Abstract
Recently markers of endothelial cell activation or injury gained increasing interest as serological parameters of disease activation in vasculitides. Among these, soluble serum thrombomodulin, ICAM-1, VCAM-1 and E-selectin are of particular interest. However, only thrombomodulin showed the expected close correlation. The objective of this study was to investigate in vitro the kinetics of these endothelial cell receptors after interaction of unstimulated or cytokine-activated polymorphonuclear neutrophils (PMN) and endothelial cells in order to find evidence explaining these different clinical findings. Over the time period of up to 48 h of incubation the kinetics of thrombomodulin, ICAM-1, E-selectin, and VCAM-1 levels in the supernatant of endothelial cells in co-culture with neutrophils were determined in vitro by ELISA under basal and partially cytokine-activated (tumour necrosis factor-alpha) conditions. Increased levels of ICAM-1, E-selectin and VCAM-1 were already found due to cytokine activation of endothelial cells alone. This increase was augmented after coincubation with neutrophils. In contrast, a significant increase of thrombomodulin in the supernatant was only found due to cell injury after cell–cell interaction of cytokine-activated endothelial cells with neutrophils. In conclusion, this in vitro model of the kinetics of soluble endothelial cell receptors after cell–cell interaction of cytokine-activated PMN and endothelial cells underlines the advantage of thrombomodulin in contrast to the adhesion molecules as a marker of endothelial damage. Therefore, soluble thrombomodulin seems to be a promising, valuable serological disease activity marker in vasculitides.
Keywords: endothelial cell damage, thrombomodulin, adhesion molecules, vasculitides, disease activity
INTRODUCTION
Vasculitides are multisystemic autoimmune disorders of unknown aetiology. Pathophysiologically they are characterized by inflammatory destruction of the vascular wall and of endothelial cells in particular [1]. Specific, pathophysiologically determined, serological markers of disease activity are still lacking. However, reliable serological markers are required due to the considerable variation of organ involvement and disease manifestations. Recently markers of endothelial cell activation or injury have gained increasing interest as serological parameters. Among these soluble serum thrombomodulin (TM), ICAM-1 (CD54), VCAM-1 (CD106), and E-selectin (CD62E) are of special interest [2,3].
TM is a transmembranous glycoprotein receptor for thrombin. Its soluble form is mainly found in the serum or culture supernatant after endothelial cell injury [4–8]. ICAM-1, VCAM-1 and E-selectin are members of the adhesion molecule family. In cases of inflammation these adhesion molecules are required for the interaction of the immunologically active cells with the local cells—in particular endothelial cells—at the inflammatory tissue side [9,10]. In vivo as well as in vitro soluble forms of these molecules are already found after cell activation with proinflammatory cytokines [2,3,11].
Recently we showed that serum TM is the most promising parameter of these soluble endothelial receptor proteins to indicate disease activity in systemic lupus erythematosus (SLE), Wegener's granulomatosis, ulcerative colitis and other systemic vasculitides [12–15]. In the present in vitro study we additionally investigate the kinetics of soluble endothelial cell receptor proteins after interaction of human endothelial cells with polymorphonuclear neutrophils (PMN) as a model, which closely mimics pathophysiological conditions, in order to find evidence for the exceptional advantage of sTM as a disease activity marker in vasculitides.
MATERIALS AND METHODS
Endothelial cell culture
Human umbilical vein endothelial cells (HUVEC) were isolated and cultured according to the method of Maruyama [16] and Jaffe [17] with minor modifications, as described previously [8]. In brief, HUVEC were isolated from human umbilical cord veins by collagenase digestion (0.1% collagenase from Clostridium histolyticum; Boehringer Mannheim, Mannheim, Germany), washed and cultured in tissue culture flasks precoated with fibronectin (5 μg/cm2; Boehringer Mannheim). They were grown in medium 199 (Sigma-Alderich Chemie GmbH, Deisenhofen, Germany; containing 200 mmol l-glutamine, 100 μg/ml endothelial growth factor (Sigma-Alderich Chemie GmbH), 100 μg/ml heparin (Sigma-Alderich Chemie GmbH), 20% fetal calf serum (FCS; Seromed; Biochrom KG, Berlin, Germany) and penicillin 50 U/ml and streptomycin 50 μg/ml (Sigma-Alderich Chemie GmbH)).
