Abstract
To date no specific serological parameter is available to assess disease activity in SLE. Soluble serum thrombomodulin is a new marker of endothelial cell injury and vasculitis. The objective of this study was to compare in vivo soluble thrombomodulin as marker of disease activity in SLE with established and recent serological parameters. One hundred and twenty-four sera of 30 patients with proven SLE with different disease activities were tested for serum levels of thrombomodulin, intercellular adhesion molecule-1 (ICAM-1), E-selectin, vascular cell adhesion molecule-1 (VCAM-1), IL-2R, IL-6, IL-10, dsDNA by ELISA and dsDNA additionally by radioimmunoassay (RIA). C-reactive protein (CRP), complement component C3, IgG, creatinine, anti-nuclear antibodies (ANA) and intermediate filament antibodies were measured by standard laboratory tests. The clinical disease activity was evaluated by the Systemic Lupus Activity Measure (SLAM). Correlations of the different serological SLE disease activity parameters with the SLAM scores revealed the highest significance for serum thrombomodulin (correlation coefficient 0.82). This was further confirmed by the intra-individual analysis of follow-up sera. In addition, a moderate correlation could be found for IL-6, IL-10, ICAM-1, CRP and erythrocyte sedimentation rate (ESR). In summary, soluble thrombomodulin is the most important serological parameter of disease activity in SLE currently available, as shown by the in vivo studies. Soluble thrombomodulin might be a valuable serological parameter for therapeutical considerations.
Keywords: systemic lupus erythematosus, disease activity, thrombomodulin, endothelial cell damage
INTRODUCTION
SLE is a systemic autoimmune disease of unknown aetiology, which involves different organ systems to a variable degree [1]. Clinically, a reliable serological parameter of the inflammatory activity of the disease is required due to considerable variation in disease manifestations. However, a pathophysiologically determined, specific serological marker of disease activity is currently missing. At present the erythrocyte sedimentation rate (ESR) [2,3], products of complement activation [3–5], and levels of C-reactive protein (CRP) [2,3,6], autoantibodies (e.g. dsDNA antibodies) [2,3,5,7,8], or cytokines such as IL-2/IL-2 receptor [8–10], IL-6 [7,11] and IL-10 [12] are used as indirect serological markers with variable degrees of significance. In general, these factors do not correlate closely enough with disease activity.
Pathophysiologically, SLE is characterized by complex alterations of the immune system (e.g. polyclonal B cell activation, autoantibodies, and T cell defects) and an immune complex vasculitis with endothelial cell damage [1,9,13]. Therefore, factors of endothelial cell activation or damage are of considerable interest. However, the levels of soluble endothelial adhesion molecules such as intercellular adhesion molecule-1 (sICAM-1; CD54) [10,14,15], vascular cell adhesion molecule-1 (sVCAM-1; CD106) [14,15], and sE-selectin (CD62E) [15,16] did not show a close correlation with disease activity in SLE.
In contrast, soluble serum thrombomodulin (sTM) was recently established as a valuable new serological marker with good correlation to disease activity in SLE [17–20]. TM is the endothelial cell transmembrane receptor for thrombin [21,22]. Soluble TM is an established marker of endothelial cell damage [23,24]. However, a direct comparison of sTM and other serological markers of disease activity in SLE is still lacking. Here we present in vivo data, comparing different serological disease activity parameters in patients with SLE and different disease activities.
PATIENTS AND METHODS
Patients
A total of 124 serum samples from 30 patients (26 female, four male; mean age 34 ± 6 years; range 16–65 years) with proven SLE were investigated in a retrospective study. At the time of diagnosis all patients fulfilled the 1982 revised American College of Rheumatology (ACR; formerly American Rheumatism Association (ARA)) criteria for the diagnosis of SLE [25]. Two to six serum samples were tested from each patient taken at times of different disease activities. The serum samples included at least one taken at the time of high active and one taken at the time of low active/inactive disease. Serum samples of 20 healthy volunteers (staff members; 14 female, six male; mean age 34 ± 9 years; range 18–41 years) were used as normal controls. Aliquots of the sera had been stored at −20°C until tested.
All patients were seen as in- or out-patients by an interdisciplinary team of specialists. At the time of sample collection the patients were treated as follows with several patients receiving combination therapy: no therapy, 14 times; 5–20 mg prednisolone daily, 75 times; > 20 mg prednisolone daily, 19 times; 50–100 mg azathioprine daily, 34 times; and cyclophosphamide pulse therapy (1000 mg), nine times.
