Abstract
Intraperitoneal administration with anti-CD86 (B7.2) MoAb into the murine model for primary SS in NFS/sld mutant mice resulted in dramatically inhibitory effects on the development of autoimmune lesions, while no significant effects were observed when the mice were administered with anti-CD80 (B7.1) MoAb. We found that spleen cells in the murine SS model treated with anti-CD86 MoAb showed a significant impairment of autoantigen-specific T cell proliferation. T cell activation markers (CD44high, CD45RBlow, Mel-14low) were significantly down-regulated in the spleen cells gated on CD4 in anti-CD86-treated mice. We detected a higher level of cytokine production of IL-4 from splenic T cells in anti-CD86-treated mice, but not of IL-2, and interferon-gamma (IFN-γ), compared with those in the anti-CD80- and PBS-treated SS model. Moreover, serum autoantibody production against α-fodrin autoantigen was almost entirely suppressed in anti-CD86-treated mice. These data provide strong evidence that in autoimmune exocrinopathy resembling SS in NFS/sld mutant mice, the CD86 costimulatory molecule plays a crucial role in the initiation and subsequent progression of Th1-mediated autoimmunity in the salivary and lacrimal glands.
Keywords: CD86 costimulation, preventive effect, α-fodrin, autoantigen, Th2 cytokine, Sjögren's syndrome
INTRODUCTION
It is well known that the initiation and maintenance of a T cell response to antigen require at least two signals [1,2]. One signal is delivered through binding of the antigen-specific T cell receptor to its MHC ligand that displays processed antigen on the surface of antigen-presenting cells (APC), and the second signal is delivered by costimulatory molecules expressed on the same APC [3–8]. Costimulatory molecules of the B7 family, including CD80 (B7.1) and CD86 (B7.2), provide key signals for the generation of T cell responses by interacting with the CD28 and CTLA-4 counter-receptors on the T cell [3,4,6,9,10]. Previous studies showing that CD80 activates antigen-primed but not naive T cells led to the suggestion that CD86-mediated costimulation is important in the initiation phase of a T cell response, whereas the role of CD80 may be to maintain and expand the ongoing T cell response [11–14].
SS is a chronic autoimmune exocrinopathy affecting the salivary and lacrimal glands and leading to clinical symptoms of dryness of the mouth and eyes (sicca syndrome) [15–17]. In primary SS patients, a wide spectrum of extraglandular manifestations may occur, including vasculitis, thyroiditis, nephritis, pneumonitis, neuropathy, and lymphoproliferation, besides sicca syndrome [16]. In our laboratories we have established an animal model for primary SS in NFS/sld mutant bearing an autosomal recessive gene with sublingual gland differentiation arrest [18,19]. Autoimmune lesions in this model are mediated by CD4+ T cells, and tissue infiltrating autoreactive T cells have revealed the presence of mRNA for Th1-type cytokines including IL-2, interferon-gamma (IFN-γ), but not for Th2-type cytokines [20,21]. Recently, we identified the 120-kD α-fodrin autoantigen from the salivary gland tissues of this murine SS model, and determined T cell responses specific to this protein besides in vitro production of IL-2 and IFN-γ [22]. It was suggested that the 120-kD α-fodrin molecule may be an important autoantigen in the pathogenesis of SS. Although blocking CD80 and CD86 has been shown to have differential effects on autoimmune responses in vivo depending upon the different disease models studied, the role of B7 costimulation on the development of Th1-mediated autoimmune exocrinopathy in the murine SS model has not yet been investigated.
This study demonstrates that the preventive effects of in vivo administration with anti-CD86 MoAb, but not with anti-CD80 MoAb, were clearly observed in the murine SS model, and analyses the mechanisms during immunotherapeutic effects through the Th1-mediated autoimmunity and cytokine balance.
