Abstract
The innate immune system of severe combined immunodeficient (SCID) mice represents an important barrier to the successful engraftment of human cells. Different genetic and pharmacological strategies improve the graft survival. Non-obese diabetic (NOD)-SCID mice are better hosts for reconstitution with human peripheral blood leucocytes (Hu-PBL) because of their reduced natural killer cell and macrophage activity next to defective T and B cell functions. We investigated effects of TM-β1, a rat monoclonal antibody recognizing the mouse IL-2 receptor β-chain, on Hu-PBL survival and function in NOD-SCID and SCID mice. Relative to untreated littermates, TM-β1 improved Hu-PBL survival in SCID and NOD-SCID mice. Moreover, TM-β1-pretreated NOD-SCID mice displayed significantly better Hu-PBL survival and tissue distribution than TM-β1-pretreated SCID mice. Irradiation of NOD-SCID mice further enhanced the effects of TM-β1. However, these animals died within 3 weeks post-grafting due to graft-versus-host disease. Secondary immune responses were evaluated with Hu-PBL from a donor immune to hepatitis B surface antigen (HBsAg). In TM-β1-pretreated NOD-SCID mice, human HBsAg-specific memory B cells produced high titres of anti-HBsAg immunoglobulin irrespective of the administration of a secondary antigen booster dose. This contrasts with secondary immune responses in TM-β1-pretreated SCID mice where high titred antigen-specific immunoglobulins were produced when the appropriate antigen booster was given. In conclusion, reducing the function of the innate immune system in immunodeficient mice improves survival of the human graft and can result in an activation of the memory B cells without the need for recall antigen exposure.
Keywords: Hu-PBL-NOD-SCID, TM-β1, NK cell
INTRODUCTION
Animal models in which human cells can survive and function represent an interesting tool for the in vivo study of normal and deranged human immune function [1]. Severe combined immunodeficient (SCID) mice, lacking functional T and B lymphocytes, were originally thought to be adequate hosts for reconstitution with human peripheral blood leucocytes (Hu-PBL). However, the reconstitution of these SCID mice is low and transient due to the innate immune system of this mouse. The macrophages, polymorphonuclear cells and especially the natural killer (NK) cells in the SCID mouse have a normal or even enhanced activity, which leads to a rapid destruction of the graft [2,3]. The targeted reduction of the murine NK cell activity with antibodies directed towards specific NK cell membrane markers (e.g. anti-asialo-GM1, anti-N.K.-1.1 and TM-β1) or against NK cell products (anti-mouse interferon-gamma (IFN-γ)) improves both the survival of the human graft and the production of human immunoglobulin [4–7]. TM-β1, a rat anti-mouse IgG2b that binds to the β-chain of the IL-2 receptor (IL-2R) which is present on a subpopulation of CD8+ T cells and on all NK cells, is of particular interest, since this MoAb induces a long-lasting depletion of murine NK cell activity in normal and SCID mice [8]. Intraperitoneal injection of 1 mg TM-β1 1 day before Hu-PBL engraftment has pronounced and long-lasting effects on the survival, distribution and function of human cells in the SCID mouse [6].
In the past few years, new mouse strains with additional defects of the innate immune system have been developed. Back-crossing of SCID onto the NOD/Lt strain resulted in the non-obese diabetic (NOD)-SCID mouse, which has a reduced NK activity, macrophage function and serum haemolytic complement activity in addition to the deficit in mature T and B cells [9]. These NOD-SCID mice are better hosts for the Hu-PBL grafts with a concomitant higher human immunoglobulin production when compared with SCID mice [10]. No data are available as to whether the further reduction of the remaining NOD-SCID NK cell activity still can result in a additional improvement of the human cell engrafting.
Antigen-specific secondary immunoglobulin responses have generally been studied in untreated and NK cell-depleted SCID mice. The induction of antigen-specific human immunoglobulin in these SCID mice is largely dependent on early immunization with a recall antigen [6,11–15], because no or only low titred antigen-specific human immunoglobulins are observed when no recall antigen is administered [6,11,12,15,16]. To our knowledge, no data on secondary immune responses are available for the NOD-SCID mouse strain.
Since immunodeficient mouse strains are increasingly used for the study of human cell function in vivo, there is a continuous need for better human–mouse chimera that are well characterized in terms of graft survival and function. We evaluated whether pretreatment with TM-β1 of NOD-SCID mice had an effect on cell survival and humoral immune functions of intraperitoneally injected Hu-PBL. The nature of the human secondary immune response in these TM-β1-pretreated mice was studied and compared with untreated mice. In addition, we examined whether total body irradiation combined with TM-β1 pretreatment further affected graft survival and function in NOD-SCID mice.
MATERIALS AND METHODS
Production and purification of TM-β1
The hybridoma cell line TM-β1, which produces a rat MoAb recognizing the murine IL-2R β-chain (CD122) was kindly provided by Dr T. Tanaka [17]. Cells were grown in a miniPerm bioreactor (Heraeus Instruments, Hanau, Germany) [18]. Antibodies were purified from the cell culture supernatant by ammonium sulphate precipitation. After dialysis against PBS buffer, the solution was sterilized through a 0.22-μm filter, aliquoted and stored at −20°C. The purity of the antibody was tested using PAGE and the concentration was determined by spectrophotometry.
SCID and NOD-SCID mice
Homozygous C.B-17 scid/scid (SCID) mice and NOD/LtSz-scid/scid (NOD-SCID) mice were bred and maintained under specific pathogen-free conditions in the mouse colony of the Department of Immunology (University Hospital Gent). The NOD-SCID strain was free of Emv30, an endogenous murine ecotropic retrovirus responsible for the induction of lethal thymomagenesis [19]. All mice were housed in sterilized cages with filter tops and fed sterilized food and water ad libitum. Mice were used when 6–8-weeks old.