Evaluation of SLE disease activity
The disease activity was retrospectively determined for each collected sample. The Systemic Lupus Activity Measure (SLAM) score [26] was used as established SLE disease activity scoring system. The SLAM score consists of 32 different laboratory or clinical parameters, which are divided into 12 subgroups: constitutional, integumentum, eye, reticulo-endothelial, pulmonary, cardiovascular, gastrointestinal, neurological, joint, nephrological/laboratory manifestations, and ad hoc observations. Each parameter is scored as 0, 1, 2, or 3 points. For some statistical evaluations the patients were divided into three subgroups with a SLAM score of 0–5 (low activity), 6–10 (moderate activity), and > 10 (high activity).
Laboratory parameter and kidney dysfunction
Marked kidney dysfunction is known to result in accumulation of different serological disease activity markers including thrombomodulin, which is difficult to distinguish from disease activity-related elevation of the respective serum levels. Therefore, in agreement with other publications, only patients were included in the study who had a serum creatinine < 2.5 mg/100 ml (=< 225 μmol/l) at the time of serum collection [19,27].
Immunological assays
A prototype two-site ELISA was used for the determination of thrombomodulin in serum samples (Thrombomodulin VarElisa, charge no. 17067; ELIAS/Pharmacia & Upjohn, Freiburg, Germany). The test was performed according to the manufacturer's instructions. Briefly, the precoated 96-well plates were washed with buffer once and than incubated with diluted samples or provided standards (25 μl serum sample and 100 μl sample buffer per well). After 1 h of incubation at room temperature the plates were washed three times and further incubated with the peroxidase-conjugated secondary anti-TM antibody (100 μl/well) for 1 h. Subsequently, the plates were washed again and incubated with the substrate solution (tetramethylbenzidine (TMB)) at room temperature in the dark. After 10 min the stop solution (2 n H2SO4) was added and the optical density (OD) measured after colour stabilization (30 min) by an automated ELISA plate reader at 450 nm (Titertek Multiscan Plus MKII; ICN/Flow, Meckenheim, Germany). The respective sample concentrations of TM were calculated in relation to the reference standard curve. The samples were tested in duplicates and the mean taken for further calculations.
Commercially available two-site ELISAs were used to determine the serum levels of dsDNA (ELIAS/Pharmacia & Upjohn), soluble IL-2 receptor (sIL-2R; Coulter-Immunotech Diagnostics, Hamburg, Germany), sIL-6 (Quantikine human IL-6; R&D Systems GmbH, Wiesbaden, Germany), sIL-10 (Quantikine human IL-10; R&D Systems GmbH), sICAM-1 (Parameter human soluble ICAM-1; R&D Systems GmbH), sE-selectin (Parameter human soluble E-selectin; R&D Systems GmbH), and sVCAM-1 (Parameter human soluble VCAM-1; R&D Systems GmbH). In addition, the levels of dsDNA were measured by radioimmunoassay (RIA; dsDNA radioimmunoassay; Amersham Buchler, Braunschweig, Germany). The assays were performed according to the manufacturers' instructions with the reagents provided. As for TM, the mean of duplicates was taken for further calculations.
Standard laboratory tests were used to measure the serum levels of CRP, ESR (at 1 h), complement component C3, creatinine, and IgG. The titre of anti-nuclear antibodies (ANA) and anti-intermediate-filament antibodies to vimentin (vim) and keratine (ker) (INFIL-ab) were determined by standard indirect immunofluorescence (IIF) technique on Hep-2 cells as described previously [28]. Fluorescein-conjugated mouse anti-human IgG antibodies (Dianova GmbH, Hamburg, Germany) were used as secondary antibodies.
Statistical analysis
If not otherwise stated the mean and s.e.m. are given. The χ2 test was used to determine the differences of disease and control groups for ANA and INFIL-ab titre. For the remaining serological parameters the non-parametric Wilcoxon–Mann–Whitney U-test was used. P ≤ 0.05 was considered significant. The same test was used to determine the significance of the different experimental groups in the in vitro tests. The multiple range Duncan's test [29] was used as multiple stage test to assess the significance of the different disease activity groups for the distinct serological disease activity parameters (Statistical Analysis System for Windows, Version 6.1; SAS Institute Inc., Cary, NC). The regression and correlation analysis (Pearson's correlation) was perfomed with WinSTAT 3.1 (Kalmia Co. Inc., Cambridge, MA). The significance of the Pearson's correlation was graded into five groups according to Landis & Kock [30]: − −, slight (0.00–0.20); −, fair (0.21–0.40); +/−, moderate (0.41–0.60); +, substantial (0.61–0.80); and ++, perfect (0.81–1.00).