MATERIALS AND METHODS
Mice and experimental protocol
Female NFS/N strain carrying the mutant gene sld (NFS/sld) [18] were reared in our specific pathogen-free mouse colony, and given food and water ad libitum. Establishment and characterization of this murine SS model in NFS/sld mice have been reported previously [19–21]. Autoimmune lesions in the murine SS model are mediated by CD4+ T cells, and tissue-infiltrating T cells have revealed the presence of mRNAs for Th1-type cytokines including IL-2 and IFN-γ, but not for Th2-type cytokines [20,21]. To analyse the preventive effect of treatment with antibodies to B7 costimulatory signal, RM80 (anti-CD80, rat IgG2a) and PO3 (anti-CD86, rat IgG2b) were used in in vivo studies. Each MoAb was injected intraperitoneally with a once a week dose of 100 μg of either anti-CD80 MoAb (RM80) (n = 8), or anti-CD86 MoAb (PO3) (n = 8). These groups were compared with controls treated with PBS alone (n = 7). At 3 weeks before disease onset, the i.p. injection schedule with these MoAbs was started, and treated until 7 weeks. At 8 weeks these mice were killed, and analysed from a variety of approaches. We compared these treated groups with 3d-thymectomized (Tx) NFS/sld mice as non-treated positive controls (n = 15), and non-Tx NFS/sld mice were used as non-treated negative controls (n = 12).
Histology and immunohistochemistry
All organs were removed from the mice, fixed with 4% phosphate-buffered formaldehyde pH 7.2 and prepared for histological examination. The sections were stained with haematoxylin and eosin (H–E). Histological grading of the inflammatory lesions was done according to the method proposed by White & Casarett [23] as follows: score 1 indicates one to five foci composed of more than 20 mononuclear cells per focus were seen; score 2, more than five such foci were seen but without significant parenchymal destruction; score 3, degeneration of parenchymal tissue; score 4, extensive infiltration of the glands with mononuclear cells and extensive parenchymal destruction. Immunohistochemical staining with MoAbs was performed on freshly frozen sections utilizing the biotin-avidin immunoperoxidase method. Briefly, frozen sections approximately 4 μm in thickness were fixed in acetone for 5 min, rinsed in PBS pH 7.2, and incubated with each of the first antibodies as follows: biotinylated rat MoAbs to CD3 (Gibco BRL, Grand Island, NY), B220, CD4, CD8, Mac-1 (Becton Dickinson, Burlingame, CA), CD28, B7.1 (CD80), and B7.2 (CD86) (PharMingen, San Diego, CA), and incubated with biotinylated anti-rat and anti-hamster IgG (Tago Inc., Burlingame, CA), followed by ABC complex reagent (Vector Labs Inc., Burlingame, CA). All control samples treated with normal rat and hamster serum (Cappel Labs, Cochranville, PA) or PBS instead of the first antibodies gave negative results. Infiltrating mononuclear cells staining positively with MoAbs including CD28, CD4, CD8 and B220, were enumerated using a 10 × 20-μm grid net disc covering an objective of area 0.16 mm2.
Isolation of mouse salivary gland epithelial cells
Primary culture of mouse salivary gland epithelial cells was prepared as described previously [24]. Briefly, the epithelial cells were from NFS/sld mice at 3–5 weeks and prepared by enzymatic digestion with 0.76 mg/ml EDTA and a mixture of collagenase (type I: 750 U/ml) and hyaluronidase (type IV: 500 U/ml), plated in 24-well plates (250 000 cells/well), and maintained in minimal essential medium (MEM) containing 10% fetal calf serum (FCS) for 10–14 days. These primary cultures contained a mixture of epithelial cells (85–95%) and fibroblasts (1–5%). Cultured cells were used for flow cytometric analysis.
Flow cytometric analysis
Spleen cells from treated and control 3d-Tx NFS/sld mice were analysed by flow cytometry, and compared with those from non-Tx NFS/sld mice. Single-cell suspensions were stained with antibodies conjugated to PE (anti-CD3, Gibco BRL; anti-CD4, Cedar Lane Labs Ltd, Ontario, Canada; B220, PharMingen), and FITC (anti-CD8, Cedar Lane Labs; Thy1.2, anti-CD80, anti-CD86, anti-CD28; anti-CD44, anti-CD45RB, and Mel-14, PharMingen), and analysed with EPICS (Coulter, Hialeah, FL). To analyse activation markers, regional lymph node (LN) cells were also prepared. Cells were gated according to size and scatter to eliminate dead cells and debris from analysis. Single-cell suspensions of spleen cells and primary cultured mouse salivary gland cells were stained with antibodies conjugated to PE (anti-I-As; PharMingen) and FITC (anti-CD80 and anti-CD86; PharMingen), and analysed with EPICS.