Pretreatment of mice and Hu-PBL reconstitution
Twenty-four hours before reconstitution with Hu-PBL, SCID or NOD-SCID mice (n = 24 per group) were injected intraperitoneally with 1 mg TM-β1 in 500 μl PBS. Control SCID or NOD-SCID (n = 24 per group) mice were injected with 500 μl PBS. In some experiments the TM-β1-pretreated NOD-SCID mice (n = 24) were irradiated with 3 Gy (gamma irradiation from a linear accelerator). Peripheral blood leucocytes (PBL) were isolated from heparinized venous blood using Ficoll–Hypaque (density = 1.077 g/ml) (Nycomed Pharma, Oslo, Norway) centrifugation and injected intraperitoneally (5 × 106 Hu-PBL/mouse in 500 μl PBS).
Human cell detection in mouse organs
At days 7 and 14 post-engraftment, blood was drawn by retro-orbital venous sinus puncture and collected in heparinized tubes. Upon centrifugation, plasma was harvested and the resuspended cell pellet was centrifuged over a Ficoll–Hypaque gradient (density = 1.077 g/ml) to remove murine erythrocytes. The latter procedure resulted in a partial enrichment of Hu-PBL within the final cell suspension since murine lymphoid cells passed through the gradient due to their higher density. Mice were killed by cervical dislocation and the peritoneal cavity was washed twice with 5 ml ice-cold PBS. Finally, spleen, thymus, lungs, inguinal lymph nodes and liver were removed for analysis. Single-cell suspensions were prepared from the spleen and lymph nodes for FACS analysis. Small parts of the spleen, liver, lungs and thymus were prepared for immunohistochemistry.
For FACS analysis, total viable cell numbers in the peritoneal lavage fluid (PELF), spleen and lymph nodes were counted by trypan blue exclusion. Flow cytometry analysis was done by gating on viable (propidium iodide-negative) mononuclear cells (human + murine). The following antibodies specific for human cell surface antigens were used: CD3, CD14, CD16, CD19, CD45 and CD56 (Becton Dickinson, San Jose, CA). All antibodies were conjugated with FITC or PE. Cells (105/sample) were incubated on ice with antibodies for 30 min followed by one wash with PBS containing 1% bovine serum albumin (BSA). At least 5000 cells were analysed on a FACScan (Becton Dickinson). In the case of PE-conjugated CD3 and CD45 MoAbs, the cell suspension was preincubated on ice for 15 min with anti-mouse Fcγ receptor antibodies to prevent aspecific colouring of mouse lymphoid cells. No human-specific colouring was observed in cell preparations derived from spleen or peritoneum of TM-β1-treated, non-reconstituted SCID or NOD-SCID mice.
For immunohistochemistry, lungs, thymus, spleen and liver were fixed in 4% paraformaldehyde and processed for paraffin embedding. Immunohistology was performed as described elsewhere [6,20]. After deparaffinization and rehydration, the 2.5 μm thick tissue sections were saturated with 20% normal human serum in Tris-buffered saline and subsequently incubated with mouse anti-human CD45 (Becton Dickinson) or mouse anti-human CD20 (Dako A/S, Glostrup, Denmark). Rabbit anti-mouse (Dako) and mouse alkaline phosphatase anti-alkaline phosphatase complex (Dako) were applied as second and third antibody. Visualization was achieved using fast red (Dako, Carpinteria, CA) before counterstaining sections with haematoxylin.
Determination of human IgG and IgM in mouse serum
Determination of total human IgG and IgM concentrations in mouse plasma was performed by ELISA. Microtitre plates (96-well; Nunc-Immunoplate Maxisorp; Nunc, Roskilde, Denmark) were coated with 100 μl (2 μg/ml PBS) rabbit anti-human IgG (Dako A/S, Glostrup, Denmark) or goat anti-human IgM (Cappel, Organon Teknika Corp., Durham, NC) for 1 h at 37°C and subsequently blocked for 2 h with 300 μl of 1% BSA in PBS at 37°C. In a third step, mouse serum or human immunoglobulin standards (Behring Diagnostics, Westwood, MA) diluted in PBS containing 0.5% BSA were added for 1 h at 37°C. After four washes, bound antibody was detected by incubating the plates with horseradish peroxidase-conjugated rabbit anti-human IgG (Dako) or goat F(ab′)2 anti-human IgM (Tago, Biosource, Camarillo, CA) for 1 h at 37°C followed by addition of tetramethylbenzidine (Sigma Chemical Co., St Louis, MO) for 30 min at room temperature. The enzymatic reaction was stopped with H2SO4 and plates were read at 450 nm. The lower detection limit was 10 and 1 ng/ml for IgG and IgM, respectively. Normal SCID or NOD-SCID serum were not reactive in either ELISA.
In vivo immunization and antigen-specific immunoglobulin detection in human and chimeric mouse plasma
In some experiments, 2 μg of aluminium hydroxide-adsorbed hepatitis B surface antigen (HBsAg; Engerix-B; Smith Kline Biologicals, Rixensart, Belgium) were injected subcutaneously in a hind leg, 1 day after Hu-PBL engraftment. The in vivo production of specific human anti-HBs immunoglobulin in human and mouse plasma was measured with the ETI-AB-AUK-3 anti-HBs enzyme immunoassay kit (Sorin Biomedica, Saluggia, Italy). Titres are expressed as U/l (detection limit 5 U/l). The in vivo production of human tetanus toxoid (TT)-specific immunoglobulin in human and SCID mouse plasma was measured using the Tetanus Toxoid Sensitive IgG Antibody Kit (Gamma S.A., Angleur, Belgium) with a detection limit of 50 U/l. The contribution of the booster dose to the synthesis of the human antigen-specific immunoglobulin responses was evaluated by calculating a booster index (BI), using the formula BI = (mean antigen-specific immunoglobulin response with antigen booster)/(mean antigen-specific immunoglobulin response without antigen booster).