RESULTS
Serological disease activity parameters
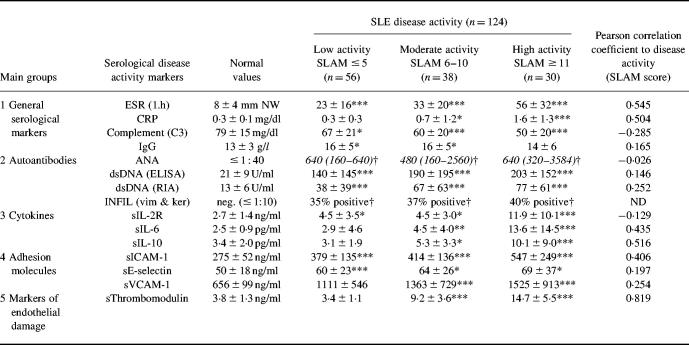
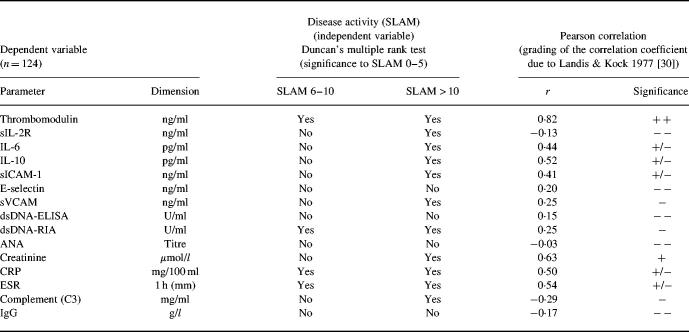
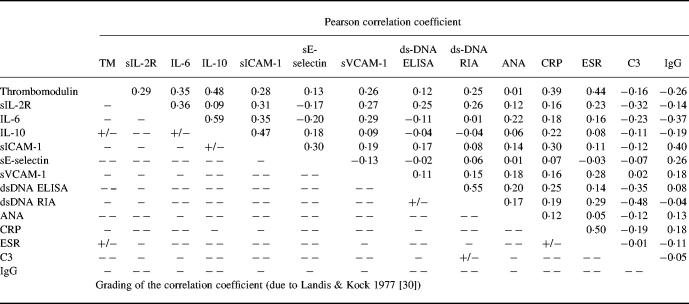
Fifty-six serum samples were collected at time of low disease activity (SLAM score 0–5), 38 serum samples at moderate disease activity (SLAM score 6–10), and 30 serum samples at high disease activity (SLAM score > 10). Table 1 summarizes the results of the different disease activity parameters. Differences with variable degrees of significance were found between the three disease activity groups of patients for all parameters tested with the exception of ANA and INFIL-ab titre, E-selectin and IgG values. The respective degrees of significance as well as the data in detail are presented in Table 1. The multiple stage correlation as well as the Pearson correlation gave the highest level of significance for sTM, pointing to an advantage of sTM as serological disease activity parameter in SLE (Tables 1 and 2) Furthermore, a cross correlation amongst all serological disease activity parameters tested confirmed these results. In addition, these correlations demonstrated the independence of sTM and the other serological parameters of disease activity and in particular the parameters of the immunological system (Table 3). A subanalysis between the different disease manifestations and the values of sTM revealed that the involvement of several organ systems contributed together to the sTM increase. In addition, a special subanalysis showed that nephritis was not only correlated with a significant increase of sTM but also with a significant increase of DNA antibody titre (P < 0.003) and decrease of C3 (P < 0.03).
Table 1.
Serological disease activity parameters in patients with SLE classified in five main groups according to historical developments
Medium ± s.d. (mean ± s.d.); italics, median (25% and 75% percentile).
*P < 0.05; **P < 0.01; ***P < 0001 (correlation to control values; Wilcoxon–Mann–Whitney U-test).