Detection of cytokines by ELISA
Levels of IL-2, IL-4 and IFN-γ in culture supernatants of spleen cells from each experimental group of mice activated with immobilized anti-CD3 MoAb (Cedar Lane Labs) for 3 days were measured by ELISA using the mIL-2 and mIL-4 ELISA systems (Amersham, Arlington Heights, IL), and IFN-γ-specific MoAb (PharMingen), respectively [22].
Proliferation assay
Single-cell suspensions of spleen cells from treated and control 3d-Tx NFS/sld mice were cultured in 96-well flat-bottomed microtitre plates (5 × 105 cells/well) in RPMI 1640 containing 10% FCS, penicillin/streptomycin, and β-mercaptoethanol. A total of 5 × 105 cells were added to a 96-well flat-bottomed microtitre plate (Nunc, Roskilde, Denmark) immobilized with or without anti-CD3 MoAb (Cedar Lane Labs), and recombinant α-fodrin protein [22]. For proliferation assay, prepared cells were cultured for 72 h under stimulation with concanavalin A (Con A) or recombinant α-fodrin protein, and pulsed with 1 μCi/well of 3H-thymidine (NEN Life Science Products, Boston, MA) during the final 20 h of the culture. 3H-thymidine incorporation was evaluated using an automated β liquid scintillation counter. Data were expressed as the mean ± s.d. determined in triplicate wells.
Western blot analysis
To detect serum autoantibodies against 120-kD α-fodrin autoantigen, the autoantigen was electrophoresed in non-reducing buffer in 10% SDS–PAGE gels, and the protein was then electrophoretically transferred to nitrocellulose which was probed with serum antibodies from treated and control 3d-Tx NFS/sld mice. Nitrocellulose membranes were incubated with peroxidase-conjugated horse anti-mouse IgG (Vector Labs). Autoantibodies were detected using ECL Western blotting reagent (Amersham) [22].
RESULTS
Pathological and immunohistochemical findings
The histopathological characteristics of autoimmune lesions developing in 3d-Tx NFS/sld mice were described previously in detail [19–21]. Briefly, autoimmune lesions in the salivary and lacrimal glands develop at 4 weeks of age or later, while no inflammatory lesions were observed in non-Tx NFS/sld mice at any age. Autoimmune exocrinopathy in the salivary and lacrimal glands aggravated gradually with advancing age. Immunohistochemical analysis to identify cell populations revealed that a majority of infiltrating cells in the salivary and lacrimal glands were CD3+ and CD4+ from disease onset, whereas a lesser number of CD8+ and B220+ cells was observed [19] (Table 1). CD28+ T cells were constitutively observed in these inflammatory lesions (61–73%) at all ages in 3d-Tx NFS/sld mice (Table 1), but not in non-Tx NFS/sld mice. A considerable proportion of CD80+ and CD86+ cells was detected exclusively in epithelial duct cells in the salivary and lacrimal glands of 3d-Tx NFS/sld mice, but not in non-Tx NFS/sld mice. Immunohistochemically, no significant difference was observed between CD80+ and CD86+ cells in the inflamed salivary gland tissues (data not shown). This is consistent with the data on flow cytometric analysis using the isolated mouse salivary gland epithelial cells from 3d-Tx NFS/sld mice (CD80, 28% and CD86, 27%) (Fig. 1). In contrast, a larger proportion of spleen cells was positive for CD86 (55%) than for CD80 (10%) in 3d-Tx NFS/sld mice (Fig. 1).
Table 1.
