Abstract
Injection of AKR/N mice with fibroblasts co-expressing MHC class II and TPO in the absence of adjuvant induces IgG-class TPO antibodies that resemble spontaneously arising human thyroid autoantibodies. We have used this model to examine the effect of iodide on TPO antibody induction as well as to analyse the interaction between T and B cells. Despite its importance as a major environmental factor in thyroid autoimmunity, variable iodide intake had no detectable effects on TPO antibody levels, lymphocytic infiltration of the thyroid or thyroid hormone levels. In terms of T cell responsiveness, splenocytes from TPO fibroblast-injected mice, but not from control mice, proliferated in response to TPO. Intriguingly, B cell-depleted splenocytes (mainly T cells without reduction of macrophages) proliferated in response to TPO only when co-cultured with irradiated autologous splenocytes from TPO fibroblast-injected mice but not from control mice. These data suggest that TPO-specific B cells are involved in antigen presentation to sensitized T cells and are supported by the ability of spleen cells from TPO cell-injected (but not control) mice to secrete TPO antibodies spontaneously in culture. In conclusion, we provide the first evidence for the presence of thyroid autoantigen-specific B cells and their ability to present their autoantigen to sensitized T cells in mice induced to develop TPO antibodies resembling autoantibodies in humans.
Keywords: antigen presentation, iodide, thyroid peroxidase, thyroid peroxidase-specific B cells, T cell proliferation
INTRODUCTION
The hallmark of human thyroid autoimmunity is the presence of IgG-class autoantibodies to a membrane-bound glycoprotein, TPO (reviewed in [1]). In contrast, thyroid autoimmunity developing spontaneously in animals is characterized by antibodies to a soluble glycoprotein, thyroglobulin (Tg) (for example [2–4]). The contrast between human and animal thyroid autoimmunity is based, at least in part, on the abundance and ease of isolation of Tg versus the relative paucity of TPO and difficulty of its purification from thyroid tissue (reviewed in [1]). However, despite the molecular cloning of both human and rodent TPO (reviewed in [1]), only a few investigations in animals have focused on TPO as an autoantigen [5,6].
In the absence of spontaneous models, autoimmunity is often induced with purified antigen and adjuvant. It is not always appreciated, however, that antibody responses elicited by this approach may show major differences from human autoantibodies. Thus, antibodies generated by conventional immunization in rabbits to Tg [7] or in mice to TPO [8] recognize diverse epitopes, whereas human autoantibodies interact with a restricted set of epitopes on the same molecule (reviewed in [9]). Similar observations have been made for the acetylcholine receptor [10] in myasthenia gravis.
Recently, we demonstrated that mice injected with fibroblasts co-expressing TPO and autologous MHC class II, but not mice injected with purified TPO and adjuvant, develop TPO antibodies that closely resemble autoantibodies in thyroid autoimmunity in terms of their extremely high affinities for TPO (Kd approx. 10−10m) and interaction with restricted epitopes in an ‘immunodominant’ region [11]. Differences of this nature may explain the ability of fibroblasts expressing class II and the thyrotropin (TSH) receptor, unlike purified receptor and adjuvant (reviewed in [12]), to elicit antibodies that mimic the stimulatory effects of TSH and cause autoimmune hyperthyroidism [13–15].
This novel approach provides the opportunity to investigate critical issues associated with thyroid autoimmunity, such as iodide intake (reviewed in [16]) and analysis of T cell responses in mice that develop TPO antibodies resembling those arising spontaneously in humans. In the present study, we found no effect of variable dietary iodine on the antibody response to TPO. However, we observed that lymphocytes from mice injected with TPO+, class II+ fibroblasts exhibit moderate proliferative responses to TPO in vitro. Moreover, and most intriguingly, T cell responses in these immunized mice appear to require TPO-specific B cells as antigen-presenting cells (APC).
MATERIALS AND METHODS
Induction of immune responses using TPO+ class II+ fibroblasts
RT4.15HP fibroblasts [17] (kindly provided by Dr R. Germain, NIH, Bethesda, MD) were stably transfected with the cDNA for human TPO [18] (pECE-hTPO together with pSV2-NEO) and selection with G418 (400 μg/ml) as described previously [11]. RT4.15HP fibroblasts express a recombinant MHC class II molecule essentially identical to MHC class II (I-Ak) of AKR/N mice [17]. Expression of cell surface TPO was determined by flow cytometry using a human monoclonal TPO autoantibody Fab (WR1.7) and PE-conjugated anti-human κ (Caltag, Burlingame, CA). The highest expressing TPO clone was expanded in Dulbecco's modified Eagle's medium (DMEM) with high glucose, 10% fetal calf serum (FCS), antibiotics (Gibco BRL, Gaithersburg, MD) in the presence of HAT medium (Sigma Chemical Co., St Louis, MO). Class II expression was confirmed by flow cytometry using FITC-conjugated anti-IA-k(aak) (Pharmingen, San Diego, CA).
Six-week-old female AKR/N mice (National Cancer Institute, Bethesda, MD) received six i.p. injections at two weekly intervals of TPO-expressing RT4.15HP fibroblasts (107 cells/injection). Fibroblasts were pretreated with 50 μg/ml mitomycin C (Sigma). Ten days after the final injection, mice were euthanized to obtain i.p. cells, spleens, blood and thyroid tissue. All animal studies were performed in accordance with the highest standards of humane care.
Variable dietary intake
Variable iodide intake involved two cycles of low iodide followed by high iodide intake as follows. Mice were fed low iodide chow (0.05 mg/kg; Harlan, Teklad, Madison, WI) for 12 days. For the first 10 of these 12 days, 0.01% methimazole (Sigma) was added to the drinking water. Subsequently, mice were fed normal mouse chow and water containing 0.05% potassium iodide for 2 days. Similar cycles of low iodide followed by high iodide, ‘Lo-I, then Hi-I’, have been shown to induce acute, transient damage in outbred mice [19].
The study involved four groups of mice (Fig. 1): (i) uninjected mice maintained throughout the study period on normal chow; (ii) mice exposed to two cycles of ‘Lo-I, then Hi-I’ intake; (iii) mice maintained on normal chow and injected on six occasions with TPO+ class II+ fibroblasts; and (iv) mice subject to variable iodide intake juxtaposed to combine the first and fourth injection of TPO+ class II+ fibroblasts with availability of high iodide.