Statistical analysis
The Statistical Package SPSS 6.1.2 (SPSS Inc., Chicago, IL) was employed. Different groups were compared using the Kruskal–Wallis H-test. When the Kruskal–Wallis significance level was P < 0.05, Mann–Whitney U-tests were applied as post hoc analysis. Correlations were analysed using the Spearman rank analysis.
RESULTS
TM-β1 pretreatment improves Hu-PBL engraftment in NOD-SCID mice
SCID and NOD-SCID mice were left untreated or were pretreated with 1 mg TM-β1. Twenty-four hours later, each animal was reconstituted by i.p. injection with 5 × 106 Hu-PBL, derived from the same donor. Human cellular engraftment and immunoglobulin production were evaluated at 7 and 14 days post-engraftment.
The NOD mutation and pretreatment with TM-β1 both had profound effects on the survival of human cells in the peritoneal cavity of SCID mice (Fig. 1). One week after the Hu-PBL transfer, higher numbers of human cells were found in the PELF of the NOD-SCID mice compared with the SCID mice. Pretreatment of SCID and NOD-SCID mice with TM-β1 markedly improved the survival of Hu-PBL in both mouse strains. Highest yields of human CD45+ cells were obtained in the TM-β1-pretreated NOD-SCID mice. After 2 weeks, the peritoneal cavities of the untreated SCID mice were almost devoid of human cells (4.9 ± 0.9 × 104), whereas the NOD-SCID mice and the TM-β1-pretreated SCID mice still harboured large amounts of human cells (1.4 ± 0.1 and 1.6 ± 0.2 × 106, respectively). Pretreatment with TM-β1 of the NOD-SCID mice resulted in a significantly better survival of human cells when compared with the three other groups (3.1 ± 0.1 × 106). The differences in human cell survival were also reflected by the percentages of human cells found in the PELF: at 1 week, percentages of human CD45+ cells in SCID and NOD-SCID mice were: without pretreatment 5.3 ± 1.9% and 13.3 ± 0.5%; and with pretreatment 30.9 ± 2.5% and 30.5 ± 2.2%, respectively. Two weeks after engraftment, in SCID and NOD-SCID mice without pretreatment 3.3 ± 0.4% and 28.5 ± 3.0%, and with pretreatment 62.3 ± 8.3% and 47.7 ± 1.7% human cells were found, respectively.
Fig. 1.
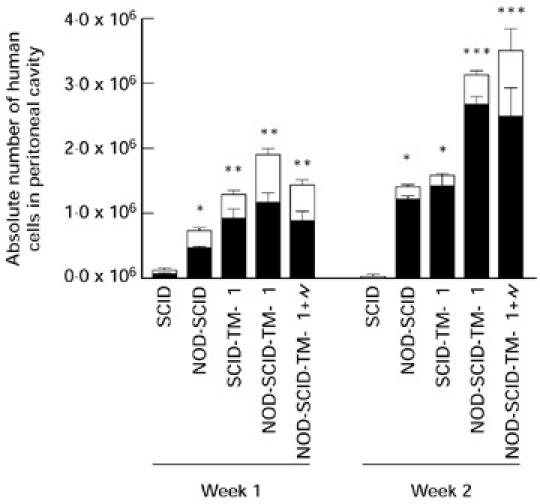
TM-β1 improves the survival of human cells in the peritoneal cavity of NOD-SCID mice when compared with SCID mice treated in the same way. SCID and NOD-SCID mice were pretreated or not 24 h before i.p. reconstitution with 5 × 106 Hu-PBL. One group of NOD-SCID mice was pretreated with TM-β1 and irradiated with 3 Gy (\object(90%,90%)="og1"). The survival of the human total (CD45+) and T (CD3+) cell populations was evaluated 7 and 14 days later by FACS analysis. Bars represent the mean values (± s.e.m.) of the absolute cell numbers recovered from the peritoneum of three mice. *P < 0.05 versus SCID; **P < 0.05 versus SCID and NOD-SCID; ***P < 0.05 versus SCID, NOD-SCID and SCID-TM-β1 mice.
Phenotypic analysis of the human cells recovered from the peritoneum showed that, irrespective of the pretreatment regimen and mouse strain, CD3+ T lymphocytes were the prevailing subset throughout the observation period (Fig. 1). After 1 week, CD19+ B cells represented 14.2 ± 1.2% in the TM-β1-pretreated SCID and NOD-SCID mice. Human NK cells (CD56+, CD16+) were also detectable after 1 week (15.8 ± 1.3%), whereas human CD14+ monocytes were not observed. Similar percentages were observed for the untreated SCID and NOD-SCID mice (data not shown). Two weeks after Hu-PBL engraftment, the percentage of human B cells (3.3 ± 0.5%) and NK cells (7.3 ± 0.7%) diminished, while there was a major survival of human T cells (87.8 ± 1.6%). In the TM-β1-pretreated animals, the T/B ratio averaged 4.8 ± 0.4 and 29.6 ± 4.2 1 and 2 weeks after engraftment, respectively, indicating the preferential survival of the human T cells.