Proportion of CD28+, CD4+, CD8+ and B220+ cells in autoimmune sialadenitis of 3d-thymectomized (Tx) NFS/sld mice
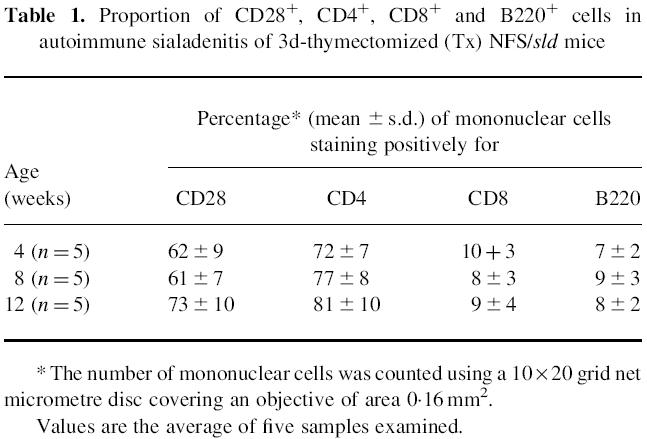
Fig. 1.
Flow cytometric analysis of CD80 and CD86 expression on isolated mouse salivary gland cells and spleen cell suspensions from 3d-thymectomized (Tx) NFS/sld mice (control) gated on I-As. While no difference was observed between CD80+ (28%), and CD86+ (27%) cells in the isolated mouse salivary gland epithelial cells, a larger proportion of spleen cells was CD86+ (55%) than of cells expressing CD80 (10%). Three experiments in each group (8 weeks old) were performed.
Effect of in vivo treatment with anti-B7 antibodies
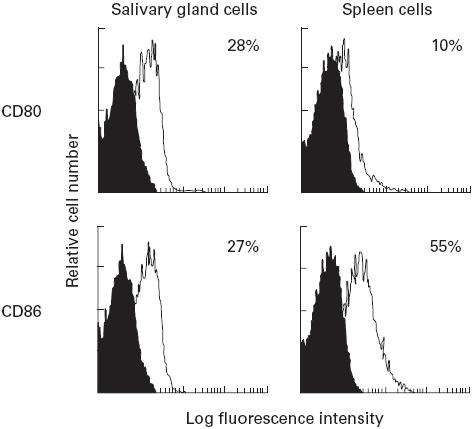
3d-Tx NFS/sld mice treated with a once a week dose of 100 μg of anti-CD80 (n = 8) showed slightly preventive effects, but not statistically significant, on the development of autoimmune lesions. In contrast, treatment of 3d-Tx NFS/sld mice with a once a week dose of 100 μg of anti-CD86 (n = 8) showed a significantly preventive effect on the development of autoimmune lesions in the salivary and lacrimal glands (P < 0.01) (Fig. 2).
Fig. 2.
Preventive effect of i.p. injection of anti-CD86 MoAb. Anti-CD80 MoAb (100 μg) (n = 8), and anti-CD86 (n = 8) were injected intraperitoneally into 3d-thymectomized (Tx) NFS/sld mice once a week (treated with MoAb from 3–7 weeks). These groups were compared with controls treated with PBS alone (n = 7). Mice with treatment were examined histopathologically at 8 weeks. Mean grade of inflammatory lesions was expressed as described in Materials and Methods. *P < 0.01, Mann–Whitney U-test.
Proliferative T cell response to α-fodrin autoantigen
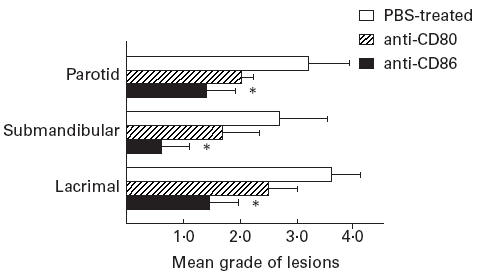
To address the role of α-fodrin autoantigen-reactive T cells, we examined the effect of in vivo treatment with anti-CD86 MoAb on proliferative T cell responses against recombinant α-fodrin autoantigen in spleen cells. We found that spleen cells in 3d-Tx NFS/sld mice treated with anti-CD86 MoAb showed a significant impairment of autoantigen-specific T cell proliferation (8 weeks old) compared with 3d-Tx NFS/sld mice treated with anti-CD80, and PBS alone (Fig. 3). No significant differences were observed in proliferative responses in these groups stimulated with Con A.
Fig. 3.