Fig. 1.
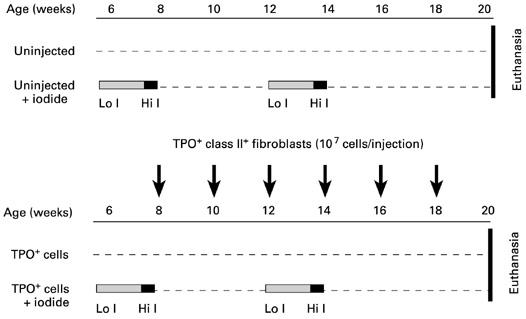
Protocol to investigate the effect of variable iodide intake on AKR/N mice that were untreated or injected on six occasions at 2-weekly intervals with viable, mitomycin C-treated TPO+ class II+ RT4.15HP fibroblasts. Lo I, Low iodide chow (see Materials and Methods) for 10 days plus 0.1% methimazole in drinking water; low iodide chow plus normal water for 2 days; Hi I, normal chow plus 0.05% potassium iodide in the drinking water for 2 days.
Thyroid histology and serum thyroxine (T4) levels
At euthanasia, the thyroid glands were removed (together with surrounding tissues), fixed in 4% paraformaldehyde pH 7.0 (Sigma) and paraffin sections stained with haematoxylin and eosin. Serum total T4 levels were measured by radioimmunoassay (Coat-a-Count, Total T4; Diagnostics Products Corp., Los Angeles, CA) and expressed as μg/dl relative to a standard curve.
Purified TPO for proliferative studies and antibody measurements
Recombinant human TPO, secreted by Chinese hamster ovary cells, was affinity-purified from culture medium as reported [20] and the buffer exchanged to PBS using Centricon 50 columns (Amicon Inc., Beverly, MA). Concentration was determined by spectrophotometry at optical density (OD) 280 nm (extinction coefficient 17.9) and purity by PAGE.
Serum antibodies to TPO (IgG class)
As previously described [11], duplicate aliquots of mouse sera (diluted 1:20 unless specified otherwise) were incubated with 125I-TPO (20 000 ct/min) labelled using iodogen to a specific activity of approx. 50 μCi/mg). Protein A (Pansorbin; Calbiochem, La Jolla, CA) was used to precipitate the antigen–antibody complex. After washing with assay buffer (0.1 m NaCl, 10 mm Tris–HCl pH 7.5, 0.1% Tween 20, 0.5% bovine serum albumin (BSA)) and centrifugation, radiolabelled TPO remaining in the pellets was counted. When indicated, non-specific 125I-TPO binding by normal mouse serum (approx. 3% of total ct/min) was subtracted in calculating the percentage 125I-TPO bound by antibodies in TPO-injected mice.
Because mouse antibodies of subclass IgG3 and particularly IgG1 bind very poorly to Protein A [21], we performed a separate assay using anti-mouse IgG (no subclass bias) coupled to a solid phase (Sac-cel; IDS, Tyne & Wear, UK). We observed a close correlation between Protein A and anti-mouse IgG solid phase in terms of their ability to precipitate125I-TPO bound by mouse sera obtained in the present study (r = 0.99, P < 0.001, n = 18).
T cell responses to TPO
Intraperitoneal cells and spleens were obtained at euthanasia. Spleens were subsequently dispersed to form single-cell suspensions and both spleen and i.p. cells were stored in liquid nitrogen. The spleens of TPO+ fibroblast-injected mice were not enlarged compared with uninjected mice and only limited numbers of splenocytes (up to a maximum of 7 × 107) were available from each mouse.
Responses to TPO were assessed in three different types of systems: (i) in preliminary studies, spleen lymphocytes at approx. 3 × 105 cells/well were incubated with increasing concentrations of TPO (1–30 μg/ml) in 96-well round-bottomed plates; (ii) in the majority of experiments, spleen cells or (in a few cases) i.p. cells were cultured at 1–2 × 105 cells/well together with an equal number of autologous, irradiated (20 Gy) spleen cells as feeders; and (iii) in some studies, B cell-depleted spleen cells (see below) were used as responders (approx. 4 × 104 cells/well) together with irradiated unseparated spleen cells (2 × 105 cells/well). Culture medium was RPMI 1640, 10% fetal bovine serum (FBS), 2 mm glutamine, 50 μg/ml gentamycin (all from the UCSF Culture Facility, San Francisco, CA), 50 μm β-mercaptoethanol (EM Science, Gibbstown, NJ),and 100 U/ml penicillin (Sigma). After 5 days at 37°C, 5% CO2, 1 μCi 3H-TdR (NEN Life Science Products, Boston, MA) was added to each well and cultures were harvested approx. 18 h later for scintillation counting using a PHD Cell Harvester (Cambridge Technology Inc., Watertown, MA) or, in some experiments, using a Tomtec Harvester 96 (Orange, CO).
Data are presented as ct/min (mean ± s.e.m. for triplicate cultures) or as a stimulation index (SI, the ratio of mean ct/min in the presence of TPO:mean ct/min in medium alone).
Depletion of B lymphocytes from spleen cell suspensions
Spleen cells from mice injected with TPO+, class II+ fibroblasts were incubated (30 min, 4°C) with biotin-conjugated anti-B220 (Pharmingen, San Diego, CA). After washing (PBS pH 7.4, 0.1% BSA), the spleen cell suspension was incubated with streptavidin-coated magnetic beads (M-280 Streptavidin; Dynal, Lake Success, NY) and incubated (30 min, 4°C). B220+ cells were then removed using a magnet to provide a B220− population. ‘B-depleted’ spleen cells were washed and used in proliferation studies (see above). Aliquots were analysed by flow cytometry (FACScan with Cellquest Software; Becton Dickinson, San Jose, CA) for T cells, B cells and macrophages using biotinylated anti-CD3 ε-chain, anti-B220 and anti-Mac-1α followed by streptavidin–FITC (all from Pharmingen).
