Abstract
PTX3 is a secreted molecule which consists of a C-terminal domain similar to classical pentraxins (e.g. C-reactive protein (CRP)) and of an unrelated N-terminal domain. Unlike the classical pentraxins, the long pentraxin PTX3 is expressed in response to IL-1β and tumour necrosis factor-alpha (TNF-α), but not to IL-6, in various cell types. The present study was designed to investigate the expression of PTX3 in RA. Dissociated RA and osteoarthritis (OA) type B synoviocytes were cultured in the presence and in the absence of inflammatory cytokines. PTX3 mRNA expression in synoviocytes was evaluated by Northern analysis. PTX3 protein levels in synovial cell cultures and synovial fluid were estimated by ELISA, and PTX3 distribution in synovial tissues by immunohistochemical techniques. OA synoviocytes were induced to express high levels of PTX3 mRNA by TNF-α, but not by other cytokines including IL-1β and IL-6. RA synoviocytes, unlike OA synoviocytes, constitutively expressed high levels of PTX3 in the absence of deliberate stimulation. The constitutive expression of PTX3 in RA synoviocytes was not modified by anti-TNF-α antibodies, IL-1 receptor antagonist or a combination of the two agents. In contrast, interferon-gamma and transforming growth factor-beta inhibited PTX3 constitutive expression in RA synoviocytes. The joint fluid from RA patients contained higher levels of immunoreactive PTX3 than controls and the synovial tissue contained endothelial cells and synoviocytes positive for PTX3 by immunohistochemistry. In conclusion, PTX3 may play a role in inflammatory circuits of RA, and its relevance as a marker of disease activity deserves further study.
Keywords: pentraxin, rheumatoid arthritis, synoviocytes, inflammation
INTRODUCTION
RA is a chronic inflammatory disease with autoimmune features in which a chronic synovitis leads to cartilage and bone destruction [1]. The main target tissue of the disease is the synovial membrane which becomes strikingly enlarged as a result of hyperplasia of the synovial lining cells and infiltration by mononuclear cells. Outgrowth of the synovial tissue (pannus) over the articular cartilage surface and into the underlying subchondral bone is implicated in the destruction of the involved joints [2]. The synovial cells are held responsible for the joint damage and remodelling through the secretion of a number of inflammatory cytokines and growth factors [3], prostaglandins [3–6], and degradative enzymes, including collagenase [7], stromelysin [8], cathepsins L, B and D [9–11]. However, despite significant advances during the past few years, the pathogenic mechanisms of RA are still obscure and controversy surrounds the relative contributions of the various cell populations and inflammatory mediators to the disease process.
PTX3 was cloned from IL-1β-treated human umbilical vein endothelial cells [12] and, independently, by Lee et al. from human fibroblasts and termed “TNF-stimulated gene 14” (TSG-14) [13]. PTX3/TSG-14 encodes a 381 amino acid, 42-kD secretory protein with a COOH-terminal domain showing up to 28% sequence identity to human C-reactive protein (CRP) and serum amyloid P-component (SAP) [12,13], including the presence of the pentraxin signature and two cysteine residues found in all pentraxins cloned so far [14]. The alignment suggests that PTX3/TSG-14 protein is a member of the pentraxin family and may be part of the acute-phase response that begins in the aftermath of injury, trauma, and infections (reviewed in [15]). PTX3/TSG-14 protein appears in the serum of mice and humans after lipopolysaccharide (LPS) injection and, unlike CRP and SAP, the major site of synthesis is extrahepatic [14,16]. After cloning of PTX3, other long pentraxins consisting of a C-terminal pentraxin domain coupled with an unrelated N-terminal portion have been identified [17–22].
In order to assess the relevance of PTX3 in human inflammation, we elected to study the constitutive and inducible expression of the PTX3 gene in synoviocytes obtained from patients with RA and from control subjects with osteoarthritis (OA). An ELISA allowed the determination of PTX3 protein in synovial fluid of RA patients.