Effect of in vivo treatment with anti-CD86 MoAb on proliferative T cell response to the recombinant α-fodrin autoantigen. A significant inhibitory effect on T cell blastogenesis to the recombinant α-fodrin autoantigen was observed in 3d-thymectomized (Tx) NFS/sld mice treated with anti-CD86 MoAb, but not in those treated with anti-CD80 (*P < 0.05, Student's t-test). No difference was observed in concanavalin A (Con A)-stimulated blastogenesis measured in spleen cells from each group of mice. Three experiments in each group of the same age were performed. Data are expressed as ct/min per culture ± s.d. in triplicate.
Flow cytometric analysis of activation markers
We reported previously that a significant proportion of splenic CD4+ T cells express activation markers (CD44high, CD45RBlow, Mel-14low) at a high level in this murine model of 3d-Tx NFS/sld [20,21]. To clarify whether the activated self-reactive T cells were impaired by the treatment with anti-CD86 MoAb in 3d-Tx NFS/sld mice, we analysed the CD4+ T cells in spleens and LN cells bearing activation markers by flow cytometry. We found that activation markers (CD44high, CD45RBlow, Mel-14low) were significantly down-regulated in both spleen and LN cells gated on CD4 in anti-CD86-treated 3d-Tx NFS/sld mice (Fig. 4). In this case, more prominent suppressive profiles were observed in regional LN cells than in spleen cells. It is possible that the effector CD4+ T cells responsible for tissue destruction appear to be more suppressed by the treatment with anti-CD86 MoAb in the regional lymphoid tissues than in the periphery.
Fig. 4.
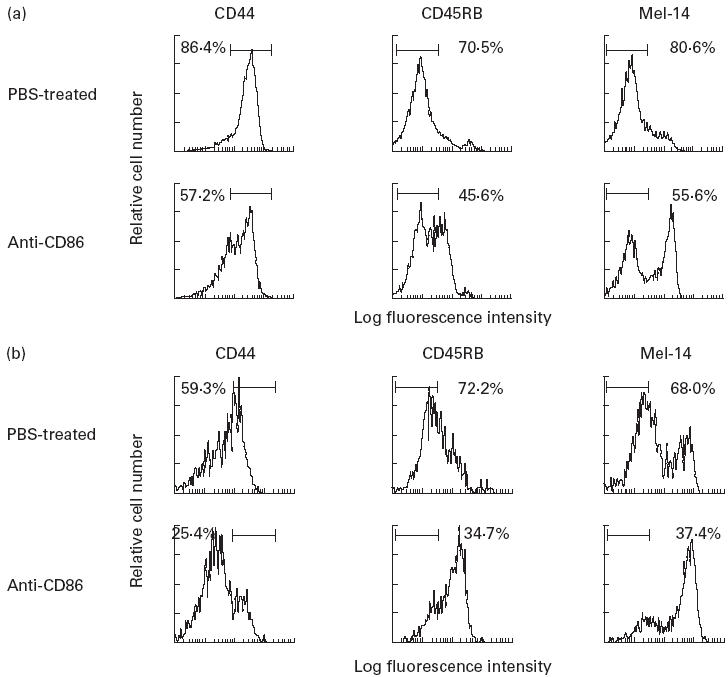
Flow cytometric analysis of activation markers in spleen cells (a) and regional lymph node (LN) cells (b) from anti-CD86-treated and PBS-treated 3d-thymectomized (Tx) NFS/sld mice gated on CD4+. Down-regulation of activation markers (CD44high, CD45RBlow, and Mel-14low) was clearly observed in both splenic and LN CD4+ cells (more prominent in LN cells) from anti-CD86-treated mice, compared with PBS-treated mice. Five mice of the same age (8 weeks old) were analysed.
Detection of cytokine production
We investigated the cytokine production in vivo, and found that anti-CD3-stimulated splenic T cell culture supernatants in the anti-CD86-treated murine SS model contained a high level of IL-4, but a lower level of IL-2 and IFN-γ compared with anti-CD80- and PBS-treated mice by ELISA (Fig. 5).
Fig. 5.
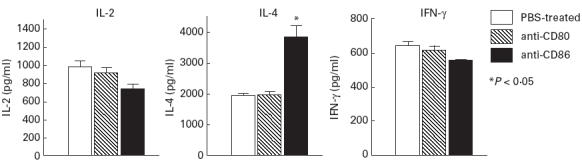
Splenic T cell culture supernatants in 3d-thymectomized (Tx) NFS/sld mice treated with anti-CD86 MoAb contained a high level of IL-4, but a lower level of IL-2, and IFN-γ by ELISA (*P < 0.05, Student's t-test). Five mice from each group (8 weeks old) were analysed.