TM-β1 pretreatment allows a high level of human cell engraftment in the spleen of NOD-SCID mice
The migration of human CD45+ cells from the peritoneal cavity towards different murine organs was investigated by FACS analysis and immunohistochemistry. In untreated SCID mice, human CD45+ cells were never detected either in blood, spleen, liver or thymus. Although we could not demonstrate a blood chimerism in NOD-SCID mice at 1 week, a small fraction of CD45+ human cells was detectable in the mouse blood 2 weeks post-engraftment (3.5 ± 1.4%). In TM-β1-pretreated SCID mice, human CD45+ cells were detectable in the mouse blood at 1 week (0.7 ± 0.1%) and more so at 2 weeks (8.2 ± 2.1%). The TM-β1-pretreated NOD-SCID mice turned out to be superior to all other groups and showed a significantly better blood chimerism at 1 and 2 weeks (1.4 ± 0.2% and 21.3 ± 8.1%, respectively). The percentage of human CD45+ cells in the PELF correlated with the blood chimerism (correlation coefficient r = 0.86, P < 0.001). Blood chimerism also correlated well with the percentage of human cells detected in the mouse spleen (r = 0.97, P < 0.001). One week after engraftment, no or very low percentages of human CD45+ cells could be detected in the spleen of untreated or TMβ1-treated mice, respectively (Table 1). Two weeks after engraftment, human cells were detectable in the spleens of untreated NOD-SCID mice (1.0 ± 0.2%). The TM-β1 pretreatment however allowed a significant human cellular infiltration which was significantly better in the spleens of NOD-SCID mice compared with the similarly treated SCID mice (18.4 ± 6.2% versus 2.5 ± 0.4%; P < 0.05). Phenotypical analysis of the human cell population in the spleen of these NOD-SCID mice revealed that only human T and B cells were present (data not shown). Again, T cells were the prevailing cell type. Two weeks after engraftment, human B cell survival was better supported in the TM-β1-pretreated NOD-SCID mice (3.0 ± 0.3% of the Hu-CD45+ cells; 1.1 ± 0.0 × 106) when compared with the TM-β1-pretreated SCID mice (0.5 ± 0.3%; 2.7 ± 1.5 × 105; P < 0.05).
Table 1.
TM-β1 pretreatment enhances human CD45+ cell distribution into the spleens of Hu-PBL-SCID and NOD-SCID mice
Mice were left untreated or were injected intraperitoneally with 1 mg TM-β1 24 h before reconstitution with 5 × 106 Hu-PBL. One group of NOD-SCID mice was pretreated with TM-β1 and irradiated with 3 Gy ( ). At indicated time points, the presence of human CD45+ cells was measured by FACS analysis. Values are expressed as mean percentage CD45+ human cells ± s.e.m. (absolute numbers of human cells ± s.e.m.).
). At indicated time points, the presence of human CD45+ cells was measured by FACS analysis. Values are expressed as mean percentage CD45+ human cells ± s.e.m. (absolute numbers of human cells ± s.e.m.).
*P < 0.05 versus SCID mice; **P < 0.05 versus SCID and NOD-SCID; ***P < 0.05 versus SCID, NOD-SCID and SCID-TM-β1 mice; ****P < 0.05 versus all other groups.
Using immunohistochemistry, we equally demonstrated a time, strain and pretreatment dependency of the degree of human cell infiltration in the peripheral tissues. TM-β1-pretreated NOD-SCID mice had more human cells in the lung, spleen, thymus and liver than TM-β1-pretreated SCID mice. The latter were in turn superior to the untreated mice (Fig. 2). The human cell infiltrates in the lungs of the SCID and NOD-SCID mice were characterized by a scattered pattern throughout the parenchyma associated with some peribronchial and perivascular cell aggregates. In the spleen and the liver, most human cells were scattered in the parenchyma or located around the blood vessels. In the thymus, the human cells were preferentially located in the cortex.
Fig. 2.
Immunohistochemical detection of human cells in the inguinal lymph nodes (a,b,c; mag. × 100) and in the lungs (d,e,f; mag. × 200) of the Hu-PBL NOD-SCID mice. NOD-SCID mice were left untreated (a,d) or were pretreated with 1 mg TM-β1 (b,e) or with 1 mg TM-β1 and irradiation (c,f). Twenty-four hours later, 5 × 106 Hu-PBL were injected intraperitoneally. Presence of human cells was evaluated 2 weeks after reconstitution. The human cells stain red following recognition of the human CD45 surface marker.
Importantly, the survival and distribution of the human cells in these TM-β1-pretreated SCID and NOD-SCID mice were not transient, but long lasting. High levels of human cell engraftment were detectable by FACS and immunohistochemistry in the mouse organs for at least 8 weeks after reconstitution (data not shown).
Survival and distribution of Hu-PBL in irradiated TM-β1-pretreated NOD-SCID mice
From the experiments outlined above, it is clear that the NOD-SCID mouse pretreated with TM-β1 represents an in vivo incubation system that strongly supports the survival and distribution of Hu-PBL. Using this combination of pretreatment (1 mg TM-β1 i.p.) and mouse strain (NOD-SCID), we analysed the survival, distribution and function of Hu-PBL in mice, which were in addition irradiated before reconstitution. Twenty-four hours before Hu-PBL cell transfer, the TM-β1-pretreated NOD-SCID mice were γ-irradiated with a sublethal dose of 3 Gy. Engraftment and tissue distribution of Hu-PBL were evaluated 7 and 14 days post-engraftment (Fig. 1 and Table 1). Seven days after Hu-PBL transfer, 69.6 ± 2.6% of all cells isolated from the PELF were positive for human CD45+. The percentage of CD45+ cells increased further to 80.7 ± 6.8% after 2 weeks. The absolute numbers of human cells (3.5 ± 0.6 × 106 CD45+ Hu-PBL after 2 weeks) were in fact comparable to those observed in the TM-β1-pretreated NOD-SCID mice without radiation described in the experiment outlined above (same blood donor). Phenotypical analysis of the human cells recovered from the peritoneum showed that, as in non-irradiated mice, the CD3+ human T cells were the predominant population (62.5 ± 2.4% and 71.1 ± 5.8% at 1 and 2 weeks, respectively). Human B cells and NK cells were also detectable throughout the experiment (data not shown).