The purity of the endothelial cell culture was checked by indirect immunofluorescence (IIF) using an anti-TM MoAb as primary antibody (Dako-Diagnostik, Hamburg, Germany). In addition, the anti-coagulant activity of the endothelial cells was investigated as proof of the quality of the grown cells as described elsewhere [18]. Only cell cultures which were > 95% pure and prolonged the coagulation time for > 120 s (= more than × 4) were used. For experiments the second passage of endothelial cells was subcultured in 24-well plates in serum-free medium at near confluent cell density. The cell cultures were partially preactivated with recombinant human tumour necrosis factor-alpha (rhTNF-α; 10 ng/ml medium; Boehringer Mannheim). This concentration of rhTNF-α had previously been tested as sufficient and optimal for the induction of the expression of adhesion molecules on endothelial cells. In addition, this concentration of rhTNF-α had previously been used in in vitro experiments [10]. In the co-culture experiments neutrophils with a final concentration of 2 × 106/ml were added to the wells. The PMN were partially preactivated with rhTNF-α (10 ng/ml) as specified below. After treatment the culture supernatant was harvested and immediately cleaved by centrifugation (10 min, 2000 g) and the supernatants stored at − 20°C. The experiments were performed in duplicates and independently repeated three times.
Isolation of PMN
Neutrophils were isolated from human peripheral blood of healthy Caucasian volunteers as described earlier [8]. In brief, neutrophils were isolated from 100 ml of anti-coagulated EDTA-blood by Polymorphprep gradient centrifugation according to the manufacturer's instructions (Immuno, Heidelberg, Germany; 1500 g for 30 min). Residual erythrocytes were removed by hypotonic lysis (0.2% NaCl for 20 s, thereafter immediately addition of 1.6% NaCl and centrifugation at 1500 g for 10 min). Finally, cells were resuspended in RPMI 1640 supplemented with 10% FCS at a concentration of 2 × 107 neutrophils/ml.
Coincubation of endothelial cells and PMN
The kinetics of soluble adhesion molecules and TM was studied using an in vitro model of coincubation of endothelial cells with PMN, whereby two consecutive 24-h periods of incubation were investigated. In the first 24-h period of incubation the endothelial cells alone were cultured in the presents or absence of rhTNF-α (10 ng/ml medium). The concentrations of the adhesion molecules ICAM-1, VCAM-1, or E-selectin as well as of TM in the culture supernatant were measured after 0, 1, 4 and 24 h of incubation.
In the second step PMN were added to the endothelial cell cultures at a final concentration of 2 × 106 cells/ml. In addition, one part of these PMN had been pretreated with rhTNF-α (10 ng/ml) for 2 h. The unstimulated as well as the activated PMN were added to the endothelial cell cultures as concentrate without additional washing in order not to alter the cells or induce artificially liberation of radicals or proteases. The respective endothelial cell receptors were determined after 0.5, 1, 4 and 24 h in the supernatant of separate culture wells.
Immunological assays
A prototype two-site ELISA was used for the determination of TM in culture supernatants (Thrombomodulin VarElisa, charge no. 17067; ELIAS/Pharmacia & Upjohn, Freiburg, Germany). The test was performed according to the manufacturer's instructions as described elsewhere [15]. Briefly, the precoated 96-well plates were washed with buffer once and than incubated with diluted samples or provided standards (50 μl culture supernantant and 75 μl sample buffer). After 1 h of incubation at room temperature the plates were washed three times and further incubated with the peroxidase-conjugated secondary anti-TM antibody (100 μl/well) for 1 h. Subsequently, the plates were washed again and incubated with the substrate solution (tetramethylbenzidine (TMB)) at room temperature in the dark. After 10 min the stop solution (2 n H2SO4) was added and the optical density (OD) measured after colour stabilization (30 min) by an automated ELISA plate reader at 450 nm (Titertek Multiscan Plus MKII; ICN/Flow, Meckenheim, Germany). The respective sample concentrations of TM were calculated in relation to the reference standard curve. The samples were tested in duplicates and the mean taken for further calculations.
