibody production, especially of the IgM isotype [33]. The pronounced ability of BWF1 splenocytes to secrete IL-5 could explain the high IgM level found in uninfected BWF1 mice, which even doubled after P. chabaudi infection, but we did not investigate whether the specificity of this enhanced IgM production related to an increase in the amount of autoantibodies. Whether or not the B1 cell subset is implicated in the inherent lupus pathogenesis of BWF1 mice remains to be established.
In addition, it is possible that P. chabaudi infection may induce the synthesis of regulatory immunoglobulin populations that equilibrate the disturbed ‘natural autoantibody network’ present in aged BWF1 mice [3,34]. The hypothesis of a mechanism attributable to an anti-idiotypic-like control is reinforced by the finding that treatment of BWF1 mice with immunoglobulin fractions, isolated from P. chabaudi-infected BALB/c mice, also caused remission of their lupus disease symptoms [6].
Taken together, these results suggest that P. chabaudi infection partially restored the immune imbalance of BWF1 mice, supported by a counter-regulatory cytokine network. The immunological changes throughout the course of P. chabaudi infection are multiple, but the results of our investigations in this complex model lead to the choice of more straightforward and controllable ways of assessing and/or reversing the regulatory defects of lupus-prone mice.
Acknowledgments
This work was supported by grant 6257 from the Association pour la Recherche sur le Cancer and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP). The expert assistance of Waltraut Lay in re-reading the manuscript and that of Mrs C. Corel in preparing it are greatly appreciated. M.N.S. is supported by a fellowship from Fundaça~o de Amparo a Pesquisa do Estado de São Paulo, FAPESP, São Paulo, Brasil.
REFERENCES
- 1.Theofilopoulos AN, Dixon J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 2.Merino RM, Iwamoto M, Fossati L, Izui S. Polyclonal B cell activation arises from different mechanisms in lupus-prone (NZB × NZW) F1 and MRL/MpJ-Ipr/Ipr mice. J Immunol. 1993;152:6509–16. [PubMed] [Google Scholar]
- 3.Hentati B, Ternynck T, Avrameas S, Ternynck T. Comparison of natural antibodies to autoantibodies arising during lupus in (NZB × NZW) F1 mice. J Autoimmun. 1991;4:341–56. doi: 10.1016/0896-8411(91)90029-c. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood BM, Herrick EM, Voller A. Can parasitic infection suppress autoimmune disease? Proc Roy Soc Med. 1970;63:19–20. [PMC free article] [PubMed] [Google Scholar]
- 5.Greenwood BM, Herrick EM, Voller A. Suppression of autoimmune disease in NZB and (NZW × NZB) F1 hybrid mice by infection with malaria. Nature. 1970;226:266–7. doi: 10.1038/226266a0. [DOI] [PubMed] [Google Scholar]
- 6.Hentati B, Sato MN, Payelle-Brogard B, Avrameas S, Ternynck T. Beneficial effect of polyclonal immunoglobulins from malaria-infected BALB/c mice on the lupus-like syndrome of (NZB × NZW) F1 mice. Eur J Immunol. 1994;24:8–15. doi: 10.1002/eji.1830240103. [DOI] [PubMed] [Google Scholar]
- 7.Sato MN, Minoprio P, Avrameas S, Ternynck T. Defects in the regulation of anti-DNA antibody production in aged lupus-prone (NZB × NZW) F1 mice: analysis of T-cell lymphokine synthesis. Immunology. 1995;85:26–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs P, Radzioch D, Stevenson MM. Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–41. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Souza JB, Williamson KH, Otani T, Playfair JH. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun. 1997;65:1593–8. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor-Robinson AW, Phillips RS, Severn A. The role of Th1 and Th2 cells in a rodent malaria infection. Science. 1993;260:1931–4. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson MM, Tam MF. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi infection in resistant and susceptible mice. Clin Exp Immunol. 1993;91:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ternynk T, Falanga PB, Unterkirscher C, Gregoire L, Pereira S, Avrameas S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudi. Int Immunol. 1991;3:29–37. doi: 10.1093/intimm/3.1.29. [DOI] [PubMed] [Google Scholar]