The migration of the human cells from the peritoneum towards the different mouse tissues was accordingly studied. In the irradiated mice, the blood chimerism reached 22.1 ± 7.9% after 1 week and 76.3 ± 4.6% after 2 weeks. Seven days after engraftment, 10.3 ± 1.3% of the leucocytes in the spleen were CD45+ human cells (1.3 ± 0.1 × 105 cells). One week later, the human cells formed the majority of the leucocytes in the spleen (70.5 ± 3.6%, 9.9 ± 0.9 × 106 cells). Phenotyping of these cells showed that 13.4 ± 0.9% of the human cells in the spleen were CD19+ B cells, while the rest were CD3+ T cells. The immunohistological analysis supported these findings and showed that the infiltration of human cells was faster and more extensive in the irradiated and TM-β1-pretreated NOD-SCID mice. Moreover, the human CD45+ cells in the spleens of these mice were in a very close arrangement. This was not surprising in view of the FACS results, since in some animals up to 93% of the spleen leucocytes were of human origin. Despite the very high percentages of human cells found in the PELF and in the spleen of these irradiated mice, absolute numbers of human cells were comparable to those found in TM-β1-pretreated mice that were not irradiated. The Hu-PBL also spread into the bone marrow with a myeloid chimerism of 1.0 ± 0.1% and 26.9 ± 4.5% after 1 and 2 weeks, respectively, and into the lymph nodes (20.1 ± 1.8% and 90.5 ± 2.2% after 1 and 2 weeks, respectively).
Importantly, we observed a marked difference in the survival of the Hu-PBL-reconstituted mice depending on the pretreatment regimen. SCID mice pretreated or not with TM-β1, and NOD-SCID mice without TM-β1 pretreatment, appeared clinically healthy during an 8-week observation period. Severe suffering with mortality from 6 weeks on was noted in the TM-β1-pretreated NOD-SCID mice. In the case of the irradiated TM-β1 NOD-SCID mice, > 50% had succumbed within 3 weeks after Hu-PBL cell transfer, while the survivors showed overt clinical signs of graft-versus-host disease (GVHD) such as ruffled fur, hunched back, diarrhoea and wasting. The organs of these mice were heavily infiltrated with human cells concomitant with splenomegaly and enlarged lymph nodes (Fig. 2). None of the irradiated mice survived 4 weeks post-engraftment.
TM-β1 enhances human IgG and IgM production in SCID and NOD-SCID mice
Within 1 week after Hu-PBL reconstitution, both human IgG and IgM concentrations exceeded 1 μg/ml serum in all mice. Throughout the observation period, the TM-β1-pretreated SCID and NOD-SCID mice produced significantly more human immunoglobulin than their untreated counterparts (Table 2). Serum IgG concentrations were always higher than IgM concentrations regardless of the pretreatment regimen or mouse strain. Relative to TM-β1-pretreated SCID mice, TM-β1-pretreated NOD-SCID mice produced more IgG but less IgM. Irradiation further enhanced human immunoglobulin production in the TM-β1-pretreated NOD-SCID mice: 2100 ± 300 μg/ml and 280 ± 20 μg/ml for IgG and IgM, respectively, 2 weeks after Hu-PBL engraftment.
Table 2.
TM-β1 pretreatment enhances human IgG and IgM production in Hu-PBL-SCID and NOD-SCID mice

Mice were pretreated with TM-β1 or were left untreated. One group of NOD-SCID mice was pretreated with TM-β1 and irradiated with 3 Gy ( ). Twenty-four hours later, 5 × 106 Hu-PBL were injected intraperitoneally. Human immunoglobulin concentrations were determined by ELISA in mouse plasma obtained 2 weeks after the Hu-PBL transfer.
). Twenty-four hours later, 5 × 106 Hu-PBL were injected intraperitoneally. Human immunoglobulin concentrations were determined by ELISA in mouse plasma obtained 2 weeks after the Hu-PBL transfer.
*P < 0.05 versus SCID mice; **P < 0.05 versus SCID and NOD-SCID; ***P < 0.05 versus SCID, NOD-SCID and SCID-TM-β1 mice; ****P < 0.05 versus all other groups; †P < 0.05 versus NOD-SCID-TM-β1.
Antigen-specific human immunoglobulin production in TM-β1-pretreated NOD-SCID mice is booster-independent
Antigen-specific immunoglobulin production was studied in untreated and TM-β1-pretreated SCID and NOD-SCID mice engrafted with Hu-PBL from a donor that was immune to HBsAg (anti-HBs titre in donor serum: 2.9 × 104 U/l) and TT (TT-Ag) (anti-TT titre in donor serum: 2.4 × 104 U/l). All mice received a single injection of 2 μg HBsAg as recall antigen the day after Hu-PBL transfer. HBsAg-specific immunoglobulin production was observed in all mice (Table 3). SCID and NOD-SCID mice treated with TM-β1 produced significantly higher anti-HBs titres than their untreated counterparts. Two weeks post-engraftment, both the TM-β1-pretreated NOD-SCID and SCID mice produced high titres of anti-HBs immunoglobulin (4.1 ± 1.2 versus 2.8 ± 0.7 × 104 U/l, respectively; P > 0.05). Remarkably, the untreated NOD-SCID mice produced less anti-HBs immunoglobulin than untreated SCID mice (1.3 ± 0.7 versus 2.2 ± 1.1 × 102 U/l, 2 weeks post-engraftment; P < 0.05). Irradiation of the TM-β1 NOD-SCID mice accordingly resulted in the production of high titred anti-HBs immunoglobulin (6.3 ± 1.4 × 104 U/l). The necessity for in vivo recall antigen administration for this secondary immune response was examined by measuring the anti-TT immunoglobulin serum titres in these mice. None of them received a TT booster. Untreated mice did not produce detectable levels of anti-TT immunoglobulin. However, after TM-β1 pretreatment, both mouse strains mounted an anti-TT immunoglobulin response within 2 weeks after engraftment. Serum titres were however multiple-fold higher in the NOD-SCID mice than in SCID mice. Accordingly, combined pretreatment of NOD-SCID mice with TM-β1 and radiation further enhanced anti-TT immunoglobulin production (3.1 ± 0.4 × 103 U/l). Thus, the pretreated NOD-SCID mice tended to produce high amounts of antigen-specific immunoglobulin even in the absence of a recall antigen. This was further evaluated in an additional experiment in which we calculated a BI using the formula BI = (mean antigen-specific immunoglobulin response with antigen booster)/(mean antigen-specific immunoglobulin response without antigen booster). TM-β1-treated SCID and NOD-SCID mice received placebo or a single injection of 2 μg HBsAg as recall antigen the day after the Hu-PBL transfer (donor immune for HBsAg). In the SCID mice strain, the BI was 11 one week after engraftment, while in the case of the NOD-SCID mice the anti-HBs immunoglobulin titres rose only two-fold upon the antigen boost (BI = 2). In the irradiated TM-β1-pretreated NOD-SCID mice, no difference in anti-HBs immunoglobulin titre was observable between the boosted and non-boosted animals (BI = 1). Accordingly, other antigen-specific human immunoglobulins (anti-TT, anti-Rubella, anti-varicella zoster, anti-toxoplasmosis, anti-measles and anti-mumps immunoglobulin) were detected in TM-β1-pretreated NOD-SCID mice, provided the Hu-PBL donor had a positive serology for these antigens (data not shown).