In vitro synthesis of TPO antibody by spleen lymphocytes
Spleen cell suspensions were cultured (approx. 6 × 105 cells/ml) in RPMI 1640 containing 10% FCS, l-glutamine and antibiotics (see above) in round-bottomed tubes. Duplicate aliquots were cultured in the presence and absence of pokeweed mitogen (PWM; 3 μl/ml; Gibco BRL). For each mouse studied, additional aliquots were frozen (–80°C) and thawed three times to provide background binding values in the TPO antibody assay (see below). After incubation for 10 days (37°C, 5% CO2), culture supernatants were harvested by centrifugation (100 g). The very low levels of TPO antibody secreted in vitro were undetectable by 125I-TPO binding (see above). Therefore, we tested these culture supernatants by ELISA using wells coated with purified TPO (1 μg/ml; see above) and, as a control, with BSA (1 μg/ml; Sigma). The assay was performed as previously described for TPO-specific mouse MoAb no. 47 [8] using horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Sigma) followed by orthophenylene diamine as substrate [22]. OD 492 nm values for the ‘freeze–thaw’ samples were subtracted from the data obtained for cells cultured in medium or with PWM.
RESULTS
TPO antibodies in AKR/N mice exposed to variable iodide intake
Uninjected AKR/N mice on regular diet (constant iodide) or exposed to variable iodide intake had undetectable IgG class TPO antibodies in their sera (approx. 2% of 125I-TPO bound). In contrast, mice injected with RT4.15HP fibroblasts co-expressing TPO and class II developed high levels of TPO antibodies (> 55%125I-TPO bound) irrespective of their iodide intake (Table 1). Detailed analysis of TPO antibody binding in these sera over a range of dilutions (1:20–1:5000) confirmed the similarity between antibodies in mice on constant or variable iodide intake. Thus, most mice had comparable high titres of TPO antibodies, but one mouse in each group had a lower titre (Fig. 2). Thyroid lymphocytic infiltration was not observed in any group of mice. Moreover, irrespective of the presence or absence of TPO antibodies, all mice had comparable serum levels of free thyroxine (P = 0.217, analysis of variance) (Table 1).
Table 1.
Serum thyroxine (T4) levels are unchanged in AKR/N mice exposed to variable iodide intake alone or in association with TPO antibodies induced by injecting RT4.15 fibroblasts co-expressing TPO and MHC class II
Variable iodide intake involved two cycles of low iodide followed by high iodide (see Materials and Methods). TPO autoantibodies are expressed as 125I-TPO bound (all sera diluted 1:20).
Values significantly different from those for untreated mice: **P = 0.008; P = 0.036 (Mann–Whitney rank sum test).
Fig. 2.
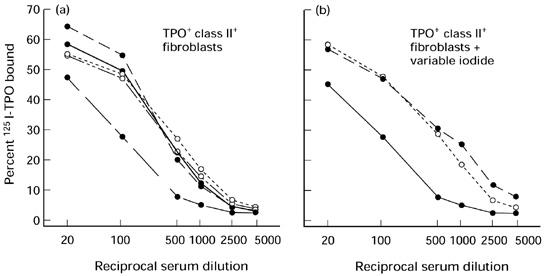
Iodide intake has no effect on TPO antibody titres in AKR/N mice injected with TPO+ class II+ RT4.15HP fibroblasts. Serial dilutions (1:20–1:5000) of TPO antibody binding in mice on a normal iodide diet (a) and on a variable iodide diet (b). Binding values are corrected for background binding by normal mouse serum (approx. 3%, see Materials and Methods).
Proliferative responses to TPO in conventional spleen cell cultures
In conventional cultures (approx. 4 × 105 cells/well), splenocytes from two TPO+ class II+ fibroblast-injected mice gave small but statistically significant proliferative responses to TPO after 5 days incubation (Fig. 3a, mice a and c). Splenocytes from only one of these two mice (mouse c) proliferated in response to TPO after a shorter incubation period (3 days; data not shown). Consequently, 5 days appeared to be the optimal incubation interval for TPO-induced proliferative responses in TPO antibody-positive mice. However, splenocyte responses from another similarly injected mouse (mouse b) were not significantly increased following incubation with TPO. Moreover, no response was detected in cultures containing higher numbers of splenocytes (approx. 8 × 105 cells/well; mouse d, Fig. 3a).
Fig. 3.
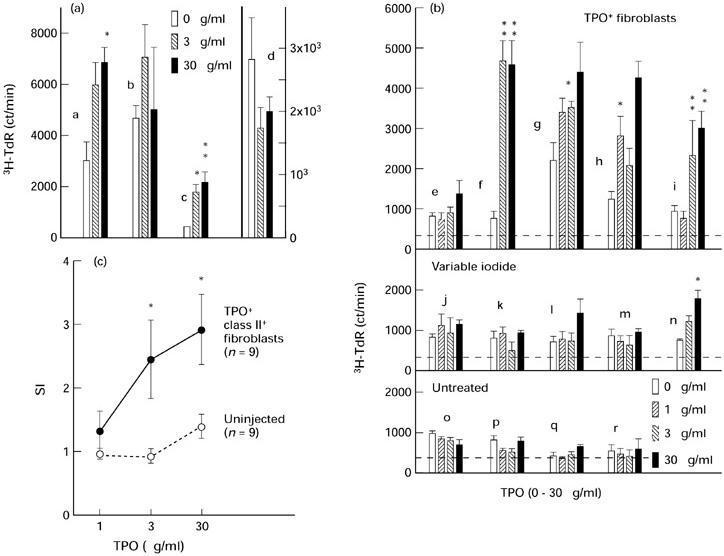
Splenocytes from AKR/N mice injected with TPO+ class II+ fibroblasts, unlike splenocytes from uninjected mice or most mice on variable iodide intake, proliferate in response to TPO in vitro. (a,b) 3H-TdR incorporation by splenocytes incubated for 5 days with increasing concentrations of TPO in conventional cultures (a) and co-cultures containing irradiated autologous splenocytes (b). Data are shown as ct/min (mean + s.e.m.) for splenocytes from individual mice (indicated by ‘a’, ‘b’, etc.) previously injected with TPO+ cells (n = 4, (a); n = 5, (b)), mice exposed to variable iodide intake (n = 5, (b)) or untreated mice on normal iodide intake (n = 4, (b)). The dashed line in (b) indicates the mean + 2 s.d. of thymidine incorporation by irradiated splenocytes used in co-cultures. Values significantly different from the response in medium without TPO: *P < 0.05; **P < 0.01 (Student's t-test). (c) Stimulation indices (SI; mean ± s.e.m.) for the response to TPO by splenocytes from mice injected with TPO+ class II+ cells (n = 9) and from uninjected mice (n = 9). Values for spleen cells from injected mice significantly higher than those for spleen cells from uninjected mice cultured with the same concentrations of TPO: *P < 0.019; Mann–Whitney rank sum test).