PATIENTS AND METHODS
Reagents
IL-1β and tumour necrosis factor-alpha (TNF-α) cDNA probes were a kind gift of Dr A. Rodriguez (Hospital de La Princesa, Madrid, Spain). Interferon-gamma (IFN-γ), transforming growth factor-beta (TGF-β), IL-4, IL-10, IL-1β, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, and IL-8 cytokines were purchased from Genzyme Diagnostics (Cambridge, MA). IL-1 receptor antagonist (IL-1Ra) and anti-TNF-α MoAb (B154.2) were a kind gift of Dr G. Trinchieri (Wistar Institute, Philadelphia, PA).
Patients
Seven patients diagnosed as having active RA according to the American College of Rheumatology criteria [23] were used as a source of synoviocytes. Synovial tissue was obtained during total knee joint replacement. Their mean age was 55 ± 5 years and the duration of the disease ranged from 6 to 12 years, with a mean of 9 years. Six sex- and age-matched patients with OA undergoing knee surgery were included as controls.
Synovial fluids were collected from 11 patients affected by active RA, and nine sex- and age-matched donors with traumatic, non-inflammatory joint effusion (< 200 cells/ml).
Synovial cell cultures
Synovial tissues were digested for 4 h at 37°C with 3 ml of 0.3% collagenase in serum-free RPMI 1640 culture medium. The cells were washed three times with PBS pH 7.4 and resuspended in RPMI 1640 supplemented with 15% heat-inactivated fetal calf serum (FCS), 2 mm l-glutamine, 50 U/ml penicillin–50 mg/ml streptomycin, and incubated at 37°C in 5% CO2/95% air.
Culture medium was tested periodically with specific commercial kits for mycoplasma contamination and for the presence of bacterial endotoxins.
The cells growing after five passages were all type B fibroblast-like synoviocytes, as suggested by the negative staining with anti-CD69, anti-CD14, anti-CD11b and anti-CD11c MoAbs, by a high uridine diphosphoglucose dehydrogenase (UDPGD) activity [24], and by their spindle-shaped, fibroblast-like morphologic appearance. At this point the medium was discarded, the cells were washed three times with PBS, and fresh medium containing 0.2% FCS was added in order to induce cell quiescence. After a further 48 h of incubation, the cells were harvested with guanidine isothiocianate or incubated with the appropriate cytokines for 6 h, as described below.
In selected experiments, fibroblasts from uninvolved skin of normal subjects were included as control.
Probes
The probe used for the detection of the PTX3 mRNA was an insert of 1300 bp containing the entire coding region of the human PTX3 gene [25]. The probes used for the detection of the IL-1β and TNF-α mRNAs were an insert of 350 bp and an insert of 600 bp, respectively, containing the 5′-coding regions of the genes. For Northern blot analysis the inserts were labelled with 32P-dCTP by the random priming standard procedures to specific activities of 5 × 108 ct/min per mg DNA.
RNA isolation and analysis
Total cellular RNA was extracted according to the guanidine isothiocyanate–caesium chloride method and hybridizations were performed as described elsewhere [26].
Cell stimulation
Quiescent cells were incubated with the appropriate cytokine for 6 h in RPMI 1640 containing 0.2% FCS. Rheumatoid synovial cells were treated with IFN-γ (100 U/ml, final concentration), TGF-β (1 ng/ml), IL-4 (280 U/ml), or IL-10 (5 ng/ml), whereas osteoarthritic synovial cells were stimulated with IL-1β (20 ng/ml), TNF-α (20 ng/ml), GM-CSF (10 ng/ml), IL-6 (50 U/ml), and IL-8 (10 ng/ml). Actinomycin D and cycloheximide were used at a concentration of 2 mg/ml and 10 mg/ml, respectively.