Detection of serum autoantibody production
Protein immunoblotting revealed that sera from the murine SS model of control NFS/sld mice were positive for the 120-kD α-fodrin protein. In contrast, serum autoantibody production against 120-kD α-fodrin autoantigen was almost entirely suppressed in the anti-CD86-treated murine SS model, but not in CD80- and PBS-treated mice (data not shown).
DISCUSSION
It has been demonstrated that CD86 is expressed earlier and at higher levels than CD80 after activation of these cells during an immune response [25–27]. Previous studies showing that CD80 activates antigen-primed but not naive T cells led to the suggestion that CD86-mediated costimulation is important in the initiation phase of a T cell response, whereas the role of CD80 may be to maintain and expand the ongoing T cell response [28–31]. Blocking CD80 has been shown to prevent experimental autoimmune encephalomyelitis (EAE), while treatment with anti-CD86 resulted in the production of effector cells of a Th1 phenotype and increased disease severity [11,32]. In contrast, blocking CD86 prevented autoimmune diabetes in non-obese diabetic (NOD) mice, while CD80 blockage worsened disease [33]. These findings suggest a differential role of CD80 and CD86 in certain situations in different autoimmune responses.
In the present study, treatment with anti-CD86 MoAb could prevent the development of autoimmune exocrinopathy in the murine SS model, demonstrating the crucial role of CD86 costimulatory molecule that may be important in eliciting autoreactive T cell responses to an organ-specific autoantigen, α-fodrin [22]. Treatment with anti-CD80 MoAb could not prevent the development of these lesions. These results are consistent with an essential timing required for the co-ordinated interaction of B7 and CD28 family receptors, as reported previously [33,34]. Recent studies have demonstrated the important contribution of two members of the B7 family molecules in directing the outcome of the T cell effector function [35,36]. Although both molecules are able to provide costimulation to T cells for proliferation, CD80 has been reported to drive Th1-type T cell responses, whereas CD86 directs a Th2-like response [11]. In our experiments, cytokine production of splenic T cells from anti-CD86-treated mice showed a Th2-like response expressing IL-4. In addition, autoantigen-mediated T cell proliferation was significantly suppressed on splenic CD4+ T cells, and T cell activation markers were clearly down-regulated in splenic and LN CD4+ T cells in the anti-CD86-treated murine SS model. Recent studies have demonstrated that CD86 provides a critical early costimulatory signal, determining whether or not the T cells will participate in a variety of immune responses [13,14,37]. Our results showing that in vivo treatment with anti-CD86 MoAb was effective in preventing autoimmune lesions strongly support a major role of CD86 as a costimulatory molecule in the initiation of an autoantigen-specific immune response in the murine SS model. It was also demonstrated that CD86 expression is increased on APC after exposure to IFN-γ [38]. Previous data of ours showed a significant expression of IFN-γ mRNA in the initial stage of this murine SS model [20,21]. We found that a larger number of spleen cells was positive for CD86 than of those expressing CD80 on flow cytometric analysis.
An animal model of primary SS in NFS/sld mutant mice is mediated by CD4+ T cells, and tissue-infiltrating T cells have revealed the presence of mRNA for Th1-type cytokines [19–21]. This is the first report to demonstrate that in the murine SS model the CD86 costimulatory molecule plays a key role in the development of Th1-mediated autoimmune disease. It is well established that several organ-specific autoimmune diseases, including EAE, are induced by autoreactive Th1 cells [39,40]. Regulatory T cells that suppress the development of Th1-mediated autoimmune diseases produce Th2 cytokines, and recovery from EAE in mice and rats is associated with Th2 cells and its cytokines [26,41]. Experimental evidence in this study suggests that anti-CD86 treatment has a negative regulatory effect on autoreactive T cells by down-regulating IL-2 production and autoantigen-specific immune responses. Thus, blockade of CD86 costimulatory signals prolongs T cell activation and plays a role in regulating peripheral T cell tolerance and differentiation. These data demonstrate that anti-CD86 MoAb administration results not only in prevention of organ-specific autoimmune lesions in vivo, but in inhibition of organ-specific autoantibody production. It was reported that treatment with anti-CD86 MoAb resulted in a marked reduction of autoantibody production in murine lupus [42]. Our data demonstrate that blockade of CD86 costimulation during autoantigen-specific T cell activation promoted the differentiation of CD4+ T cell subsets into IL-4-producing T cells. It was shown that IL-4 regulates the differentiation of CD4+ T cells by stimulating precursor cells to mature into Th2-type cells while inhibiting the generation of Th1-type cells [43,44].
