Abstract
The β2-adrenergic receptor (β2-AR) belongs to the group of G-protein coupled receptors and is present mainly on skeletal and cardiac muscle cells and lymphocytes. The gene encoding β2-AR (ADRB2) displays a moderate degree of heterogeneity in the human population. The distribution of polymorphisms at amino acid positions 16, 27 and 164 is changed in asthma, hypertension and obesity. We have earlier reported a decreased density of the β2-AR on peripheral blood mononuclear cells and the presence of β2-AR antibodies in patients with MG. Since certain polymorphisms affect the function of the β2-AR, it was of interest to analyse these in MG. Using allele-specific polymerase chain reaction amplification, we revealed an over-representation of homozygosity for Arg16 and a lower prevalence of homozygosity for Gly16 in MG patients compared with healthy individuals. The increased frequency of homozygosity for Arg16 was due to a contribution from patients with generalized MG but not from patients with only ocular disease. Homozygosity for Glu27 was negatively associated with both the presence of β2-AR antibodies and severity of disease. Moreover, acetylcholine receptor (AChR) antibodies were more often present in patients being homozygous for Gln27. Our results imply that homozygosity for Arg16 confers susceptibility to generalized MG, and that certain polymorphisms at amino acid position 27 are associated with subgroups of patients.
Keywords: myasthenia gravis, β-adrenergic receptor, gene polymorphisms, β-adrenergic receptor antibody, acetylcholine receptor antibody
INTRODUCTION
Certain MHC polymorphisms appear to be risk factors for most autoimmune diseases. HLA-B8 and DR3 have the strongest association with MG, while DR2, DR4 and certain DQ haplotypes are associated with the disease when patients are stratified according to age of disease onset and thymic histopathology. However, these associations are weak, suggesting that other genes may be of importance. The genes for the nicotinic acetylcholine receptor (AChR) [1,2], immunoglobulin heavy chains [3,4] and T cell receptor (TCR) [5,6] are some of the non-MHC candidate genes that have been evaluated. In addition, there are also associations between gene polymorphisms of CTLA-4 [7] and IL-1β [8]. Since the contribution of different genes varies in different subgroups of patients, MG could be considered as a polygenic disease.
The β2-adrenergic receptor (β2-AR) is a G protein-coupled receptor which has the typical structure with an extracellular amino terminal, seven transmembrane domains and an intracellular carboxyl-terminal. The gene encoding the β2-AR (ADRB2) is an intronless gene that is present at q31-32 of chromosome 5 and was cloned in 1987 [9]. Three loci at amino acid positions 16, 27 and 164 have been found to alter receptor functions significantly. Agonist-promoted down-regulation was enhanced in receptors homozygous for glycine16 (Gly16) compared with those homozygous for arginine16 (Arg16). Receptors homozygous for glutamic acid27 (Glu27) were resistant to such down-regulation when compared with those homozygous for glutamine27 (Gln27) [10]. The receptors homozygous for isoleucine164 (Ile164) had markedly decreased ligand binding and coupling properties compared with those homozygous for threonine164 (Thr164). Cardiac dysfunction was observed in transgenic mice having the Ile164 form of β2-AR [11]. Two of the polymorphisms at the amino acid positions 16 and 27 are on the amino terminal while that at amino acid position 164 is in the fourth transmembrane domain. The distribution of the polymorphisms is changed in asthma, hypertension and obesity. The importance of the β2-AR for the regulation of the immune system as well as skeletal and cardiac functions implies a potential importance of genetic variations of this gene also in MG.
Patients with MG have T and B cells that are stimulated to cytokine secretion by peptides from the β2-AR [12], they have a less than normal density of such receptors on peripheral blood mononuclear cells (PBMC) [13] and in 25% also serum antibodies against both the β2-AR and the β1-AR [14]. The β2-AR can thus be considered to be one of the autoantigens in MG. That polymorphisms in the genes coding for the autoantigen can be of importance has been demonstrated in the gene for AChR in MG [1,2] and the gene for insulin in insulin-dependent diabetes mellitus [15].