Table 3.
The effect of TM-β1 pretreatment on the recall immune responses in Hu-PBL-SCID and NOD-SCID mice
Mice were pretreated with TM-β1 or were left untreated. Twenty-four hours later, 5 × 106 Hu-PBL were injected intraperitoneally. One group of NOD-SCID mice was pretreated with TM-β1 and irradiated with 3 Gy ( ). The Hu-PBL donor was immune for hepatitis B surface antigen (HBsAg) (2.9 × 104 U/l) and for tetanus toxoid (TT) (2.4 × 104 U/l). Another 24 h later, all mice were boosted with 2 μg HBsAg. Anti-HBs- and anti-TT-specific human immunoglobulin concentrations were determined by ELISA in plasma obtained 2 weeks after reconstitution. Values are expressed as means (± s.e.m.).
). The Hu-PBL donor was immune for hepatitis B surface antigen (HBsAg) (2.9 × 104 U/l) and for tetanus toxoid (TT) (2.4 × 104 U/l). Another 24 h later, all mice were boosted with 2 μg HBsAg. Anti-HBs- and anti-TT-specific human immunoglobulin concentrations were determined by ELISA in plasma obtained 2 weeks after reconstitution. Values are expressed as means (± s.e.m.).
*P < 0.05 versus untreated SCID and NOD-SCID; **P < 0.05 versus SCID, NOD-SCID and SCID-TM-β1 mice; †P < 0.05 versus NOD-SCID mice.
DISCUSSION
SCID mice become suitable hosts for human haematopoietic and lymphoid cell grafts only when the grafts can survive and function normally for an extended period (i.e. weeks). Different pretreatment regimens of SCID mice have resulted in an improved human cellular engraftment. Long-term survival of functional human lymphoid cells in SCID mice has been reported following the targeted reduction of the endogenous murine NK activity using TM-β1 antibodies directed towards the murine IL-2R β-chain [6]. The development of new mouse strains (e.g. SCID-Bg, Bg-nu-XID and NOD-SCID) all characterized by additional immune defects next to T and B cell deficiency has provided researchers with improved in vivo incubation systems for the Hu-PBL grafts [9,21,22]. NOD-SCID mice, especially, are convincingly better hosts for Hu-PBL grafts compared with SCID mice [10].
In this study, we confirm these findings and demonstrate that Hu-PBL engraftment in NOD-SCID mice can be further enhanced by treatment of the host with TM-β1 prior to reconstitution. Markedly higher numbers of human cells were recovered from the peritoneal cavity of NOD-SCID mice that received TM-β1 pretreatment compared with untreated counterparts or TM-β1-pretreated SCID mice. The human cells recovered from mouse peritoneal cavities consisted mainly of T cells and to a much lesser extent B cells and NK cells. This finding is in agreement with observations in untreated SCID and NOD-SCID mice [10]. Accordingly, the distribution of human cells in the circulation (blood chimerism) and in the different tissues (spleen, lungs, liver and thymus) was greater in TM-β1-treated mice and always higher in NOD-SCID than in SCID mice. The fact that TM-β1 has marked effects on the survival of human cells in the NOD-SCID mouse suggests that the remaining NK activity (with concomitant IFN-γ production and macrophage activation [7]) in the NOD-SCID mouse still has a major impact on graft survival and on the migration of the human cells from the peritoneum. These findings all represent an area of obvious clinical relevance, since they stress the importance of host NK activity in the graft rejection process. The combination of TM-β1 and radiation in NOD-SCID mice resulted in a further improvement in the relative survival and especially the spreading of human cells from the peritoneum. In these irradiated TM-β1-pretreated NOD-SCID mice, the absolute numbers of surviving human cells in the peritoneum were in the same range as that in the non-irradiated TM-β1-pretreated NOD-SCID mice. The enhanced spreading of the human cells in the irradiated mice therefore suggests an effect on the Hu-PBL distribution via the inhibition of the mouse peritoneal leucocyte population rather than via the stimulation of the human graft growth. In addition, the presence of human cells in these engrafted mice is the result not only of survival and spreading, but also of graft proliferation. Indeed, in the TM-β1-pretreated NOD-SCID mice (regardless of irradiation), the number of human cells harvested from the spleen alone exceeded the number of cells used to engraft the animal. This suggests a critical interplay between graft survival, spreading and proliferation. The enormous accumulations of human cells throughout the different tissues, however, had vast consequences on the health of these mice. While TM-β1-pretreated SCID mice remained in good health (with observations up to 8 weeks post-engraftment), TM-β1-pretreated NOD-SCID mice developed lethal GVHD within 6 weeks, while supplemental irradiation of the mice induced a mortality of 50% within 3 weeks.