†P < 0.05 (difference from control values, χ2 test).
Table 2.
Pearson'apos;s correlation and Duncan'apos;s multiple range test between serological disease activity parameters and disease activity in patients with SLE
The significance of the Pearson'apos;s correlation was graded into five groups according to Landis & Kock [30]: − −, slight (0.00–0.20); −, fair (0.21–0.40); +/−, moderate (0.41–0.60); +, substantial (0.61–0.80); and + +, perfect (0.81–1.00).
Table 3.
Cross-correlation of the different serological disease activity parameters in patients with SLE
The significance of the Pearson's correlation was graded into five groups according to Landis & Kock [30]: − −, slight (0.00–0.20); −, fair (0.21–0.40); +/−, moderate (0.41–0.60); +, substantial (0.61–0.80); and + +, perfect (0.81–1.00).
Next we investigated sTM as well as the adhesion molecules sICAM-1, sE-selectin, and sVCAM-1 as markers of disease activity in more detail due to their importance as parameters of endothelial cell activation and cell damage. The levels of sICAM-1, sVCAM-1, and to a minor extent sE-selectin were elevated in SLE patients compared with control values. However, there was only a weak correlation with disease activity due to a broad variability amongst patients with similar disease activities (Table 1, Fig. 1). In contrast, sTM showed a much better correlation with disease activity (r = 0.82). In addition, the intra-individual kinetics of these parameters further demonstrated the superiority of sTM. Serum samples were available for all 30 patients at low active or inactive (SLAM score 0–5) and active (SLAM score > 5) stages of disease activity. The mean of the respective serological values was taken if more than one sample at the same stage of disease activity was available in individual patients. All 30 patients showed lower sTM values at low active/inactive compared with high active stage of disease. In contrast, the sVCAM-1, sICAM-1 and sE-selectin values decreased only in 73%, 70%, and 53% of patients, respectively (Fig. 2).
Fig. 1.
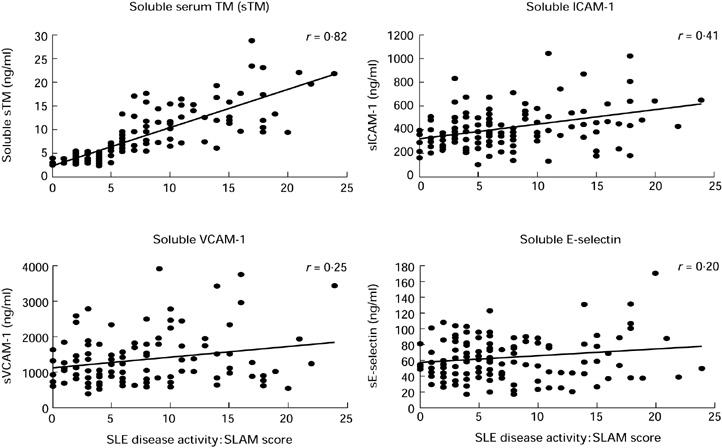
The serological values and correlations with SLE disease activity are shown for thrombomodulin (TM) as well as for the adhesion molecules intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1). The SLE disease activity was determined by the SLAM score.
Fig. 2.
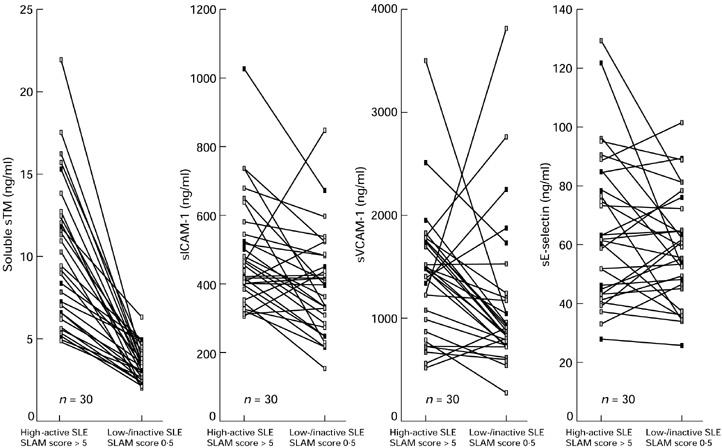
The serological levels of thrombomodulin (TM) and adhesion molecules (intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1)) are compared in 30 patients with proven SLE at active (SLAM score > 5) and low active/inactive (SLAM score 0–5) stage of disease (more details in Patients and Methods). The best correlation of the intra-individual kinetics with the course of disease is found for the serum TM levels.