IL-1Ra at a final concentration of 300 ng/ml, or B154.2 anti-TNF-α MoAb at a 1:2000 final dilution, were added separately or in combination in RPMI 1640 with 0.2% FCS and their effect was evaluated at 24 h.
Following this incubation, the cells were harvested with guanidine isothiocyanate–caesium chloride and the total RNA was extracted as described above.
PTX3 protein determination by ELISA
PTX3 was measured using a sandwich ELISA (G. Peri, unpublished). In brief, 96-well ELISA plates (Nunc, Roskilde, Denmark) were coated with 70 ng of rat MoAb MNB4 anti-PTX3 in 100 μl of coating buffer and incubated overnight at 4°C. After incubation, the plates were extensively washed with Dulbecco's PBS pH 7.2 containing 0.05% Tween 20 (washing buffer), and 300 μl of 5% dry milk in washing buffer were then added to block non-specific binding sites. After 2 h incubation at room temperature, the plates were again washed three times with washing buffer, and 50 μl of either purified human recombinant PTX3 standards (100 pg/ml to 2 ng/ml, diluted in RPMI 1640/2% bovine serum albumin (BSA)), cell supernatants, or biologic fluids were added in triplicate to each well and incubated for 2 h at 37°C. The plates were washed three times with washing buffer, and 100 μl of biotin-conjugated anti-PTX3 rabbit polyclonal diluted 1:2000 in washing buffer were added. The plates were incubated for 1 h at 37°C, and then washed three times with 200 μl of washing buffer. One hundred microlitres per well of peroxidase-streptavidin conjugated to dextran back-bone (AMDEX, Copenhagen, Denmark) diluted 1:4000 were subsequently added and the plates incubated for 1 h at room temperature. After incubation, the plates were washed four times before the addition of 100 μl of the chromogen substrate ABTS. Plates were read at 405 nm in an automatic ELISA reader. The ELISA assay did not cross-react with CRP.
Immunohistochemical staining
Five-micrometre thick paraffin-embedded synovial tissue sections were rehydrated and pretreated in a 750-W microwave oven in 0.01 m sodium citrate buffer pH 7.3 for 5 min and allowed to cool for 20 min. Endogenous peroxidase activity was blocked in 0.3% alcoholic hydrogen peroxide for 30 min. Sections were treated with 3% normal rabbit serum for 30 min at room temperature and then incubated overnight at 4°C with mouse MoAb 1C8 anti PTX3 diluted 1:20 in PBS. After washing with PBS, the sections were incubated with an appropriate biotinylated rabbit anti-mouse immunoglobulin antibody for 30 min at room temperature and then with peroxidase–avidin–biotin complex (Vectastain ABC Elite kit; Vector Labs, Burlingame, CA) for 30 min at room temperature. 3′3-diaminobenzidine (0.05%; Sigma) in 0.05 m Tris–HCl pH 7.6 containing 0.01% H2O2 was used as the chromogen and sections were counterstained using Mayer's haematoxylin. Appropriate negative controls were performed omitting the primary antiserum and replacing it with normal rabbit serum or with supernatant fluid from Chinese hamster ovary (CHO) 2.1 cell cultures.
Statistical analysis
Data are expressed as a mean + 1 s.d., and were compared by Mann–Whitney U-test. P < 0.05 was considered statistically significant.
RESULTS
Expression of the PTX3 gene in RA and OA synoviocytes
After 48 h of quiescence, type B synoviocytes grown from OA synovial tissue showed a low basal expression of the PTX3 gene, essentially similar to that found in fibroblasts [16]. In contrast, in resting rheumatoid synovial cells PTX-3 gene was strongly expressed (Fig. 1a,b). In some experiments (see Fig. 3), Northern analysis of PTX3 showed a double band. Since PTX3 exons are quite spaced out, the most likely explanation is that the doublet represents a precursor molecule with higher molecular weight present when PTX3 is over-expressed.
Fig. 1.