Commercially available two-site ELISAs were used to determine the levels of sICAM-1 (Parameter human soluble ICAM-1; R&D Systems GmbH, Wiesbaden, Germany), sE-selectin (Parameter human soluble E-Selectin; R&D Systems GmbH), and sVCAM-1 (Parameter human soluble VCAM-1; R&D Systems GmbH) in the culture supernatant (dilution 1:2).
Statistical analysis
If not otherwise stated the mean and s.e.m. are given. The non-parametric Wilcoxon–Mann–Whitney U-test was used to determine the significance between the different culture conditions in the distinct in vitro experimental settings. P ≤ 0.05 was considered significant. The analysis was perfomed with WinSTAT 3.1 (Kalmia Co. Inc., Cambridge, MA).
RESULTS
Kinetics of soluble receptors after cytokine activation of endothelial cells during the preincubation period
Thrombomodulin
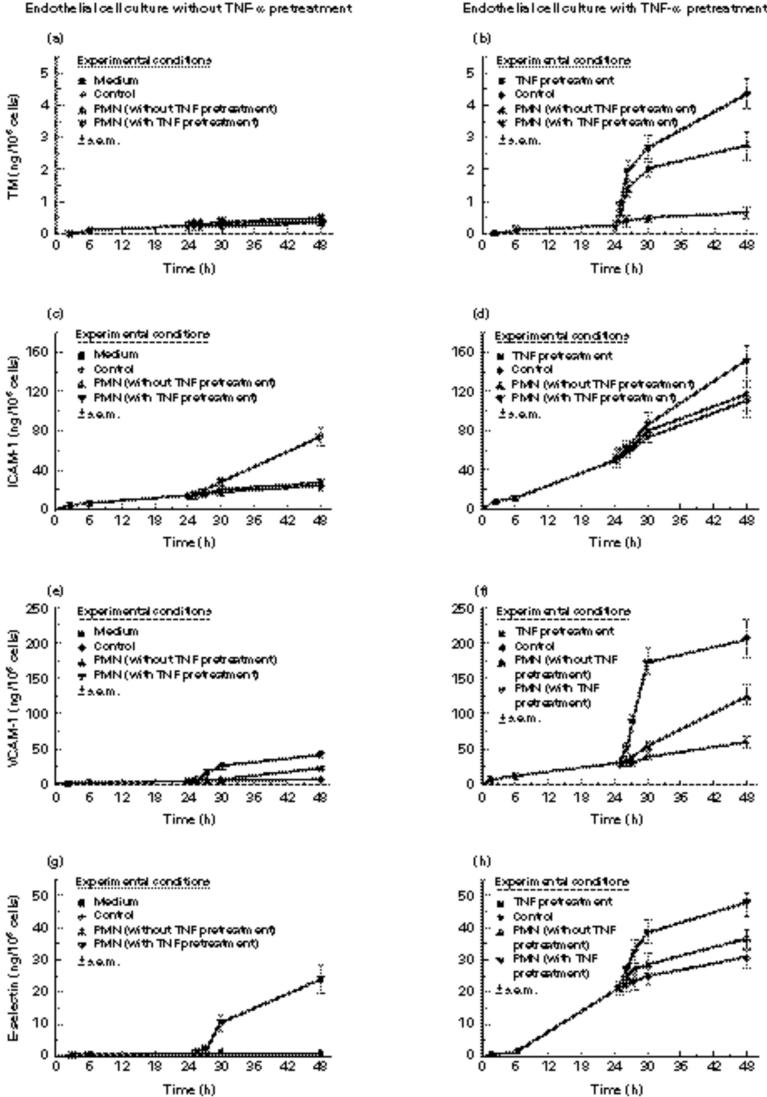
During the first 24 h of preincubation no significant changes or increase of TM levels occurred in the supernatant either of the controls or of the cytokine-activated endothelial cell cultures (Fig. 1a,b).
Fig. 1.