DISCUSSION
Our data provide in vivo evidence for sTM as an excellent serological parameter of disease activity in patients with SLE. sTM shows the best correlation to disease activity by comparing established and recent serological parameters. The serological parameters of disease activity in SLE can be divided into the following groups according to the historical development: general indirect markers, autoantibodies, cytokines, adhesion molecules and finally markers of endothelial cell damage.
Initially, only general, unselective parameters of disease activity were available such as ESR, CRP, or levels of immunoglobulins and complement components. Overall, these parameters show a weak correlation to disease activity only and are influenced by a multiplicity of factors [2–6]. Furthermore, the CRP response of patients with SLE was characterized in most cases by a weak increase only [6]. Finally, the levels of several complement components have proved to be of special value in the subgroup of patients with nephritis [4,5].
A characteristic feature of SLE is the polyclonal B cell activation and the occurence of a variety of autoantibodies. Particularly, autoantibodies to dsDNA have proved to be of high value for the diagnosis of SLE [7,8]. In contrast, the titre of dsDNA antibodies is of limited value as a serological parameter of disease activity [5,7,8,25]. Continuously elevated titres are found in a substantial subgroup of SLE patients independent of the clinical disease activity or relapse [5,7,31]. In addition, several autoantibodies occur of very restricted or minor clinical importance. Anti-endothelial cell antibodies (AECA) and INFIL-ab are present in up to 50% of the patients but they do not correlate well with the course of disease. The titre of distinct autoantibodies may be of interest for the definition of subgroups such as patients with kidney involvement (AECA; [32]), joint involvement (INFIL-ab; [33]), or drug-induced SLE (anti-histone antibodies; [3]).
Pro- and anti-inflammatory cytokines mediate cell–cell interactions. They play an important role in the initiation and maintenance of inflammatory autoimmune processes. Soluble cytokine levels are potential parameters to monitor disease activity in SLE patients. However, the serological levels of different cytokines or their respective receptors were shown to be of limited value. In general, the levels of investigated cytokines such as IL-2/IL-2R (as marker of T cell activation), IL-6 or IL-10 were higher in active than in low active/inactive SLE, but did not correlate well with the course of the disease. They might be useful in certain subgroups of SLE patients only [3,8–12]. The limited value of serological cytokine levels as activity parameters of SLE is probably due to the established abnormalities of T cell function, the deficient cytokine production, the altered Th1/Th2 cell relation in SLE, and the substantial differences between local cytokine secretion and systemic distribution [9,34].
The results of the present study confirm the restricted significance of the general unselective markers, autoantibodies, and cytokines as serological disease activity markers in SLE. Recently, markers of endothelial cell activation or injury have gained increasing importance. This is mainly due to the established endothelial cell activation and damage as an important pathophysiological feature of SLE.
ICAM-1, E-selectin and VCAM-1 are important adhesion molecules for the interaction of endothelial cells with leucocytes in inflammation [35]. ICAM-1 is constitutively expressed on a large variety of cells. The expression is induced by proinflammatory cytokines. VCAM-1 is found on a limited number of activated cells, including endothelial cells. E-selectin is mainly expressed on cytokine-activated endothelial cells in a transient fashion [35,36]. In addition to the membrane-bound receptor, soluble forms of adhesion molecules can be found in the supernatant of activated endothelial cells in vitro or in serum in vivo, probably as a result of active shedding or membrane injury [36–38]. However, the data of this study as well as other studies show a weak correlation between soluble adhesion molecule levels and disease activity of SLE only. Therefore, levels of soluble adhesion molecules are of limited value as disease activity parameters in SLE as well [10,14–16].