Taken together, these data support a crucial role for CD86 costimulation engagement in the regulation of both tolerance induction and T cell differentiation in the murine SS model in vivo. Treatment with anti-CD86 MoAb may block the interaction of CD28 and subsequent progression of Th1-mediated autoimmunity, resulting in an increase in the promoting signals through IL-4 production.
REFERENCES
- 1.Bretcher P. The two-signal model of lymphocyte activation twenty-one years later. Immunol Today. 1992;13:74–6. doi: 10.1016/0167-5699(92)90138-W. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 3.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. J Exp Med. 1993;177:845–50. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azuma M, Ito D, Yagita H, Okumura K, Phillip JH, Lanier LL, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–9. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 5.Linsley PS, Clark EA, Ledbetter JA. The T cell antigen, CD28, mediates adhesion with B cells by interacting with the activation antigen B7. Proc Natl Acad Sci USA. 1990;87:5031–5. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Ann Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 7.Linsley PS, Grreen JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) binds with similar avidity but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 8.Sansom DM, Hall ND. B7/BB1, the ligand for CD28, is expressed on repeatedly activated human T cells in vivo. Eur J Immunol. 1993;23:295–8. doi: 10.1002/eji.1830230148. [DOI] [PubMed] [Google Scholar]
- 9.Linsley PS, Green JA, Tan P, Gradshaw J. Co-expression and functional co-operation of CTLA-4 and CD28 on activated T lymphocyte. J Exp Med. 1992;176:1595–604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linsley PS, Greene JL, Tan P, Gradshaw J, Ledbetter JA. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 12.Lenschow DJ, Herold KC, Rhee L, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–93. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 13.Tsuyuki S, Tsuyuki J, Einsle K, Kopf M, Coyle AJ. Costimulation through B7-2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J Exp Med. 1997;185:1671–9. doi: 10.1084/jem.185.9.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, Heeger PS, Fairchild RL. Distinct roles for B7-1 and B7-2 determinants during priming of effector CD8+ Tcl and regulatory CD4+ Th2 cells for contact hypersensitivity. J Immunol. 1997;159:4217–26. [PubMed] [Google Scholar]
- 15.Bloch K, Buchanan W, Wohl M, Bunim J. Sjögren's syndrome. A clinical, pathological and serological study of sixty-two cases. Medicine. 1965;44:187–231. [PubMed] [Google Scholar]
- 16.Moutsopoulos HM, Chused TM, Mann DL, Klippel JH, Fauci AS, Frank MM, Lawley TJ, Hamburger MI. Sjögren's syndrome (sicca syndrome): current issues. Ann Intern Med. 1980;92:212–6. doi: 10.7326/0003-4819-92-2-212. [DOI] [PubMed] [Google Scholar]
- 17.Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986;29:577–85. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi Y, Kojima A, Hata M, Hirokawa K. A new mutation involving the sublingual gland in NFS/N mice. Partially arrested mucous cell differentiation. Am J Pathol. 1988;132:187–91. [PMC free article] [PubMed] [Google Scholar]
- 19.Haneji N, Hamano H, Yanagi K, Hayashi Y. A new animal model for primary Sjögren's syndrome in NFS/sld mutant mice. J Immunol. 1994;153:2769–77. [PubMed] [Google Scholar]
- 20.Hayashi Y, Haneji N, Hamano H, Yanagi K, Takahashi M, Ishimaru N. Effector mechanism of experimental autoimmune sialadenitis in the mouse model for primary Sjögren's syndrome. Cell Immunol. 1996;171:217–25. doi: 10.1006/cimm.1996.0196. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Mimura Y, Hamano H, Haneji N, Yanagi K, Hayashi Y. Mechanism of the development of autoimmune dacryoadenitis in the mouse model for primary Sjögren's syndrome. Cell Immunol. 1996;170:54–62. doi: 10.1006/cimm.1996.0133. [DOI] [PubMed] [Google Scholar]