The kinetics are shown for soluble thrombomodulin (TM) (a,b), ICAM-1 (c,d), VCAM-1 (e,f), and E-selectin (g,h) in the supernatant of human umbilical vein endothelial cell culture over 24 h or 48 h. ▪, Control cultures in the first 24-h period of incubation; •, control cultures in the second 24-h period of incubation. The endothelial cells were partially stimulated with recombinant human tumour necrosis factor-alpha rhTNF-α (b,d,f,h) or co-cultured with neutrophils (▴). The latter had been partially prestimulated with rhTNF-α as well (▾). The experiments were performed in duplicates and repeated three times.
ICAM-1
In contrast to TM a minor increase of ICAM-1 (P < 0.05) was already found in the supernatant of the control culture. However, marked and significantly increased levels of ICAM-1 were measured in the presence of rhTNF-α (P < 0.01) (Fig. 1c,d).
VCAM-1
In the control culture no significant changes of VCAM-1 levels were detected. However, the levels rose significantly after cytokine activation of endothelial cells (P < 0.01) (Fig. 1e,f).
E-selectin
The E-selectin values of the control cultures remained constant during the preincubation period. In contrast, the activation of the endothelial cells resulted in a significant increase of E-selectin in the supernatant (P < 0.01) (Fig. 1g,h).
Co-incubation of endothelial cells and PMN
Thrombomodulin
The second 24-h period of coincubation of endothelial cells with PMN did not lead to a significant increase of TM in the cultures with unstimulated endothelial cells. In contrast, a significant increase of TM in the supernatant occurred after prestimulation of the endothelial cells with rhTNF-α and PMN coincubation (P < 0.01). This TM increase was additionally augmented in the case of prestimulated PMN (Fig. 1a,b).
ICAM-1, VCAM-1, E-selectin
In contrast, compared with the values at the end of the 24-h preincubation period, significantly increased levels of the adhesion molecules ICAM-1, E-selectin, and VCAM-1 were already found in the co-culture if one of the two cell types was prestimulated with rhTNF-α (P < 0.01). This increase could be augmented by preincubation of both cell types with rhTNF-α (Fig. 1c–h). Furthermore, a significant but weaker increase was found in the rhTNF-α-pretreated control cultures for all three adhesion molecules ICAM-1, VCAM-1 and E-selectin (P < 0.05). In addition, the pre-existing ICAM-1 increase continued in the untreated control culture (Fig. 1c–h).
Control cultures of PMN showed no detectable levels of the respective receptors either under unstimulated or under activated conditions.
DISCUSSION
Vasculitides are an inhomogeneous group of multisystemic autoimmune diseases. They are characterized by inflammatory damage of distinct vessels of different sizes and of endothelial cells in particular. Pathophysiologically several immunological mechanisms are involved. These include local secretion of proinflammatory cytokines like TNF-α or IL-1, cytokine activation of endothelial cells as well as leucocytes, increased expression of adhesion molecules, antibody or immune complex-mediated local inflammation, and increased cellular cytotoxicity (e.g. PMN to endothelial cells) [1,10,19–21].
Therefore, recently serological parameters of endothelial cell activation, injury or leucocyte interaction have increasingly gained interest as disease activity markers of vasculitides [2,3]. However, in general only a weak significance was found in vivo for these soluble endothelial cell markers with the exception of serum TM, which showed a promising close correlation to the disease activity of several vasculitides. In the present study we investigated in vitro the kinetics of these endothelial cell receptors after interaction of unstimulated or cytokine-activated PMN with endothelial cells in order to provide in vitro evidence for the different clinical relevance of these endothelial receptors. Only the TM levels in the supernatant reflected an endothelial cell injury after PMN–endothelial cell interaction due to their independence of the general cytokine activation. In contrast, adhesion molecule levels were increased not only due to cell destruction but also due to cytokine activation.