Pathophysiologically SLE is characterized by an immune complex vasculitis and increased cytotoxicity of polymorphonuclear neutrophils (PMN) to endothelial cells [1,39]. Recently, sTM was established as marker of endothelial cell damage [23,24]. Thrombomodulin is the transmembranous glycoprotein receptor for thrombin mainly expressed on endothelial cells and syncytiotrophoblasts. The TM–thrombin complex acts as important anticoagulant resulting in accelerated activation of protein C [21,22]. After physiological activation and reaction the complex is internalized and degraded. In vitro studies including 51Cr-release assays could confirm that soluble TM is a reliable marker of endothelial cell damage independent of physiological activation [23,24]. In addition, cytokine stimulation of endothelial cells results in decreased TM expression on the cell surface due to TM internalization with subsequent degradation and suppression of TM transcription and translation [40,41]. Nevertheless, previously we showed in endothelial cell cultures in vitro and in patients receiving recombinant human tumour necrosis factor-alpha (rhTNF-α) for therapy, that soluble TM is a reliable marker of endothelial cell damage due to endothelial–leucocyte adhesion and interaction after cytokine activation [42]. In summary, sTM is an excellent and promising marker of endothelial cell damage in vasculitides. The latter is highlighted by our present study comparing in parallel established and recent serological markers of SLE disease activity, including the intra-individual follow up at times of different disease activity. The clinical importance of sTM has further been documented recently in studies correlating sTM with disease activity in patients with various diseases resulting in vasculitides or vascular injury. These included patients with SLE [17–22], ulcerative colitis [43], Wegener's granulomatosis [44,45], Takayasu's arteritis [44], Behçet's disease [44], giant cell arteritis [44], panarteritis nodosa [46], microscopic polyangiitis [45], diabetic microangiopathy [46], sepsis [47,48], malaria [49,50], and disseminated intravascular coagulation [48,51]. However, a marked variation of TM concentration (up to 10-fold) is found comparing the different reports. This might be due to the different standards used in the individual test kits, based on either recombinate or purified TM from human placenta. Therefore, an international standardization is required.
In conclusion, our in vivo data highlight sTM as the best serological activity marker in SLE available at present. sTM reflects closely endothelial cell damage due to vasculitis and therefore is closely related to the immunopathophysiological alterations in SLE. Our comparative study suggests that sTM may also represent a promising serological parameter for therapeutical considerations and decisions.
Acknowledgments
The authors would like to thank Dr I. Zuna (German Cancer Research Centre, Heidelberg, Germany) for statistical analysis.
REFERENCES
- 1.Koffler D. Immunpathogenesis of systemic lupus erythematosus. Ann Rev Med. 1974;25:149–64. doi: 10.1146/annurev.me.25.020174.001053. [DOI] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Joseph L, et al. Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail. Arthritis Rheum. 1996;39:370–8. doi: 10.1002/art.1780390304. [DOI] [PubMed] [Google Scholar]
- 3.Spronk PE, Limburg PC, Kallenberg CG. Serological markers of disease activity in systemic lupus erythematosus. Lupus. 1995;4:86–94. doi: 10.1177/096120339500400202. [DOI] [PubMed] [Google Scholar]
- 4.Manzi S, Rairie JE, Carpenter AB, et al. Sensitivity and specificity of plasma and urine complement split products as indicators of lupus disease activity. Arthritis Rheum. 1996;39:1178–88. doi: 10.1002/art.1780390716. [DOI] [PubMed] [Google Scholar]
- 5.Swaak AJG, Groenwald J, Bronsveld W. Predicitive value of complement profiles and anti-ds-DNA in systemic lupus erythematosus. Ann Rheum Dis. 1986;45:359–66. doi: 10.1136/ard.45.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabay C, Roux-Lombard P, de-Moerloose P, et al. Absence of correlation between interleukin 6 and C-reactive protein blood levels in systemic lupus erythematosus compared with rheumatoid arthritis. J Rheumatol. 1993;20:815–21. [PubMed] [Google Scholar]