Our study of the polymorphisms in ADRB2 showed significant differences between MG patients and healthy individuals with regard to the variants at amino acid positions 16 and 27 of the gene.
SUBJECTS AND METHODS
Study groups
One hundred and forty-five Swedish Caucasian MG patients and 96 ethnically matched healthy individuals were studied.
Ninety-two female and 53 male MG patients were included. The clinical evaluation was done using the Osserman–Oosterhuis classification [16], and using the most severe stage ever present in the patients since start of disease. Ten patients had only ocular symptoms (stage I), 75 had mild generalized disease (stage IIA) and 60 had severe generalized disease (stage IIB-III). Ninety-eight of the patients were thymectomized, 19 had normal thymic histology, 55 had hyperplasia and 24 had thymomas. The onset of the disease was between 2 and 59 years. The 60 patients with severe disease had been or were treated with immunosuppressive drugs. One hundred and thirty-five patients were treated with cholinesterase inhibitors. Nineteen patients were treated with the β2-AR-stimulating drug terbutaline sulphate which temporarily improves skeletal muscle function [17]. The evaluation of clinical stages and evidence for cardiovascular diseases was done by one of us (R.P.).
DNA extraction
Genomic DNA was extracted from EDTA preserved whole blood by a standard proteinase K digestion and phenol/chloroform method.
β2-AR genotyping
Allele-specific polymerase chain reaction (PCR) was used with primers as described [18]. PCR reactions were carried out in a volume of 20 μl. Temperature cycling was 94°C for 30 s, 61°C for 45 s and 72°C for 45 s for 30 cycles for the polymorphisms of amino acid positions 16 and 27. Annealing temperature at 46°C for 45 s was used for the polymorphism of amino acid position 164. Ten microlitres of the PCR products were visualized on a 1.0% agarose gel, stained with ethidium bromide.
ELISA for β2-AR antibodies
The ELISA assay was performed on 87 MG patients as previously described [14,19]. The serum antibody data for these patients have been described previously [14].
Statistical analysis
Mann–Whitney U-test was used for comparing the values of antibody concentrations between two groups and χ2 test with Yates' correction was used for comparing the prevalence of different receptor gene polymorphisms between two groups. Odds ratios (OR) and 95% confidence intervals (95% CI) for relative risks were calculated after use of Fisher's exact test when necessary. All P values were corrected for the number of comparisons made (Pc). A Pc value < 0.05 was considered to be significant. Both P and Pc values are shown. Agreement between the observed genotypes and those predicted by the Hardy– Weinberg equilibrium was assessed by χ2 test.
RESULTS
Polymorphisms at amino acid positions 16, 27 and 164: increased prevalence of homozygosity for Arg16 in patients with generalized MG
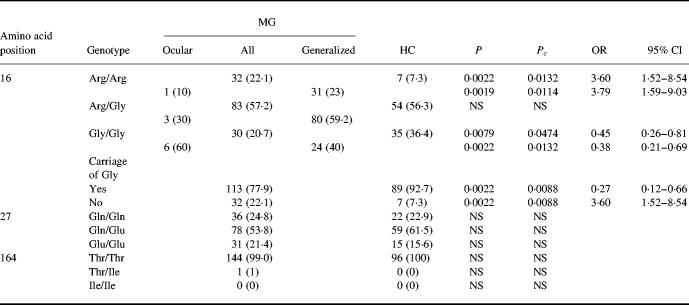
The prevalence of polymorphisms at amino acid positions 16, 27 and 164 in MG patients and healthy individuals and the genotypic frequencies of β2-AR at amino acid position 16 in MG patients with ocular and generalized disease are presented in Table 1. The frequency of homozygosity for Arg16 was higher and the frequency of homozygosity for Gly16 was lower in patients than in healthy individuals. Patients with generalized disease had a higher prevalence of homozygosity for Arg16 and lower prevalence of homozygosity for Gly16 compared with healthy individuals, while there was no difference between patients with ocular MG and healthy individuals. The frequencies of gene polymorphisms at amino acid positions 27 and 164 did not differ between patients with ocular and generalized MG and healthy individuals. The number of homozygous and heterozygous alleles that were found was not different from that predicted by the Hardy– Weinberg relationship.