Cytokine activation of endothelial cells is known to lead to a time-dependent increase of adhesion molecules in the culture supernatant due to receptor shedding or secretion [2,3,10,22]. This was confirmed by our in vitro studies. However, the additional coincubation of endothelial cells with PMN resulted in a marked increase of the soluble adhesion molecule levels, if one or both of the cell types were activated by proinflammatory cytokines. This rapid increase of soluble adhesion molecules is probably due to the adhesion-related leucocyte–endothelial cell interaction and receptor shedding based on the observation of the morphological integrity of the endothelial cells. Prolonged coincubation (4–24 h) results in progressive endothelial cell damage due to neutrophil-derived proteases and reactive oxygen products. This effect can be further augmented by additional supplementation of the culture medium with serum of patients. In contrast, the cytokine stimulation of PMN alone induces the synthesis of oxygen radicals or proteases but does not result in a PMN burst [8,23–28]. The direct cytotoxic mechanism due to cell–cell interaction additionally contributes to the occurence of soluble adhesion molecules. In summary, these multiple causes for the occurence of soluble adhesion molecules—including receptor shedding, direct endothelial damage, and probably receptor secretion—represent important reasons to explain their limited value as disease activity parameters in SLE.
In contrast, TM is a receptor of the haematological/haemostasiological system and is completely independent of the immunologically based systems of the adhesion molecules. TM is the transmembranous glycoprotein receptor for thrombin mainly expressed on endothelial cells and syncytiotrophoblasts. The TM–thrombin complex acts as an important anti-coagulant resulting in accelerated activation of protein C [4,5]. After physiological activation and binding the complex is internalized and degraded. In vitro studies including 51Cr-release assays could confirm that soluble TM is a reliable marker of endothelial cell damage independent of physiological activation [6,7,29]. In addition, cytokine stimulation of endothelial cells results in decreased TM expression on the cell surface due to TM internalization with subsequent degradation. Furthermore, the cytokine activation results in a suppression of TM transcription and translation [18,30–33]. However, previously we showed, in vitro in endothelial cell cultures and in vivo in patients receiving therapeutically rhTNF-α, that soluble TM is a reliable marker of endothelial cell damage occurring due to endothelial–leucocyte adhesion and interaction after cytokine activation [8]. The in vitro data presented here confirm that a significant increase of TM is only due to an endothelial cell injury after co-culture of PMN with endothelial cells, which previously had additionally been activated by proinflammatory cytokines.
In conclusion, this in vitro model of the kinetics of soluble endothelial cell receptors after cytokine-triggered PMN and endothelial cell interaction underlines the advantage of TM compared with the adhesion molecules as a marker of endothelial damage. Therefore, soluble TM might prove to be a valuable serological disease activity marker in vasculitides.
Acknowledgments
Dedicated to Professor Dr med. K.-H. Meyer zum Büschenfelde, University of Mainz, on the occasion of his 70th birthday.
REFERENCES
- 1.Pall AA, Savage COS. Mechanisms of endothelial cell injury in vasculitis. Springers Semin Immunopathol. 1994;16:23–37. doi: 10.1007/BF00196711. [DOI] [PubMed] [Google Scholar]
- 2.Gearing AJH, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–12. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 3.Gearing AJ, Hemingway I, Pigott R, et al. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann NY Acad Sci. 1992;667:324–31. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- 4.Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci USA. 1981;78:2249–52. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75:329–36. [PubMed] [Google Scholar]
- 6.Ishii H, Yama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemostas. 1991;65:618–23. [PubMed] [Google Scholar]
- 7.Sawada K, Yamamoto H, Yago H, et al. A simple assay to detect endothelial cell injury; measurement of released thrombomodulin from cells. Exp Mol Pathol. 1992;57:116–23. doi: 10.1016/0014-4800(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 8.Boehme MWJ, Deng Y, Raeth U, et al. Release of thrombomodulin from endothelial cells by concerted action of TNF-α and neutrophils: in vivo and in vitro studies. Immunol. 1996;87:134–40. [PMC free article] [PubMed] [Google Scholar]