Intraperitoneal cells co-cultured with irradiated autologous splenocytes
Recently, it has been reported that adoptive transfer into naive recipients of i.p. cells (but not splenocytes) from mice previously injected with TSH receptor expressing class II+ RT4.15 fibroblasts accelerated thyroid dysfunction [14]. On this basis, we examined the proliferative response to TPO of i.p. cells from mice injected with TPO-expressing class II+ fibroblasts. Only limited numbers of i.p. cells were available from each mouse. Consequently, we co-cultured ‘responder’ i.p. cells with an equal number (approx. 2 × 105 cells/well) of irradiated autologous spleen cells as ‘feeders’ in the presence or absence of TPO. No proliferative responses to TPO were observed (data not shown). In retrospect, this result was not surprising, because of the very small number of T cells (approx. 2% estimated by flow cytometry) within the i.p. population. The control cultures for these negative data, however, provided useful information and led to experiments described below.
Splenocyte responses to TPO when co-cultured with irradiated autologous splenocytes
In examining the potential proliferative response of i.p. cells, parallel control studies used spleen cells as responders co-cultured with irradiated autologous spleen cells. Under these conditions, splenocytes from four of five mice injected with TPO+ class II+ fibroblasts (animals f, g, h and i) responded significantly to one or more concentrations of TPO (Fig. 3b, upper panel). Comparable 3H-TdR incorporation was observed for animals on normal diet (mice f and g) and mice on variable iodide intake (mice h and i). In contrast, splenocytes from only one of five mice (mouse n) on variable iodide intake gave a statistically significant response to TPO (Fig. 3b, middle panel) and splenocytes from none of four uninjected mice responded to TPO (Fig. 3b, lower panel).
As there was no major effect of dietary iodide intake on the splenocyte proliferative responses to TPO, we pooled the data for uninjected mice (n = 9) for comparison with the data for mice injected with TPO+ class II+ fibroblasts (n = 9; four mice studied in conventional cultures and five mice studied in co-cultures containing irradiated splenocytes). The proportion of mice whose splenocytes proliferated in response to TPO was significantly higher among TPO cell-injected mice (six of nine animals) than in the group of uninjected mice (one of nine animals) (Fisher's exact test, P = 0.05). Moreover, proliferation (expressed as SI) was significantly increased at 3 and 30 μg/ml TPO for splenocytes from TPO fibroblast-injected versus uninjected mice (Fig. 3c; P < 0.019, Mann–Whitney rank sum test).
B cells from TPO-immunized mice appear to be required for the response of sensitized T cells to TPO
The very high affinity (Kd approx. 10−10 m) of TPO antibodies induced by injection of TPO+ class II+ fibroblasts, in contrast to conventional immunization with purified TPO and adjuvant, led us to hypothesize that B cells may play a role in TPO presentation to sensitized T cells in this model [11]. We investigated this possibility by depleting B cells from splenocytes of TPO cell-injected mice. We first confirmed by flow cytometry that our protocol for removal of B220+ cells effectively decreased the B cell proportion from approx. 66% to 7%. Accordingly the proportion of T cells and macrophages was increased (Fig. 4).
Fig. 4.
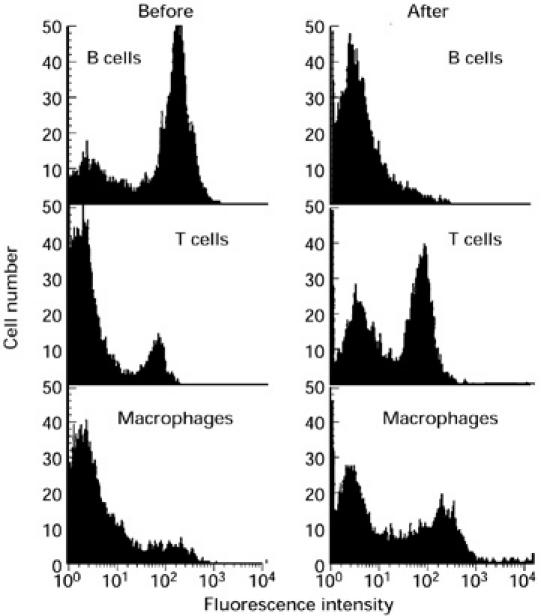
Removal of B220+ cells from spleen cells depletes B cells and enriches for T cells and macrophages. Spleen cells were analysed by flow cytometry before and after removal of cells labelled with biotinylated anti-B220 using streptavidin-coated beads. B cells, T cells and macrophages were labelled with biotinylated antibodies (anti-B220, anti-CD3ε and anti-Mac-1α, respectively) and detected with streptavidin–FITC (Materials and Methods).
To assess proliferation, B-depleted splenocytes were cultured in the absence or presence of TPO together with irradiated unseparated spleen cells from TPO fibroblast-injected mice or from uninjected mice (Fig. 5a). Because relatively low numbers of splenocytes are available from TPO cell-injected mice (< 107 cells/animal, approx. 15% of which are T cells), it was necessary to pool splenocytes from three TPO cell-injected mice for each experiment. A significant proliferative response to TPO was observed when B-depleted splenocytes (pooled from mice t and u) were co-cultured with irradiated splenocytes from the same TPO fibroblast-injected mice but not from control mice (Fig. 5b). Similar observations were made with splenocytes pooled from mice h, t and u (Fig. 5c). The lower counts (approx. 10-fold) in the latter study probably reflect a smaller recovery of T cells after B cell depletion. These studies with pooled T cells supplement the above evidence for a proliferative response to TPO from splenocytes of individual mice (see above). More importantly, TPO induced T cell proliferation only in the presence of irradiated splenocytes from TPO-injected mice.