Table 1.
Genotypes of the β2-adrenergic receptor (β2-AR) in patients with MG and in healthy individuals (HC)
Percentages are shown in parentheses.
OR, Odds ratio; 95% CI, 95% confidence interval; NS, not significant.
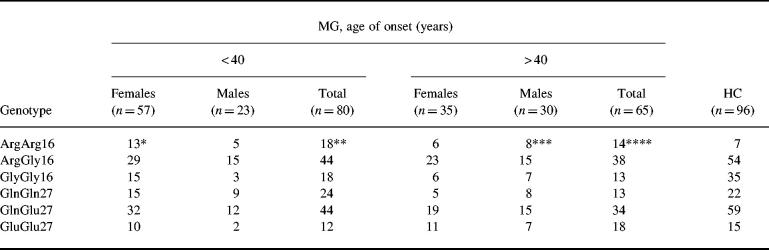
The prevalence of the gene polymorphisms related to age at disease onset and gender is shown in Table 2. Patients with early disease onset had a significantly higher frequency of homozygosity for Arg16. A tendency of increased frequency of homozygosity for Arg16 was found in female patients with early and male patients with late disease onset.
Table 2.
Genotypes at amino acid positions 16 and 27 in patients with MG with early and late onset disease and in healthy individuals (HC)
*P = 0.0114; Pc > 0.05; odds ratio (OR) = 3.76; 95% confidence interval (CI) = 1.40–10.09 compared with HC.
**P = 0.0048; Pc = 0.0288; OR = 3.69; 95% CI = 1.45–9.37 compared with HC.
***P = 0.0083; Pc = 0.0498; OR = 4.62; 95% CI = 1.51–14.13 compared with HC.
****P = 0.0154; Pc > 0.05; OR = 3.49; 95% CI = 1.32–9.21 compared with HC.
The prevalence of gene polymorphisms in patients with different thymic histopathology is shown in Table 3. There was a tendency to an increased frequency of homozygosity for Arg16 in patients with thymoma or thymic hyperplasia. There was also a tendency to a decreased frequency of homozygosity for Gly16 in patients with thymic hyperplasia.
Table 3.
Genotypes at amino acid positions 16 and 27 in patients with MG and different thymic histopathology
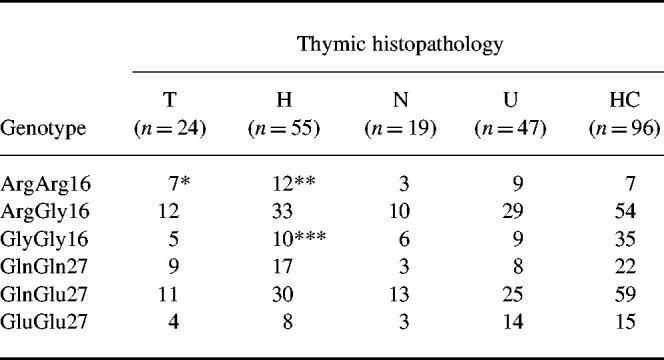
HC, Healthy individuals; T, thymoma; H, hyperplasia; N, normal thymic histology; U, not thymectomized.
*P = 0.0073; Pc > 0.05; odds ratio (OR) = 5.23; 95% confidence interval (CI) = 1.63–16.86 compared with HC.
**P = 0.0194; Pc > 0.05; OR = 3.55; 95% CI = 1.30–9.65 compared with HC.
***P = 0.0259; Pc > 0.05; OR = 0.38; 95% CI = 0.17–0.86 compared with HC.
The prevalence of gene polymorphisms at amino acid position 16 did not differ between patients with and without β2-AR antibodies and AChR antibodies or between patients with severe and mild disease.