The effects of TM-β1 on the cellular reconstitution in NOD-SCID mice were accompanied by comparable effects on the production of human IgG and IgM. The TM-β1-pretreated animals produced significantly more human immunoglobulin than their untreated littermates. Human IgG production was significantly higher in pretreated NOD-SCID mice relative to pretreated SCID mice, while IgM concentrations were lower. This suggests differences in the metabolism of the human immunoglobulin or in the stimulation of the human memory B cells between the NOD-SCID and SCID mouse strains after TM-β1. Remarkably, human immunoglobulin production as well as Hu-PBL infiltration in the spleen of the untreated NOD-SCID mice was significantly lower when compared with TM-β1-pretreated SCID mice, despite the comparable Hu-PBL survival in the peritoneum (2 weeks post-engraftment). The residual NK activity in these untreated NOD-SCID mice therefore affects not only Hu-PBL survival, but also the distribution of the engrafted cells as well as the production of human immunoglobulin. Human immunoglobulin production in irradiated TM-β1-pretreated NOD-SCID mice was of comparable magnitude compared with the TM-β1-treated NOD-SCID mice. Overall, these results suggest that radiation pretreatment in the TM-β1 NOD-SCID mice has vast effects on the survival/proliferation and distribution of the human cells, while its beneficial effect on human immunoglobulin production is less clear.
The chimeric SCID and NOD-SCID mice represent interesting tools to produce human immunoglobulin with varying specificities outside the human body. The human B cells producing the immunoglobulin specificity of interest can be isolated from Hu-PBL reconstituted mice and immortalized either through cellular or molecular biology techniques [12,15], generating human immunoglobulin-producing cell clones. In SCID mice it has been demonstrated that the generation of antigen-specific human immunoglobulin depends not only on the survival and migration of the human cells, but also on the presence of a selected recall antigen at an early and not a later stage of engraftment [11–15,23,24]. In contrast to the observations in SCID and TM-β1-pretreated SCID mice, we found that the production of antigen-specific immunoglobulin in the TM-β1 NOD-SCID mice was much less dependent on the administration of an antigen boost. A broad repertoire of human antibodies emerged in these mice, since human immunoglobulin could be detected for other antigen specificities such as TT, mumps, varicella zoster virus, rubella, measles and toxoplasmosis (as long as the Hu-PBL donor had positive serum titres for these pathogens). The combination of an overwhelming survival of human cells and xenoreactivity is probably the driving force for this booster-independent immunoglobulin production. Radiation pretreatment in the TM-β1-pretreated NOD-SCID mice completely abolished the need for an antigen boost, since no difference in antigen-specific immunoglobulin production was observed between immunoglobulin-boosted and non-boosted mice.
The characteristics of the different Hu-PBL-SCID and NOD-SCID mouse models used in our study can be graphically represented (Fig. 3). Two series of characteristics appear that are inversely correlated. On the one hand there are the survival and spreading of the human lymphoid cells and the Hu-Ig production that go along with the vigour of the GVHD. On the other hand, there are the life span of the animals and the booster dependency, both inversely related to the severity of the GVHD. Untreated SCID mice have a poor human cellular survival and only develop antigen-specific immunoglobulin when a booster with a recall antigen is administered, while the irradiated TM-β1-NOD-SCID mice have an overwhelming human cellular survival and antigen-specific immunoglobulin production even in the absence of an antigen booster. The former mouse is suitable for the study of booster antigen-dependent secondary immune responses, while the latter opens possibilities for the development of human immunoglobulin-secreting cell lines. The TM-β1-pretreated SCID mouse occupies an intermediate position and is characterized by a good cellular survival combined with a fast and high-titred antigen-specific immunoglobulin production, provided the mice receive an antigen booster. The choice of the appropriate mouse model should therefore depend on the goals that are faced and the research questions that are asked.
Fig. 3.

Representation of the influence of different pretreatment regimens in SCID and NOD-SCID mice on the survival and spreading of transplanted Hu-PBL, on human immunoglobulin production, on the life span of the transplanted animals, on the severity of graft-versus-host disease (GVHD) and on the necessity of a booster dose for antigen-specific immunoglobulin production.
Acknowledgments
The presented experimental work was approved by the local ethical committees of the University of Gent (Ethical Committee for Laboratory Animals, project 96/5 and Committee for Medical Ethics, project 96/58). K.G.T. is supported by the Fund for Scientific Research, Flanders (FWO-Vlaanderen). J. Plum, G. Leclercq and M. Desmet (Immunology Department, University Hospital, Gent) are thanked for providing the possibilities to produce the TM-β1 antibody. T. Boterberg is thanked for his help with the irradiation of the mice. The authors are grateful to H. Spits and K. Weijer for the NOD/LtSz-scid/scid mouse strain. L. Verhoye, A. Neesen, I. De Borle and M. Mouton are thanked for their excellent technical assistance.