Fig. 5.
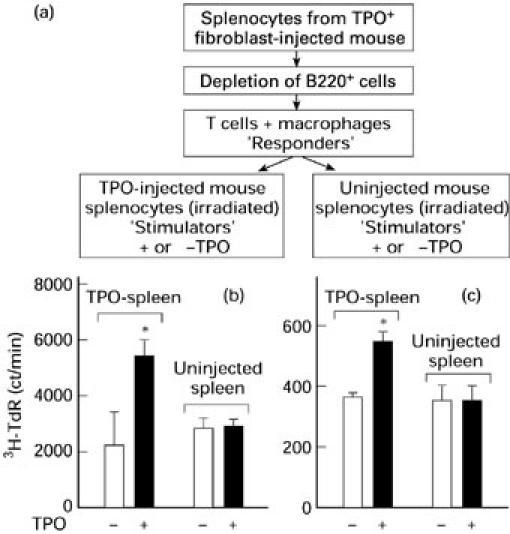
Splenic T cell proliferation in response to TPO requires irradiated ‘feeder’ splenocytes from TPO+ class II+ fibroblast-injected mice. (a) Protocol for studying proliferation to TPO by B-depleted spleen cells containing T cells and macrophages (‘Responders’). These cells were co-cultured with irradiated unseparated spleen cells from TPO fibroblast-injected mice or from control mice. (b,c) 3H-TdR uptake by B-depleted responder splenocytes. Responder cells from three mice were pooled (mice t and u, (b); mice h, t and u, (c)) and cultured with irradiated spleen cells from TPO fibroblast-injected mice or control mice with or without TPO. Data are the mean ± s.e.m. ct/min. Values significantly different from cultures without TPO: *P < 0.03 (t = 2.59, (b)); *P < 0.01 (t = 3.12, (c)).
These data suggest that TPO-specific B cells in the irradiated splenocytes play a role in TPO presentation to T cells sensitized in vivo, for two reasons. First, there was no reduction in the macrophage population in the B-depleted responding cells and the macrophage and dendritic cell populations in the irradiated ‘feeder’ splenocytes were unmanipulated. Second, in contrast to B cells, there is no mechanism for specific TPO uptake by macrophages and dendritic cells. The only APC with specificity for TPO can be the B cells in the irradiated ‘feeders’ from the mice injected 2 weeks previously with TPO-expressing fibroblasts.
TPO antibody synthesis by spleen lymphocytes in vitro
Support for TPO-specific B cells as APC would be the ability of spleen lymphocytes from TPO+ class II+ fibroblast-injected mice to synthesize TPO antibodies in culture. Indeed, splenocyte culture supernatants (10 days) from TPO fibroblast-injected contained low, but significantly higher, levels of IgG-class antibodies to TPO than supernatants from uninjected mice splenocyte supernatants (Fig. 6; P < 0.019, Mann–Whitney rank sum test). In contrast, no difference was observed with ELISA wells coated with BSA. Moreover, spontaneous TPO antibody production was reduced by PWM. This pattern of spontaneous TPO antibody production and inhibition by the mitogen is characteristic of thyroid autoantibody synthesis by thyroid-infiltrating lymphocytes (for example see [23]).
Fig. 6.
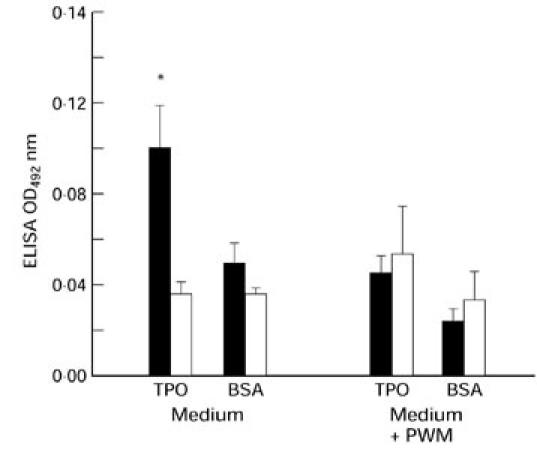
Evidence for TPO antibody synthesis by cultures of splenocytes from TPO+ class II+ fibroblast-injected mice. Supernatants were collected from splenocytes cultured for 10 days in the presence or absence of pokeweed mitogen (PWM). Cultures of splenocytes from TPO cell-injected (▪, n = 4) or uninjected (□, n = 6) mice were analysed by ELISA using plates coated with recombinant TPO or bovine serum albumin (BSA). Data are expressed as optical density (OD) 492 (mean + s.e.m.) after subtraction of background levels determined in parallel cultures for each donor mouse by freeze–thaw (× 3) of splenocyte suspensions.
DISCUSSION
TPO antibodies with high affinities and epitopes similar to spontaneously arising autoantibodies in human disease are induced by injecting AKR/N mice with TPO+ class II+ fibroblasts, but not by conventional immunization with purified TPO and adjuvant [11]. One objective of the present study was to use this new approach to examine the effect of iodide on TPO antibody induction. Second, we wished to analyse the relationship between T and B cells in a TPO antibody response comparable to that in human disease. In terms of our first objective, we found that variable iodide intake had no evident effect on TPO antibody induction or thyroid function in this model. On the other hand, we observed that splenocytes from TPO cell-injected mice (but not control mice) proliferated in response to TPO. Most importantly, antigen-specific B cells appeared to be involved in antigen presentation to T cells from mice with an established TPO antibody response.
Both aspects of our present study concern critical issues in human autoimmune thyroid disease. Dietary iodine is a major environmental factor in thyroid autoimmunity (reviewed in [16]). Thus, increased iodide intake can cause either hypothyroidism or iodide-induced thyrotoxicosis in humans (reviewed in [24]). In iodide-induced hypothyroidism, the individuals most susceptible to iodide are those with thyroid autoantibodies [30]. Iodine also has major effects in animal models of spontaneous and induced thyroiditis. For example, exposure to iodide enhances spontaneous thyroiditis and Tg autoantibody levels in Obese strain chickens [25], BB rats [26–28] and NOD-H-2h4 mice [4]. Moreover, the antigenicity of Tg is influenced by its iodide content [4,25].