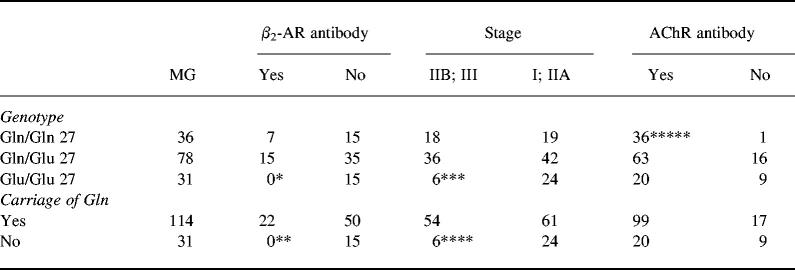
Homozygosity for Glu27: negative association with β2-AR antibodies, severity of disease and need for immunosuppressive therapy
The frequencies of β2-AR genotypes at amino acid position 27 in MG patients stratified with regard to presence of β2-AR antibodies, AChR antibodies and severity of disease are shown in Table 4. No patient with β2-AR antibodies was homozygous for Glu27. Mild disease, defined as stage I1 and IIA, was more often present in patients homozygous for Glu27, and these patients were or had been less often treated with immunosuppressive drugs. AChR antibodies were more prevalent in patients homozygous for Gln27.
Table 4.
Genotypes and carriage of Gln at amino acid position 27 in patients with MG stratified by presence of antibodies against the β2-adrenergic receptor (β2-AR) and the acetylcholine receptor (AChR) and severity of disease
Stage IIB; III, Severe disease; stage I; IIA, mild disease.
*P = 0.0097; Pc > 0.05 compared with patients without β2-AR antibody.
**P = 0.0097; Pc = 0.0388 compared with patients without β2-AR antibody.
***P = 0.0116; Pc > 0.05 compared with patients with mild disease.
****P = 0.0116; Pc = 0.0464 compared with patients with mild disease.*****P = 0.0051; Pc = 0.0204 compared with patients without AChR antibody.
There was a strong linkage disequilibrium between Arg16 and Gln27 in both healthy individuals (P < 0.0001) and MG patients (P = 0.0002), as shown in Table 5.
Table 5.
Association between carriage of Arg at amino acid position 16 and carriage of Gln at amino acid position 27
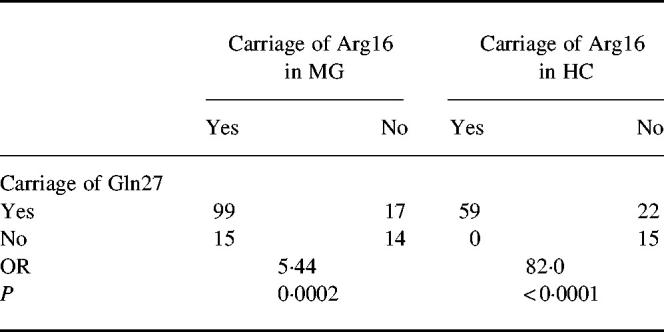
HC, Healthy individuals; OR, odds ratio.
No correlation between cardiovascular diseases and gene polymorphisms
Thirty-six out of 145 MG patients had cardiovascular diseases. Among them, 21 had symptomatic heart disease and 15 hypertension. The frequencies of the polymorphisms at amino acid positions 16 and 27 did not differ between patients suffering from either hypertension or heart disease and those without cardiovascular diseases.
The frequencies of gene polymorphisms did not differ between HLA-B8, DR2, DR3 and DR4-positive and -negative patients. There was no difference between patients with and without treatment with cholinesterase inhibitors or β2-AR agonists.
DISCUSSION
In the present study, we have revealed a new genetic association with the ADRB2 on chromosome 5 and showed that MG is associated with homozygosity for Arg16 (OR 3.6). The association between MG and homozygosity for Arg16 is present in female patients with early disease onset and males with late disease onset and in patients with generalized disease but not in ocular myasthenia. It is of special interest that patients with early and late onset disease had similar associations, since the MHC associations are clearly different for these disease subgroups [20,21]. This emphasizes that polymorphism at amino acid position 16 may constitute an additional genetic factor contributing to the susceptibility for MG.