- 7.TerBorg EJ, Horst G, Hummel EJ, et al. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33:634–43. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 8.TerBorg EJ, Horst G, Limburg PC, et al. Changes in plasma levels of interleukin-2 receptor in relation to disease exacerbations and levels of anti-ds-DNA and complement in systemic lupus erythematosus. Clin Exp Immunol. 1990;82:21–6. doi: 10.1111/j.1365-2249.1990.tb05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linker-Israeli M, Bakke AC, Kitridou RC, et al. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE) J Immunol. 1983;130:2651–5. [PubMed] [Google Scholar]
- 10.Kling E, Bieg S, Boehme M, et al. Circulating intercellular adhesion molecule 1 as a new activity marker in patients with systemic lupus erythematosus. Clin Invest. 1993;71:299–304. doi: 10.1007/BF00184731. [DOI] [PubMed] [Google Scholar]
- 11.Spronk PE, ter-Borg EJ, Limburg PC, et al. Plasma concentration of IL-6 in systemic lupus erythematosus; an indicator of disease activity. Clin Exp Immunol. 1992;90:106–10. doi: 10.1111/j.1365-2249.1992.tb05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houssiau FA, Lefebvre C, Vanden-Berghe M, et al. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–5. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Cozar FJ, Molina IJ, Cuadrado MJ, et al. Defective B7 expression on antigen-presenting cells underlying T-cell activation abnormalities in systemic lupus erythematosus (SLE) patients. Clin Exp Immunol. 1996;104:72–9. doi: 10.1046/j.1365-2249.1996.d01-648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason JC, Kapahi P, Haskard DO. Detection of increased levels of circulating intercellular adhesion molecule 1 in some patients with rheumatoid arthritis but not in patients with systemic lupus erythematosus. Lack of correlation with levels of circulating vascular cell adhesion molecule 1. Arthritis Rheum. 1993;36:519–27. doi: 10.1002/art.1780360412. [DOI] [PubMed] [Google Scholar]
- 15.Spronk PE, Bootsma H, Huitema MG, et al. Levels of soluble VCAM-1, soluble ICAM-1, and soluble E-selectin during disease exacerbations in patients with systemic lupus erythematosus (SLE); a long term prospective study. Clin Exp Immunol. 1994;97:439–44. doi: 10.1111/j.1365-2249.1994.tb06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson CW, Beall LD, Hunder GG, et al. Serum ELAM-1 is increased in vasculitis, scleroderma, and systemic lupus erythematosus. J Rheumatol. 1993;20:809–14. [PubMed] [Google Scholar]
- 17.Takaya M, Ichikawa Y, Kobayashi N, et al. Serum thrombomodulin and anticardiolipin antibodies in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1991;9:495–9. [PubMed] [Google Scholar]
- 18.Kawakami M, Kitani A, Hara M, et al. Plasma thrombomodulin and a2-plasmin inhibitor–plasmin complex are elevated in active systemic lupus erythematosus. J Rheumatol. 1992;19:1704–9. [PubMed] [Google Scholar]
- 19.Boehme MWJ, Nawroth PP, Kling E, et al. Serum thrombomodulin. A novel marker of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1994;37:572–7. doi: 10.1002/art.1780370419. [DOI] [PubMed] [Google Scholar]
- 20.Karmochkine M, Boffa MC, Piette JC, et al. Increase in plasma thrombomodulin in lupus erythematosus with antiphospholipid antibodies. Blood. 1992;79:837–8. [PubMed] [Google Scholar]
- 21.Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci USA. 1981;78:2249–52. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75:329–36. [PubMed] [Google Scholar]
- 23.Ishii H, Yama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemostas. 1991;65:618–23. [PubMed] [Google Scholar]
- 24.Sawada K, Yamamoto H, Yago H, et al. A simple assay to detect endothelial cell injury; measurement of released thrombomodulin from cells. Exp Mol Pathol. 1992;57:116–23. doi: 10.1016/0014-4800(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 25.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 26.Liang MH, Socher SA, Larson MG, et al. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–18. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 27.Hergesell O, Andrassy K, Geberth S, et al. Plasma thrombomodulin levels are dependent on renal function. Thromb Res. 1993;72:455–8. doi: 10.1016/0049-3848(93)90246-k. [DOI] [PubMed] [Google Scholar]
- 28.Boehme MWJ, Kataaha PK, Holborow EJ. Autoantibodies to intermediate filaments in sera of patients with Schistosoma mansoni infection. Clin Exp Immunol. 1989;77:230–3. [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan DB. Multiple range and multiple F-tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 30.Landis JR, Kock GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 31.Gladman DD, Urowitz MB, Keystone EC. Serologically active clinically quiescent systemic lupus erythematosus. A discordance between clinical and serologic features. Am J Med. 1979;66:210–5. doi: 10.1016/0002-9343(79)90529-1. [DOI] [PubMed] [Google Scholar]