Despite evidence for the importance of iodide in humans and murine models, we found no difference between antibody levels or T cell responses in either TPO cell-injected or uninjected mice on constant or variable iodide intake. Previous investigations have demonstrated acute thyroid epithelial cell necrosis and inflammation in mice maintained on a low iodide diet, injected with high doses of iodide and studied 48 h later [19]. In contrast, our mice were studied 6 weeks after the two cycles of similar low, followed by high, dietary iodide intake. The lack of an iodide effect on TPO antibody induction and T cell responsiveness is likely to be related to the absence of thyroid lymphocytic infiltration in our model. Unlike observations of enhanced thyroid autoantibody production in vitro following exposure to exogenous iodine [29], our data suggest that a response to iodide requires an interaction between the thyroid and the immune system.
Although the lack of thyroid inflammation in AKR/N mice injected with class II+ TPO+ fibroblasts is not fully understood, it is consistent with the similar ‘Shimojo’ model for generating stimulating TSHR antibodies by injecting the same strain of mice with fibroblasts co-expressing MHC class II and the human TSHR [13,14,30]. The development of Graves'-like hyperthyroidism in some mice by the Shimojo approach indicates antibody cross-reactivity with the mouse receptor. It is well known that TSHR autoantibodies in some Graves' sera recognize the mouse TSHR (reviewed in [12]). In contrast, human autoantibodies to TPO usually do not cross-react with TPO from non-primates [1]. Thus, it is possible that the response to fibroblasts bearing human TPO may not break tolerance to murine TPO and cause thyroid damage. Also, H2-k strain (like AKR/N) mice do not develop thyroid inflammation even after conventional immunization with purified porcine TPO and adjuvant [8].
Thyroid infiltration alone represents only one facet of human autoimmune thyroid disease. Moreover, thyroiditis is relatively non-specific, as it also occurs in some thyroid carcinomas [31]. Clinical disease (hypothyroidism) rarely if ever develops in the conventional models of thyroiditis induced using antigen and adjuvant. The major characteristic of thyroid autoimmunity in humans is the presence of high-affinity autoantibodies that interact with restricted epitopes on TPO. Consequently, the second and more important aspect of our study was to examine the in vitro responsiveness to TPO of splenocytes from mice injected with TPO+ class II+ fibroblasts, at a time when all animals had TPO autoantibodies. Proliferation in response to TPO (presumably reflecting sensitized T cells) was detected using splenocytes from two-thirds of TPO+ class II+ fibroblast-injected mice. The magnitude of the proliferative responses was relatively small, reaching a maximal SI of approx. 6.0 in two mice. In contrast, SI values of 5.0–10.0 are induced by Tg in primary cultures of lymph node lymphocytes from mice immunized with Tg and Freund's complete adjuvant [32].
Although our model relates to antibody production, not thyroiditis, in our view it is closer to some features of spontaneously arising human thyroid autoimmunity than other induced models for the following reasons. First, we examined T cell responses in splenocytes from mice that develop TPO antibodies gradually over a period of 3 months following exposure to low levels of TPO (approx. 0.5 μg/mouse distributed over six injections) [11]. In contrast, T cell studies in response to conventional immunization with Tg are usually performed 7–10 days after a single injection of high concentrations of antigen (approx. 60 μg/mouse) using lymphocytes from draining lymph nodes. An important second difference was that our protocol avoids the use of adjuvant, ‘the immunologist's dirty little secret’ [33]. Bacterial products enhance expression of costimulatory molecules [34]. Finally, in our approach the immunogen was provided as a functional cell surface protein similar to TPO in vivo.
The weak T cell proliferative responses that we observed are consistent with the similarly modest responses of primary lymphocyte cultures from autoimmune thyroid disease patients in response to eukaryotic preparations of intact native TPO and Tg (for example see [35]). We suggest that similar weak responses may be characteristic of other organ-specific autoimmune diseases. Indeed, a variety of approaches is commonly used to amplify such modest responses in human autoimmunity. Thus, supplementation with IL-2 can enhance proliferation in primary cultures in response to Tg [35] and to TPO in some [36,37] but not all [38] TPO-specific T cell lines or clones. Synthetic peptides, as opposed to native protein, may also induce higher responses in primary cultures. However, as illustrated for the acetylcholine receptor in myasthenia gravis, use of peptides frequently fails to expand T cells that recognize the intact antigen responsible for the response in vivo [39].
The most intriguing finding in our mouse model is that it provides the first evidence for both the presence of thyroid autoantigen-specific B cells and their ability to present their autoantigen to autologous T cells. Thus, B cell-depleted splenocytes (mainly T cells without reduction of macrophages) from TPO antibody-positive mice proliferated in response to TPO only when co-cultured with irradiated autologous splenocytes from TPO fibroblast-injected mice, not from control mice. Spontaneous synthesis of TPO antibodies by splenocytes from TPO fibroblast-injected mice but not from uninjected mice also supports a role for TPO-specific B cells in antigen presentation to T cells. These data are consistent with previous observations. First, the spleen is an important site of Tg antibody synthesis in rats immunized with Tg and adjuvant or subjected to thymectomy and sublethal irradiation [40]. Second, B cells from mice primed with Tg and adjuvant present low concentrations of Tg extremely efficiently to a Tg-specific T cell hybridoma [41].
It is not known which APC are involved in initiating the immune response to TPO+ MHC class II+ fibroblasts. In accordance with the Bottazzo–Feldman hypothesis of antigen presentation by thyroid cells induced to express ‘aberrant’ MHC class II [42], the fibroblasts themselves may function as APC. On the other hand, as discussed previously [11], we cannot exclude the possibility that other APC (macrophages or dendritic cells) process TPO from fibroblasts (alive but rendered incapable of dividing) for presentation to naive T cells. Our current observations suggesting a role for B cells in antigen presentation apply to sensitized T cells from mice with an on-going, high-affinity IgG-class antibody response to TPO.