Associations to the polymorphisms at amino acid position 27 emerged when patients were stratified according to severity of disease and the presence of β2-AR antibodies and AChR antibodies. No patient with β2-AR antibodies in serum was homozygous for Glu27. An explanation for this might be that individuals homozygous for Glu27 do not express the receptor in the fully mature form, in contrast to those homozygous for Gln27 [10]. The receptor homozygous for Glu27 might thus be less immunogenic and less capable of inducing antibody formation in vivo. In asthma, there is a strong association between the polymorphisms at amino acid position 27 and IgE levels [22]. MG patients homozygous for Glu27 also had a less severe disease than other patients. Our previous study showed that patients with β2-AR antibodies generally had a more severe disease and were more often treated with immunosuppressive drugs [14]. This finding is thus now confirmed at the gene level. Thus, these gene polymorphisms seem to affect the immune response in the disease, possibly via the receptor on the cells of the immune system.
A genetic predisposition to MG is suggested by the high concordance rate in identical twins [23,24]. The contribution of MHC genes has been extensively evaluated [20,21], but the positive associations with the extended DR3 haplotype are rather weak, with average OR for HLA-B8 and HLA-DR3 of 3.3 and 2.0, respectively. This suggests the contribution of other genetic factor(s). We have earlier described associations to the gene for tumour necrosis factor-alpha (TNF-α) and non-MHC associations to the ‘high secretor genotypes' of the IL-1β gene located at chromosome 2 [8]. The polymorphism of the β2-AR has been extensively studied and certain polymorphisms are associated with asthma [22,25–27], hypertension [28] and obesity [29]. The functional basis for these associations is incompletely known. The β2-AR contains significant amounts of N-linked carbohydrate and the glycosylation of β2-AR determines the surface expression of the receptor [30]. Positions 16 and 27 are close to the glycosylation sites and may be important for cellular processing and β2-AR expression. How Arg16, which is less common in the normal population than Gly16 [22], relates to susceptibility to MG remains to be elucidated. Our finding of a linkage disequilibrium between gene polymorphisms at amino acid positions 16 and 27 is in accordance with others [31]. It is interesting to note that although a strong linkage was found in both MG and healthy individuals, this association was weaker in MG (OR = 5.44) than in healthy individuals (OR = 82). This might indicate a greater susceptibility to develop MG once linkage is broken.
Thus, this study of the prevalence of β2-AR gene polymorphisms showed that homozygosity for Arg16 was associated with generalized disease, while homozygosity for Glu27 was associated with mild disease and with lack of β2-AR antibodies. The β2-AR gene polymorphisms thus constitute an additional non-MHC association in myasthenia gravis.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council, the Foundations of the Karolinska Institute and the Palle Ferb Foundation.