- 32.D'cruz DP, Houssiau FA, Ramirez G, et al. Antibodies to endothelial cells in systemic lupus erythematosus: a potential marker for nephritis and vasculitis. Clin Exp Immunol. 1991;85:254–61. doi: 10.1111/j.1365-2249.1991.tb05714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaschek MA, Boehme M, Jouquan J, et al. Relation of antivimentin antibodies to anti-cardiolipin antibodies in systemic lupus erythematosus. Ann Rheum Dis. 1988;47:708–16. doi: 10.1136/ard.47.9.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richaud-Platin Y, Alcover-Varela J, Liorente L. High levels of TH2 cytokine gene expression in systemic lupus erythematosus. Rev Invest Clin. 1995;47:267–72. [PubMed] [Google Scholar]
- 35.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 36.Gearing AJH, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–12. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 37.Gearing AJ, Hemingway I, Pigott R, et al. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann NY Acad Sci. 1992;667:324–31. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 38.Pigott R, Dillon LP, Hemingway IH, et al. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine-activated cultured endothelial cells. Biochem Biophys Res Commun. 1992;187:584–9. doi: 10.1016/0006-291x(92)91234-h. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto Y, Nakano K, Yoshinoya S, et al. Endothelial cell destruction by polymorphonuclear leukocytes incubated with sera from patients with systemic lupus erythematosus (SLE) Scand J Rheumatol. 1992;21:209–14. doi: 10.3109/03009749209099226. [DOI] [PubMed] [Google Scholar]
- 40.Moore KL, Esmon CT, Esmon NL. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989;73:159–65. [PubMed] [Google Scholar]
- 41.Lentz SR, Tsiang M, Sadler JE. Regulation of thrombomodulin by tumor necrosis factor-α: comparison of transcriptional and posttranscriptional mechanisms. Blood. 1991;77:542–50. [PubMed] [Google Scholar]
- 42.Boehme MWJ, Deng Y, Raeth U, et al. Release of thrombomodulin from endothelial cells by concerted action of TNF-α and neutrophils: in vivo and in vitro studies. Immunol. 1996;87:134–40. [PMC free article] [PubMed] [Google Scholar]
- 43.Boehme MWJ, Autschbach F, Zuna I, et al. Elevated serum levels and reduced immunohistochemical expression of thrombomodulin in active ulcerative colitis. Gastroenterol. 1997;113:107–17. doi: 10.1016/s0016-5085(97)70086-6. [DOI] [PubMed] [Google Scholar]
- 44.Boehme MWJ, Schmitt WH, Youinou P, et al. Clinical relevance of elevated serum thrombomodulin and soluble E-selectin in patients with Wegener's granulomatosis and other systemic vasculitides. Am J Med. 1996;101:387–94. doi: 10.1016/S0002-9343(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 45.Hergesell O, Andrassy K, Nawroth P. Elevated levels of markers of endothelial cell damage and markers of activated coagulation in patients with systemic necrotizing vasculitis. Thromb Haemost. 1996;75:892–8. [PubMed] [Google Scholar]
- 46.Tanaka A, Ishii H, Hiraishi S, et al. Increased thrombomodulin values in plasma of diabetic men with microangiopathy. Clin Chem. 1991;37:269–72. [PubMed] [Google Scholar]
- 47.Takakuwa T, Endo S, Nakae H, et al. Plasma levels of TNF-alpha, endothelin-1 and thrombomodulin in patients with sepsis. Pharmacol. 1994;84:261–9. [PubMed] [Google Scholar]
- 48.Endo S, Inada K, Nakae H, et al. Blood levels of endothelin-1 and thrombomodulin in patients with disseminated intravascular coagulation and sepsis. Res Com Mol Pathol Pharmacol. 1995;90:277–88. [PubMed] [Google Scholar]
- 49.Boehme MWJ, Werle E, Kommerell B, et al. Serum levels of adhesion molecules and thrombomodulin as indicator of vascular injury in severe Plasmodium falciparum malaria. Clin Invest. 1994;72:598–603. doi: 10.1007/BF00227452. [DOI] [PubMed] [Google Scholar]
- 50.Hemmer CJ, Bierhaus A, Riedesel J, et al. Elevated thrombomodulin plasma levels as a result of endothelial involvement in Plasmodium falciparum malaria. Thromb Haemost. 1994;72:457–64. [PubMed] [Google Scholar]
- 51.Asakura H, Jokaji H, Saito M, et al. Plasma levels of soluble thrombomodulin increase in cases of disseminated intravascular coagulation with organ failure. Am J Hematol. 1991;38:281–7. doi: 10.1002/ajh.2830380406. [DOI] [PubMed] [Google Scholar]