- 9.Rothlein R, Czajkowski M, O'neill MM, et al. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. J Immunol. 1988;141:1665–9. [PubMed] [Google Scholar]
- 10.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 11.Pigott R, Dillon LP, Hemingway IH, et al. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine-activated cultured endothelial cells. Biochem Biophys Res Commun. 1992;187:584–9. doi: 10.1016/0006-291x(92)91234-h. [DOI] [PubMed] [Google Scholar]
- 12.Boehme MWJ, Nawroth PP, Kling E, et al. Serum thrombomodulin. A novel marker of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1994;37:572–7. doi: 10.1002/art.1780370419. [DOI] [PubMed] [Google Scholar]
- 13.Boehme MWJ, Autschbach F, Zuna I, et al. Elevated serum levels and reduced immunohistochemical expression of thrombomodulin in active ulcerative colitis. Gastroenterol. 1997;113:107–17. doi: 10.1016/s0016-5085(97)70086-6. [DOI] [PubMed] [Google Scholar]
- 14.Boehme MWJ, Schmitt WH, Youinou P, et al. Clinical relevance of elevated serum thrombomodulin and soluble E-selectin in patients with Wegener's granulomatosis and other systemic vasculitides. Am J Med. 1996;101:387–94. doi: 10.1016/S0002-9343(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 15.Boehme MWJ, Raeth U, Galle PR, et al. Serum thrombomodulin—a reliable marker of disease activity in systemic lupus erythematosus: advantage over established serological parameters to indicate disease activity. Clin Exp Immunol. 2000;119:189–195. doi: 10.1046/j.1365-2249.2000.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruyama Y. The human endothelial cell tissue culture. Z Zellforsch Mikrosk Anat. 1963;60:69–79. doi: 10.1007/BF00329383. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe EA, Nachman RL, Becker CG, et al. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740–5. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koffler D. Immunpathogenesis of systemic lupus erythematosus. Ann Rev Med. 1974;25:149–64. doi: 10.1146/annurev.me.25.020174.001053. [DOI] [PubMed] [Google Scholar]
- 20.Carlos T, Kovach N, Schwartz B, et al. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991;77:2266–71. [PubMed] [Google Scholar]
- 21.Hashimoto Y, Nakano K, Yoshinoya S, et al. Endothelial cell destruction by polymorphonuclear leukocytes incubated with sera from patients with systemic lupus erythematosus (SLE) Scand J Rheumatol. 1992;21:209–14. doi: 10.3109/03009749209099226. [DOI] [PubMed] [Google Scholar]
- 22.Leeuwenberg JFM, Smeets EF, Neefjes J, et al. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunol. 1992;77:543–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss SJ. Tissue destruction by neutrophils. N Eng J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 24.Via CS, Allen RC, Welton RC. Direct stimulation of oxygenation activity by serum from patients with systemic lupus erythematosus: a relationship to disease activity. J Rheumatol. 1984;11:745–53. [PubMed] [Google Scholar]
- 25.Zeck-Kapp G, Kapp A, Busse R, et al. Interaction of granulocytes and endothelial cells upon stimulation with tumor necrosis factor-α: an ultrastructural study. Immunobiology. 1990;181:267–75. doi: 10.1016/s0171-2985(11)80518-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamada O, Moldow CF, Sacks T, et al. Deleterious effects of endotoxin on cultured endothelial cells: an in vitro model of vascular injury. Inflammation. 1981;5:115–26. doi: 10.1007/BF00914201. [DOI] [PubMed] [Google Scholar]
- 27.Varani J, Ginsburg I, Schuger L, et al. Endothelial cell killing by neutrophils: synergistic interaction of oxygen products and proteases. Am J Pathol. 1989;135:435–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan CF. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–60. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe H, Okajima K, Okabe H, et al. Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro. J Lab Clin Med. 1994;123:874–81. [PubMed] [Google Scholar]
- 30.Moore KL, Esmon CT, Esmon NL. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989;73:159–65. [PubMed] [Google Scholar]
- 31.Conway EM, Rosenberg RD. Tumor necrosis factor suppresses transcription of the thrombomodulin gene in endothelial cells. Mol Cell Biol. 1988;8:5588–92. doi: 10.1128/mcb.8.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirokawa K, Aoki N. Regulatory mechanisms for thrombomodulin expression in human umbilical vein endothelial cells in vitro. J Cell Physiol. 1991;147:157–65. doi: 10.1002/jcp.1041470120. [DOI] [PubMed] [Google Scholar]
- 33.Lentz SR, Tsiang M, Sadler JE. Regulation of thrombomodulin by tumor necrosis factor-α: comparison of transcriptional and posttranscriptional mechanisms. Blood. 1991;77:542–50. [PubMed] [Google Scholar]