- 13.Abrams JS, Roncarolo MG, Yssel H, Andersson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 14.Prat M, Gribaudo G, Comoglio PM, Cavallo G, Landolfo S. Monoclonal antibodies against murine γ interferon. Proc Natl Aca Sci USA. 1984;81:4515–9. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minoprio P, El Cheik MC, Murphy E, Hontebeyrie-Joskowicz M, Coffman R, Coutinho A, O'garra A. Xid-associated resistance to experimental Chagas' disease is IFN-γ dependent. J Immunol. 1993;152:4200–8. [PubMed] [Google Scholar]
- 16.Krug MS, Bertger SL. First-strand cDNA synthesis primed with oligo (dt) Methods Enzymol. 1987;152:316–25. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- 17.Murphy E, Hieny S, Sher A, O'garra A. Detection of in vivo expression of interleukin-10 using a semi-quantitative polymerase chain reaction method in Schistosoma mansoni infected mice. J Immunol Methods. 1993;162:211–23. doi: 10.1016/0022-1759(93)90386-l. [DOI] [PubMed] [Google Scholar]
- 18.Altman A, Theofilopoulos AN, Weiner R, Katz DH, Dixon FJ. Analysis of T cell function in autoimmune murine strains. Defects in production of and responsiveness to interleukin 2. J Exp Med. 1981;154:791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob CO, van der Meide PH, Mcdevitt HO. In vivo treatment of (NZB × NZW) F1 lupus-like nephritis with monoclonal antibody to γ interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Inobe J-I, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+ cells mediate active suppression. J Immunol. 1995;155:910–6. [PubMed] [Google Scholar]
- 21.Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β mediated oral tolerance. J Exp Med. 1996;183:2605–16. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz E, Dudler J, Lotz M, Baird SM, Berry CC, Eisenberg RA, Carson DA. Modulation of disease activity in murine systemic lupus erythematosus by cytokine gene delivery. Lupus. 1995;4:286–92. doi: 10.1177/096120339500400409. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo CA, Chang Y, Prud'homme GJ. TGF-β1 somatic gene therapy prevents autoimmune disease in nonobese diabetic mice. J Immunol. 1998;161:3950–6. [PubMed] [Google Scholar]
- 24.Jacob CO, MacDevitt HO. Tumour necrosis factor-α in murine autoimmune ‘lupus’ nephritis. Nature. 1988;331:356–8. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- 25.Gordon C, Ranges GE, Greenspan JS, Wosfy D. Chronic therapy with recombinant tumor necrosis factor-α in autoimmune NZB/NZWF1 mice. Clin Immunol Immunopathol. 1989;52:421–34. doi: 10.1016/0090-1229(89)90157-8. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Jacob CO. Regulation of MHC class II antigen expression. Opposing effects of tumor necrosis factor-α on IFN-γ-induced HLA-DR and Ia expression depends on the maturation and differentiation stage of the cell. J Immunol. 1991;146:899–905. [PubMed] [Google Scholar]
- 27.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/NZW F1 mice. J Exp Med. 1994;179:305–10. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cell. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 29.Snaper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 30.Jongstra-Bilen J, Vukusic B, Boras K, Wither JE. Resting B cells from autoimmune lupus-prone New Zealand Black and (New Zealand Black × New Zealand White) F1 mice are hyper-responsive to T cell-derived stimuli. J Immunol. 1997;159:5810–20. [PubMed] [Google Scholar]
- 31.Hardy R, Carmarck CE, Li YS, Hayakawa K. Distinctive developmental origins and specificities of murine CD5+ B cells. Immunol Rev. 1994;137:91–118. doi: 10.1111/j.1600-065x.1994.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton AM, Lehuen A, Kearney JF. Immunofluorescence analysis of B-1 cell ontogeny in the mouse. Int Immunol. 1994;6:355–61. doi: 10.1093/intimm/6.3.355. [DOI] [PubMed] [Google Scholar]
- 33.Umland SP, Go N, Cupp JE, Howard M. Responses of B cells from autoimmune mice to IL-5. J Immunol. 1989;142:1528–35. [PubMed] [Google Scholar]
- 34.Avrameas S, Ternynck T. The natural autoantibodies system: between hypotheses and facts. Mol Immunol. 1993;30:1133–42. doi: 10.1016/0161-5890(93)90160-d. [DOI] [PubMed] [Google Scholar]