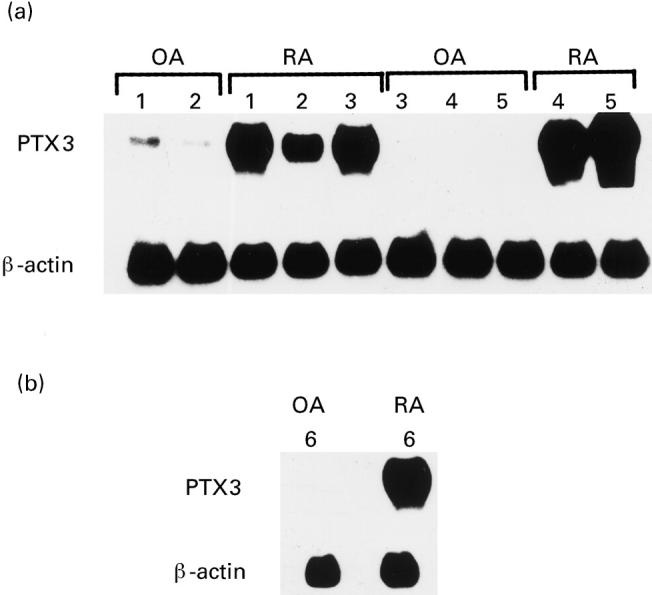
Northern blot analysis showing PTX3 expression in type B synoviocytes from six patients with RA and six patients with osteoarthritis (OA). Total RNA was prepared and analysed by Northern blot analysis as described in Patients and Methods. Expression of β-actin mRNA is shown for normalization of the quantities of RNA loaded in gel lanes. (a,b) Distinct Northern analysis with different patients and at different times.
Fig. 3.
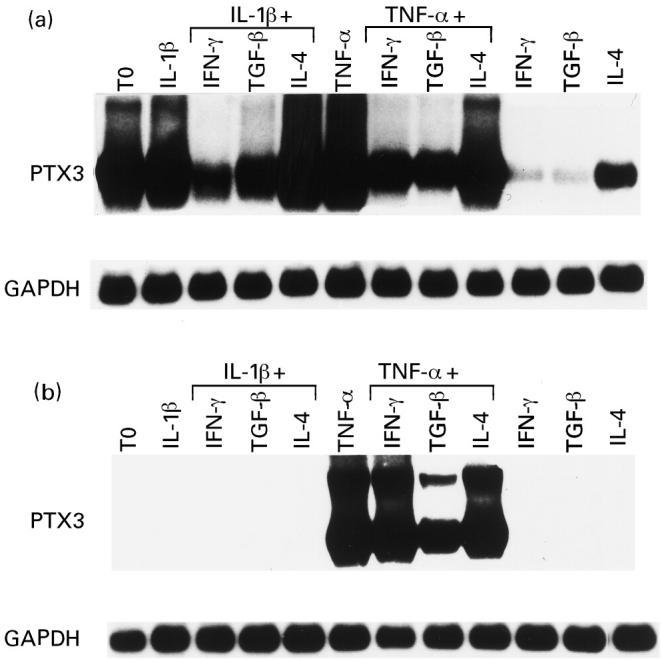
Northern blots showing the effects of different cytokines on PTX3 expression in RA synoviocytes (a), and in osteoarthritis (OA) synoviocytes (b). Cytokine concentrations and times of stimulation are detailed in Patients and Methods. Expression of GAPDH mRNA is shown for normalization of the quantities of RNA loaded in gel lanes.