There is increasing evidence that B cells play a role in presentation of protein antigens. Thus, insulin-dependent diabetes type I, the classic example of a T cell-mediated disease, does not develop in non-obese diabetic (NOD) mice lacking B cells [43]. The explanation for this phenomenon appears to be that B cells present islet cell autoantigens to T cells which ultimately damage the islet cells [44]. However, the role of B cells is not limited to their ability to capture minute amounts of antigen via their immunoglobulin receptors. Perhaps the most dramatic effect of antigen-specific B cells, at least in vitro, is their ability to influence the peptides made available for presentation to T cells. For example, human B cell clones specific for different epitopes on tetanus toxoid enhance or suppress presentation of different peptides to T cells [45]. Similar observations have recently been made for Tg in mice using a T cell hybridoma [46]. Moreover, it has been suggested that processing of antibody–antigen complexes may expose ‘cryptic’ T cell epitopes and lead to autoimmunity [47]. In our view [9], the most important outcome of the influence of antibodies on the peptides presented to T cells in organ-specific autoimmunity may be the restricted epitopic recognition by autoantibodies which is characteristic of human organ-specific autoimmunity.
In conclusion, using a mouse model for the induction of TPO antibodies resembling those arising spontaneously in human thyroid autoimmunity, we provide evidence for the role of TPO-specific B cells in antigen presentation to autologous T cells. Our findings suggest that antigen uptake via high-affinity, membrane-bound IgG on B cells may play a role in the maintenance and/or expansion of sensitized T cells specific for TPO (and perhaps other thyroid autoantigens) in humans.
Acknowledgments
This work was supported by the National Institutes of Health Grants DK 36182 and DK54684.
REFERENCES
- 1.McLachlan SM, Rapoport B. The molecular biology of thyroid peroxidase: cloning, expression and role as autoantigen in autoimmune thyroid disease. Endocr Rev. 1992;13:192–206. doi: 10.1210/edrv-13-2-192. [DOI] [PubMed] [Google Scholar]
- 2.Bigazzi PE, Rose NR. Spontaneous autoimmune thyroiditis in animals as a model of human disease. Prog Allergy. 1975;19:245–74. [PubMed] [Google Scholar]
- 3.Wick G, Most J, Schauenstein K, et al. Spontaneous autoimmune thyroiditis—a bird's eye view. Immunol Today. 1985;6:359–65. doi: 10.1016/0167-5699(85)90095-7. [DOI] [PubMed] [Google Scholar]
- 4.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H2h4 mice. Clin Immunol Immunopathol. 1996;81:287–92. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 5.Kotani T, Umeki K, Hirai K, Ohtaki S. Experimental murine thyroiditis induced by porcine thyroid peroxidase and its transfer by the antigen-specific T cell line. Clin Exp Immunol. 1990;80:11–8. doi: 10.1111/j.1365-2249.1990.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLachlan SM, Atherton MC, Nakajima Y, et al. Thyroid peroxidase and the induction of autoimmune thyroid disease. Clin Exp Immunol. 1990;79:182–8. doi: 10.1111/j.1365-2249.1990.tb05176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nye L, Pontes de Carvalho LC, Roitt IM. Restrictions in the response to autologous thyroglobulin in the human. Clin Exp Immunol. 1980;41:252–63. [PMC free article] [PubMed] [Google Scholar]
- 8.Ruf J, Toubert M, Czarnocka B, Durand-Gorde J, Ferrand M, Carayon P. Relationship between immunological structure and biochemical properties of human thyroid peroxidase. Endocrinol. 1989;125:1211–8. doi: 10.1210/endo-125-3-1211. [DOI] [PubMed] [Google Scholar]
- 9.McLachlan SM, Rapoport B. Genetic and epitopic analysis of thyroid peroxidase (TPO) autoantibodies: markers of the human thyroid autoimmune response. Clin Exp Immunol. 1995;101:200–6. doi: 10.1111/j.1365-2249.1995.tb08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich F, Vincent A, Roberts A, Newsom-Davis J. Epitopes on human acetylcholine receptor defined by monoclonal antibodies and myasthenia gravis sera. Autoimmunity. 1988;1:285–97. doi: 10.3109/08916938809010682. [DOI] [PubMed] [Google Scholar]
- 11.Jaume JC, Guo J, Wang Y, Rapoport B, McLachlan SM. Cellular thyroid peroxidase (TPO), unlike purified TPO and adjuvant, induces antibodies in mice that resemble autoantibodies in human autoimmune thyroid disease. J Clin Endocrinol Metab. 1999;84:1651–7. doi: 10.1210/jcem.84.5.5666. [DOI] [PubMed] [Google Scholar]
- 12.Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin receptor: interaction with thyrotropin and autoantibodies. Endocr Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- 13.Shimojo N, Kohno Y, Yamaguchi K-I, et al. Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci USA. 1996;93:11074–9. doi: 10.1073/pnas.93.20.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kita M, Ahmad L, Marians RC, et al. Regulation and transfer of a murine model of thyrotropin receptor antibody mediated Graves' disease. Endocrinol. 1999;140:1392–8. doi: 10.1210/endo.140.3.6599. [DOI] [PubMed] [Google Scholar]
- 15.Jaume JC, Rapoport B, McLachlan SM. Lack of female bias in a mouse model of autoimmune hyperthyroidism (Graves' disease) Autoimmunity. 1999;29:269–72. doi: 10.3109/08916939908994746. [DOI] [PubMed] [Google Scholar]
- 16.Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev. 1994;15:788–830. doi: 10.1210/edrv-15-6-788. [DOI] [PubMed] [Google Scholar]