REFERENCES
- 1.Garchon HJ, Djabiri F, Viard JP, Gajdos P, Bach JF. Involvement of human muscle acetylcholine receptor α-subunit gene (CHRNA) in susceptibility to myasthenia gravis. Proc Natl Acad Sci USA. 1994;91:4668–72. doi: 10.1073/pnas.91.11.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heckmann JM, Morrison KE, Emeryk-Szajewska B, Strugalska H, Bergoffen J, Willcox N, Newson-Davis J. Human muscle acetylcholine receptor α-subunit gene (CHRNA1) association with autoimmune myasthenia gravis in black, mixed-ancestry and Caucasian subjects. J Autoimmun. 1996;9:175–80. doi: 10.1006/jaut.1996.0021. [DOI] [PubMed] [Google Scholar]
- 3.Gilhus N, Pandey JP, Gaarder PI, Aarli JA. Immunoglobulin allotypes in myasthenia gravis patients with thymoma. J Autoimmun. 1990;3:299–305. doi: 10.1016/0896-8411(90)90148-l. [DOI] [PubMed] [Google Scholar]
- 4.Demain A, Willcox N, Janer M, Welsh K, Newson-Davis J. Immunoglobulin heavy chain gene associations in myasthenia gravis: new evidence for disease heterogeneity. J Neurol. 1992;239:53–6. doi: 10.1007/BF00839214. [DOI] [PubMed] [Google Scholar]
- 5.Mantegazza R, Okersberg JR, Baggi F, Antozzi C, Illeni MT, Pellegris G, Cornelio F, Steinman L. Increased incidence of certain TCR and HLA genes associated with myasthenia gravis in Italians. J Autoimmun. 1990;3:431–40. doi: 10.1016/s0896-8411(05)80010-1. [DOI] [PubMed] [Google Scholar]
- 6.Oksenberg JR, Sherritt M, Begovich AB, Erlich HA, Bernard CC, Cavalli-Sforza LL, Steinman L. T-cell receptor Vα and Cα alleles associated with multiple sclerosis and myasthenia gravis. Proc Natl Acad Sci USA. 1989;86:988–92. doi: 10.1073/pnas.86.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang DR, Liu L, Noren K, Xia SQ, Trifunovic J, Pirskanen R, Lefvert AK. Genetic association of Ctla-4 to myasthenia gravis with thymoma. J Neuroimmunol. 1998;88:192–8. doi: 10.1016/s0165-5728(98)00119-2. [DOI] [PubMed] [Google Scholar]
- 8.Huang DR, Pirskanen R, Hjelmström P, Lefvert AK. Polymorphisms in IL-1β and IL-1 receptor antagonist genes are associated with myasthenia gravis. J Neuroimmunol. 1998;81:76–81. doi: 10.1016/s0165-5728(97)00161-6. [DOI] [PubMed] [Google Scholar]
- 9.Kobilka BK, Dixon RAF, Frielle T, et al. cDNA for the human β2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci USA. 1987;84:46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green S, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human β2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 11.Turki J, Lorenz JN, Green SA, Donnelly ET, Jacinto M, Liggett SB. Myocardial signaling defects and impaired cardiac function of a human β2-adrenergic receptor polymorphism expressed in transgenic mice. Proc Natl Acad Sci USA. 1996;93:10483–8. doi: 10.1073/pnas.93.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi Q, He W, Matell G, Pirskanen R, Magnusson Y, Eng H, Lefvert AK. T and B lymphocytes reacting with the extracellular loop of the β2-adrenergic receptor (β2AR) are present in the peripheral blood of patients with myasthenia gravis. Clin Exp Immunol. 1996;103:133–40. doi: 10.1046/j.1365-2249.1996.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu BY, Yi Q, Pirskanen R, Matell G, Eng H, Lefvert AK. Decreased β2-adrenergic receptor density on peripheral blood mononuclear cells in myasthenia gravis. J Autoimmun. 1997;10:401–6. doi: 10.1006/jaut.1997.0141. [DOI] [PubMed] [Google Scholar]
- 14.Xu BY, Pirskanen R, Lefvert AK. Antibodies against β1 and β2-adrenergic receptors in myasthenia gravis. J Neuroimmunol. 1998;91:82–8. doi: 10.1016/s0165-5728(98)00159-3. [DOI] [PubMed] [Google Scholar]
- 15.Bennet ST, Todd JA. Human type I diabetes and the insulin gene: principles of mapping polygenes. Annu Rev Genet. 1996;30:343–70. doi: 10.1146/annurev.genet.30.1.343. [DOI] [PubMed] [Google Scholar]