REFERENCES
- 1.Tary-Lehmann M, Saxon A, Lehmann P. The human immune system in hu-PBL-SCID mice. Immunol Today. 1995;16:529–33. doi: 10.1016/0167-5699(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 2.Ansell JD, Bancroft BJ. The biology of the SCID mutation. Immunol Today. 1989;10:322–5. doi: 10.1016/0167-5699(89)90181-3. [DOI] [PubMed] [Google Scholar]
- 3.Shibata S, Asano T, Noguchi A, Naito M, Ogura A, Doi K. Peritoneal macrophages play an important role in eliminating human cells from severe combined immunodeficient mice transplanted with human peripheral blood lymphocytes. Immunology. 1998;93:524–32. doi: 10.1046/j.1365-2567.1998.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shpitz B, Chambers CA, Singhal AB, et al. High level functional engraftment of severe combined immunodeficient mice with human peripheral blood lymphocytes following pretreatment with radiation and anti-asialo GM1. J Immunol Methods. 1994;169:1–15. doi: 10.1016/0022-1759(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 5.Christianson SW, Greiner DL, Schweitzer IB, Gott B, Beamer GL, Schweitzer PA, Hesselton RAM, Shultz LD. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol. 1996;171:186–99. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 6.Tournoy KG, Depraetere S, Meuleman P, Leroux-Roels GG, Pauwels RA. Murine IL-2 receptor beta chain blockade improves human leukocyte engraftment in SCID mice. Eur J Immunol. 1998;28:3221–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3221::AID-IMMU3221>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Shibata S, Asano T, Noguchi A, Kimura H, Ogura A, Naiki M, Doi K. Enhanced engraftment of human peripheral blood lymphocytes into anti-murine interferon-gamma monoclonal antibody-treated CB-17-scid mice. Cell Immunol. 1998;83:60–9. doi: 10.1006/cimm.1997.1238. [DOI] [PubMed] [Google Scholar]
- 8.Ehl S, Nuesh R, Tanaka T, Myasaka M, Hengartner H, Zinkernagel R. A comparison of efficacy and specificity of three NK depleting antibodies. J Immunol Methods. 1996;199:149–53. doi: 10.1016/s0022-1759(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 9.Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–91. [PubMed] [Google Scholar]
- 10.Greiner DL, Shultz LD, Yates J, et al. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am J Pathol. 1995;146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 11.Nonoyama S, Smith FO, Ochs HD. Specific antibody production to a recall or a neoantigen by Scid mice reconstituted with human peripheral blood lymphocytes. J Immunol. 1993;151:3894–901. [PubMed] [Google Scholar]
- 12.Carlsson R, Martensson C, Kalliomaki S, Ohlin M, Borrebaeck CAK. Human peripheral blood lymphocytes transplanted into SCID mice constitute an in vivo culture system exhibiting several parameters found in a normal humoral immune response and are a source of immunocytes for the production of human monoclonal antibodies. J Immunol. 1992;148:1065–71. [PubMed] [Google Scholar]
- 13.Pestel J, Jeannin P, Delneste Y, Dessaint JP, Cesbron JY, Capron A, Tsicopoulos A, Tonnel AB. Human IgE in SCID mice reconstituted with peripheral blood mononuclear cells from Dermatophagoides pteronyssinus-sensitive patients. J Immunol. 1994;153:3804–10. [PubMed] [Google Scholar]
- 14.Sandhu J, Shpitz B, Gallinger S, Hozumi N. Human primary immune response in SCID mice engrafted with human peripheral blood lymphocytes. J Immunol. 1994;152:3802–13. [PubMed] [Google Scholar]
- 15.Duchosal MA, Eming SA, Fisher P, et al. Immunization of hu-PBL-SCID mice and the rescue of human monoclonal Fab fragments through combinatorial libraries. Nature. 1995;355:258–62. doi: 10.1038/355258a0. [DOI] [PubMed] [Google Scholar]
- 16.Duchosal MA, Eming SA, McConahey PJ, Dixon FJ. Characterization of hu-PBL-SCID mice with high human immunoglobulin serum levels and graft-versus-host disease. Am J Pathol. 1992;141:1097–113. [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Tsudo M, Karasuyama H, Kitamura F, Kono T, Hatakeyama M, Taniguchi T, Miyasaka M. A novel monoclonal antibody against murine IL-2 receptor beta-chain. Characterization of receptor expression in normal lymphoid cells and EL-4 cells. J Immunol. 1991;147:2222–8. [PubMed] [Google Scholar]
- 18.Falkenberg FW, Weichert H, Krane M, Bartels I, Palme M, Nagels HO, Fiebig H. In vitro production of monoclonal antibodies in high concentration in a new and easy to handle modular minifermenter. J Immunol Methods. 1995;179:13–29. doi: 10.1016/0022-1759(94)00266-y. [DOI] [PubMed] [Google Scholar]
- 19.Serreze DV, Leiter EH, Hanson MS, Christianson SW, Shultz LD, Hesselton RM, Greiner DL. Emv30null NOD-scid mice. An improved host for adoptive transfer of autoimmune diabetes and growth of human lymphohematopoietic cells. Diabetes. 1995;44:1392–8. doi: 10.2337/diab.44.12.1392. [DOI] [PubMed] [Google Scholar]
- 20.Duez C, Tsicopoulos A, Janin A, et al. An in vivo model of allergic inflammation: pulmonary human cell infiltrate in allergen-challenged allergic Hu-SCID mice. Eur J Immunol. 1996;25:1088–93. doi: 10.1002/eji.1830260520. [DOI] [PubMed] [Google Scholar]
- 21.Shibata S, Asano T, Ogura A, Hashimoto N, Hayakawa J, Uetsuka K, Nakayama H, Doi K. SCID-bg mice as xenograft recipients. Lab Anim. 1997;31:163–8. doi: 10.1258/002367797780600107. [DOI] [PubMed] [Google Scholar]
- 22.Jicha DL, Yannelli JR, Custer M, Colandrea J, Taubenberger J, Mule JJ, Rosenberg SA. The persistence of human peripheral lymphocytes, tumor infiltrating lymphocytes, and colon adenocarcinomas in immunodeficient mice. J Immunother. 1992;11:19–29. doi: 10.1097/00002371-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Albert SE, McKerlie C, Pester A, Edgell BJ, Carlyle J, Petric M, Chamberlain JW. Time dependent induction of protective anti-influenza immune responses in human peripheral blood lymphocyte SCID mice. J Immunol. 1997;159:1393–403. [PubMed] [Google Scholar]
- 24.Martensson C, Kristensson K, Kalliomaki S, Borrebaick K, Carlsson R. Antigen-specific human immunoglobulin production in SCID mice transplanted with human peripheral lymphocytes is dependent on CD4+ CD45RO+ T cells. Immunology. 1994;83:171–9. [PMC free article] [PubMed] [Google Scholar]