- 22.Haneji N, Nakamura T, Takio K, et al. Identification of α-fodrin as a candidate autoantigen in primary Sjögren's syndrome. Science. 1997;276:604–7. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 23.White SC, Casarett GW. Induction of experimental autoallergic sialadenitis. J Immunol. 1974;112:178–85. [PubMed] [Google Scholar]
- 24.Ishimaru N, Saegusa K, Yanagi K, Haneji N, Saito I, Hayashi Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjögren's syndrome through Fas-mediated apoptosis. Am J Pathol. 1999;155:173–81. doi: 10.1016/S0002-9440(10)65111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–40. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenschow DJ, Su GHT, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, Miler J, Bluestone JA. Expression and functional significance of an additional ligand for CTLA4. Proc Natl Acad Sci USA. 1993;90:11054–8. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenschow DJ, Sperling AI, Cooke MP, et al. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–7. [PubMed] [Google Scholar]
- 28.Chen C, Faherty DA, Gault A, Connaughton SE, Power GD, Godfrey DI, Nabavi N. Monoclonal antibody 2D10 recognizes a novel T cell costimulatory molecule on activated murine B lymphocytes. J Immunol. 1994;152:2105–14. [PubMed] [Google Scholar]
- 29.Chen C, Gault A, Shen L, Nabavi N. Molecular cloning and expression of early T cell costimulatory molecule-1 and its characterization as B7-2 molecule. J Immunol. 1994;152:4929–36. [PubMed] [Google Scholar]
- 30.Damle NK, Klussman K, Leytze G, Aruffo A, Linsley PS, Ledbetter JA. Costimulation with integrin ligands intercellular adhesion molecule-1 or vascular cell adhesion molecule-1 augments activation-induced death of antigen-specific CD4+ T lymphocytes. J Immunol. 1993;151:2368–79. [PubMed] [Google Scholar]
- 31.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 32.Gallon L, Chandraker A, Issazadeh S, Peach R, Linsley PS, Turka LA, Sayegh MH, Khoury SJ. Differential effects of B7-1 blockade in the rat experimental autoimmune encephalomyelitis model. J Immunol. 1997;159:4212–6. [PubMed] [Google Scholar]
- 33.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rache MK, Scott DE, Quigley L, Gray GS, Abe R, June CH, Perrin PJ. Distinct roles for B7-1 (CD80) and B7-2 (CD86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195–203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 36.Gause WC, Mitro V, Via C, Linsley P, Urban JF, Jr, Greenwald RJ. Do effector and memory T helper cells also need B7 ligand costimulatory signals? J Immunol. 1997;159:1055–8. [PubMed] [Google Scholar]
- 37.Keane-Myers A, Gause WC, Linsley PS, Chen S-J, Wills-Karp M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J Immunol. 1997;158:2042–9. [PubMed] [Google Scholar]
- 38.Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowley RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecules (B7-1 and B7-2) expression on murine dendric cells. J Immunol. 1994;152:5208–19. [PubMed] [Google Scholar]
- 39.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway JCA. Surface expression of α4 integrin by CD4 T cells is required for their entry into brain parenchyme. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuchroo VK, Martin CA, Greer JM, Ju S-T, Sobel RA, Dorf ME. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. J Immunol. 1993;151:4371–82. [PubMed] [Google Scholar]
- 41.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental allergic encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor-β, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakajima A, Azuma M, Kodera S, et al. Preferential dependence of autoantibody production in murine lupus on CD86 co-stimulatory molecule. Eur J Immunol. 1995;25:3060–9. doi: 10.1002/eji.1830251112. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann TM, Sad S. The expanding universe of T-cell sunsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 44.Lakkis FG, Konieczny BT, Saleem S, et al. Blocking the CD28–B7 T cell costimulation pathway induces long term cardiac allograft acceptance in the absence of IL-4. J Immunol. 1997;158:2443–8. [PubMed] [Google Scholar]