- 16.Oosterhuis HHGH. Studies in myasthenia gravis. Part 1. A clinical study of 180 patients. J Neurol Sci. 1964;1:512–46. doi: 10.1016/0022-510x(64)90171-6. [DOI] [PubMed] [Google Scholar]
- 17.Jonkers I, Swerup C, Pirskanen R, Bjelak S, Matell G. Acute effects of intravenous injection of beta-adrenoreceptor and calcium channel antagonists and agonists in myasthenia gravis. Muscle Nerve. 1996;19:959–65. doi: 10.1002/(SICI)1097-4598(199608)19:8<959::AID-MUS4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Turki J, Pak J, Green SA, Martin RJ, Liggett SB. Genetic polymorphisms of the β2-adrenergic receptor in nocturnal and nonnocturnal asthma. Evidence that Gly16 correlates with the nocturnal phenotype. J Clin Invest. 1995;95:1635–41. doi: 10.1172/JCI117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng H, Magnusson Y, Matell G, Lefvert AK, Saponja R, Hoebeke J. β2-adrenergic receptor antibodies in myasthenia gravis. J Autoimmun. 1992;5:213–27. doi: 10.1016/0896-8411(92)90201-z. [DOI] [PubMed] [Google Scholar]
- 20.Hjelmström P, Giscombe R, Lefvert AK, Pirskanen R, Kockum I, Landin-Olsson M, Sanjeevi CB. Different HLA-DQ are positive and negative associated in Swedish patients with myasthenia gravis. Autoimmunity. 1995;22:59–65. doi: 10.3109/08916939508995300. [DOI] [PubMed] [Google Scholar]
- 21.Vieira ML, Caillat-Zucman S, Gajdos P, Cohen-Kaminsky S, Castiu A, Bach JF. Identification by genomic typing of non-DR3 HLA class II genes associated with myasthenia gravis. J Neuroimmunol. 1993;47:115–22. doi: 10.1016/0165-5728(93)90021-p. [DOI] [PubMed] [Google Scholar]
- 22.Liggett SB. Polymorphisms of the β2-adrenergic receptor and asthma. Am J Respir Crit Care Med. 1997;156:S156–S162. doi: 10.1164/ajrccm.156.4.12tac-15. [DOI] [PubMed] [Google Scholar]
- 23.Murphy J, Murphy SF. Myasthenia gravis in identical twins. Neurology. 1986;36:78–80. doi: 10.1212/wnl.36.1.78. [DOI] [PubMed] [Google Scholar]
- 24.Pirskanen R, Tiilikainen A, Hokkanen E. Histocompatibility (HLA) antigens associated with myasthenia gravis. Ann Clin Rev. 1972;4:304–6. [PubMed] [Google Scholar]
- 25.Liggett SB. Genetics of β2-adrenergic receptor variants in asthma. Clin Exp Allergy. 1995;25:89–94. doi: 10.1111/j.1365-2222.1995.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 26.Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the β2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993;8:334–9. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- 27.Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between β2-adrenoceptor polymorphism and susceptibility to bronchodilator desensitisation in moderately severe stable asthmatics. Lancet. 1997;350:995–9. doi: 10.1016/S0140-6736(97)03211-X. [DOI] [PubMed] [Google Scholar]
- 28.Svetkey LP, Timmons PZ, Emovon O, Anderson NB, Preis L, Chen Y–T. Association of hypertension with β2- and α2c10-adrenergic receptor genotype. Hypertension. 1996;27:1210–5. doi: 10.1161/01.hyp.27.6.1210. [DOI] [PubMed] [Google Scholar]
- 29.Large V, Hellström L, Reymisdottir S, Lönnqvist F, Eriksson P, Lannfelt L, Arner P. Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Invest. 1997;100:3005–13. doi: 10.1172/JCI119854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rands E, Candelore MR, Cheung AH, Hill WS, Strader CD, Dixion RAF. Mutational analysis of β-adrenergic receptor glycosylation. J Biol Chem. 1990;265:10759–64. [PubMed] [Google Scholar]
- 31.Dewar JC, Wheatley AP, Venn A, Morrison JFJ, Britton J, Hall IP. β2-adrenoceptor polymorphisms are in linkage disequilibrium, but are not associated with asthma in an adult population. Clin Exp Allergy. 1998;28:442–8. doi: 10.1046/j.1365-2222.1998.00245.x. [DOI] [PubMed] [Google Scholar]