- 17.Germain RN, Ashwell JD, Lechler RI, et al. ‘Exon-shuffling’ maps control of antibody- and T-cell-recognition sites to the NH2-terminal domain of the class II major histocompatibility polypeptide Aβ. Proc Natl Acad Sci USA. 1985;82:2940–4. doi: 10.1073/pnas.82.9.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnusson RP, Chazenbalk GD, Gestautas J, et al. Molecular cloning of the complementary deoxyribonucleic acid for human thyroid peroxidase. Mol Endocrinol. 1987;1:856–61. doi: 10.1210/mend-1-11-856. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud I, Colin I, Many M-C, Denef J-F. Direct toxic effect of iodide in excess on iodine-deficient thyroid glands: epithelial necrosis and inflammation associated with lipofuscin accumulation. Exp Mol Pathol. 1986;44:259–71. doi: 10.1016/0014-4800(86)90040-7. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, McLachlan SM, Hutchison JS, Rapoport B. The greater glycan content of recombinant human thyroid peroxidase of mammalian than of insect cell origin facilitates purification to homogeneity of enzymatically active protein remaining soluble at high concentration. Endocrinol. 1998;139:999–1005. doi: 10.1210/endo.139.3.5782. [DOI] [PubMed] [Google Scholar]
- 21.Harlow E, Lane D. A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. Antibodies. [Google Scholar]
- 22.Chazenbalk GD, Costante G, Portolano S, McLachlan SM, Rapoport B. The immunodominant region on human thyroid peroxidase recognized by autoantibodies does not contain the monoclonal antibody 47/c2l linear epitope. J Clin Endocrinol Metab. 1993;77:1715–8. doi: 10.1210/jcem.77.6.7505290. [DOI] [PubMed] [Google Scholar]
- 23.McLachlan SM, McGregor A, Rees Smith B, Hall R. Thyroid-autoantibody synthesis by Hashimoto thyroid lymphocytes. Lancet. 1979;i:162–3. doi: 10.1016/s0140-6736(79)90559-2. [DOI] [PubMed] [Google Scholar]
- 24.Ingbar SH. Autoregulation of the thyroid. Response to iodide excess and depletion. Mayo Clin Proc. 1972;47:814–23. [PubMed] [Google Scholar]
- 25.Bagchi N, Brown TR, Urdanivia E, Sundick RS. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science. 1985;230:325–7. doi: 10.1126/science.4048936. [DOI] [PubMed] [Google Scholar]
- 26.Allen EM, Appel MC, Braverman LE. The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinol. 1986;118:1977–81. doi: 10.1210/endo-118-5-1977. [DOI] [PubMed] [Google Scholar]
- 27.Mooij P, de Wit HJ, Drexhage HA. An excess of dietary iodine accelerates the development of a thyroid-associated lymphoid tissue in autoimmune prone BB rats. Clin Immunol Immunopathol. 1993;69:189–98. doi: 10.1006/clin.1993.1169. [DOI] [PubMed] [Google Scholar]
- 28.Cohen SB, Weetman AP. The effect of iodide depletion and supplementation in the Buffalo strain rat. J Endocrinol Invest. 1988;11:625–7. doi: 10.1007/BF03350197. [DOI] [PubMed] [Google Scholar]
- 29.Weetman AP, McGregor AM, Campbell H, Lazarus JH, Ibbertson HK, Hall R. Iodide enhances IgG synthesis by human peripheral blood lymphocytes in vitro. Acta Endocrinol (Copenh) 1983;103:210–5. doi: 10.1530/acta.0.1030210. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi K-I, Shimojo N, Kikuoka S, et al. Genetic control of anti-thyrotropin receptor antibody generation in H-2k mice immunized with thyrotropin receptor-transfected fibroblasts. J Clin Endocrinol Metab. 1997;82:4266–9. doi: 10.1210/jcem.82.12.4589. [DOI] [PubMed] [Google Scholar]
- 31.Scopsi L, Collini P, Sampietro G, Boracchi P, Pilotti S. Prognostic impact of thyroid lymphocytic infiltration in patients with medullary thyroid carcinoma. Thyroid. 1996;6:613–7. doi: 10.1089/thy.1996.6.613. [DOI] [PubMed] [Google Scholar]
- 32.Kong YM, Simon LL, Creemers P, Rose N. In vitro T cell proliferation and cytotoxicity in murine autoimmune thyroiditis. Mount Sinai J Med. 1986;53:46–52. [PubMed] [Google Scholar]
- 33.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov R, Janeway CA. Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–3. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 35.Rasooly L, Rose NR, Saboori AM, Ladenson PW, Burek CL. Iodine is essential for human T cell recognition of human thyroglobulin. Autoimmunity. 1998;27:213–9. doi: 10.3109/08916939808993833. [DOI] [PubMed] [Google Scholar]
- 36.Dayan CM, Londei M, Corcoran AE, et al. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci USA. 1991;88:7415–9. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullins RJ, Chernajovsky Y, Dayan C, Londei M, Feldmann M. Transfection of thyroid autoantigens into EBV-transformed B cell lines. J Immunol. 1994;152:5572–80. [PubMed] [Google Scholar]
- 38.Martin A, Magnusson RP, Kendler DL, Concepcion E, Ben-Nun A, Davies TF. Endogenous antigen presentation by autoantigen-transfected Epstein-Barr virus-lymphoblastoid cells. J Clin Invest. 1993;91:1567–74. doi: 10.1172/JCI116362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuo H, Batocchi A-P, Hawke S, et al. Peptide-selected T cell lines from myasthenia gravis patients and controls recognize epitopes that are not processed from whole acetylcholine receptor. J Immunol. 1995;155:3683–92. [PubMed] [Google Scholar]
- 40.Weetman AP, McGregor AM, Rennie DP, Hall R. Sites of autoantibody production in rats with thyroiditis. Immunol. 1982;46:465–72. [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchings P, Rayner DC, Champion BR, et al. High efficiency antigen presentation by thyroglobulin-primed murine splenic B cells. Eur J Immunol. 1987;17:393–8. doi: 10.1002/eji.1830170314. [DOI] [PubMed] [Google Scholar]
- 42.Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;ii:1115–8. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- 43.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new ‘speed congenic’ stock of NOD.Igμnull mice. J Exp Med. 1996;184:2049–53. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–8. [PubMed] [Google Scholar]
- 45.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–63. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai Y, Carayanniotis KA, Eliades P, et al. Enhancing or suppressive effects of antibodies on processing of a pathogenic T cell epitope in thyroglobulin. J Immunol. 1999;162:6987–92. [PubMed] [Google Scholar]
- 47.Lanzavecchia A. How can cryptic epitopes trigger autoimmunity? J Exp Med. 1995;181:1945–8. doi: 10.1084/jem.181.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]