PTX3 expression was unchanged in cells analysed after the eighth passage (data not shown), suggesting that it was not maintained by cytokines produced by mononuclear/phagocytes, usually contaminating only the earlier passages of type B synoviocytes [27]. However, in order to rule out that secretion of very small and undetectable amounts of IL-1β and TNF-α by cultured synoviocytes could stimulate PTX3 gene expression through an autocrine pathway, synoviocytes were incubated either with the IL-1Ra, or with a MoAb against TNF-α, or with a combination of these agents before Northern analysis. Neither IL-1Ra nor anti-TNF-α antibody nor a combination of the two affected the basal PTX3 over-expression (data not shown), although under the same experimental conditions anti-TNF-α MoAb and IL-1Ra completely blocked the induction of monocyte chemotactic protein-1 (MCP-1) and PTX3 itself in human monocytes exposed to IL-1β and TNF-α, respectively (unpublished data). Furthermore, the same blots which showed PTX3 message (Fig. 1) were hybridized with IL-1β and TNF-α probes with negative results (data not shown).
Thus, these experiments demonstrate that RA but not OA type B synoviocytes display high levels of PTX3 mRNA.
Cytokine modulation of PTX-3 gene expression in RA and OA synoviocytes
To elucidate whether cytokines modulate PTX3 expression in synovial cells in vitro, OA synoviocyte cultures were stimulated with recombinant human IL-1β, TNF-α, IL-6, GM-CSF, IL-8, which are normally present in RA joints [1,3]. Although IL-1β and TNF-α have been demonstrated to be able to induce PTX3 gene expression in skin fibroblasts [16] and in other cell types [12,13,25], only TNF-α was active on OA synoviocytes (Fig. 2a).
Fig. 2.
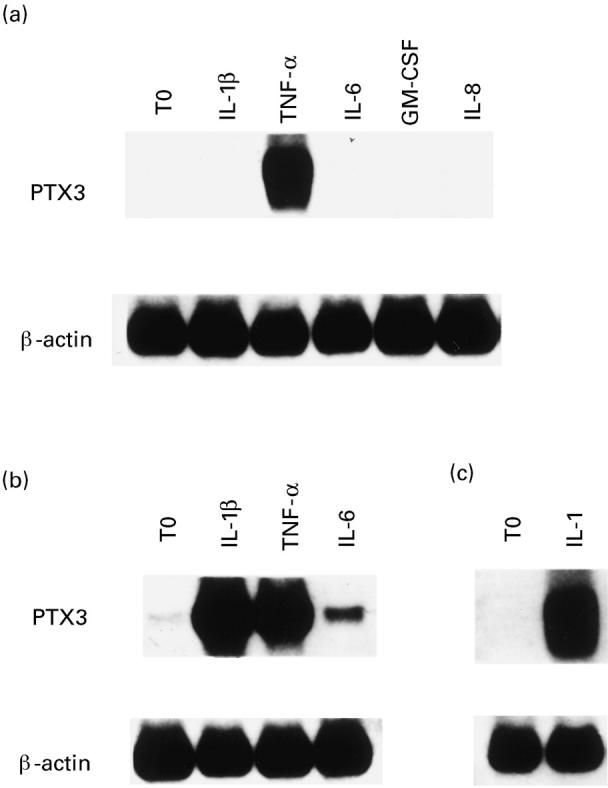
Northern blots showing the effects of different cytokines on PTX3 expression by osteoarthritis (OA) synoviocytes (a) and normal skin fibroblasts (b). (c) Induction of IL-8 mRNA in OA synoviocytes by the preparation of the IL-1β used in all the PTX3 experiments. Cytokine concentrations and times of stimulation are detailed in Patients and Methods. Expression of GAPDH mRNA is shown for normalization of the quantities of RNA loaded in gel lanes.
This finding could not be ascribed to the IL-1β preparation used since, as expected, we observed a positive response in skin fibroblasts (Fig. 2b) and obtained the same negative result when synoviocytes were stimulated with three different batches of IL-1β (data not shown). Furthermore, IL-1β was able to induce IL-8 mRNA in the same synoviocytes (Fig. 2c). Actinomycin D, but not cycloheximide, inhibited TNF-α-induced stimulation of PTX3 expression in OA synoviocytes, suggesting dependency on gene transcription (data not shown).
The down-regulation of PTX3 was investigated in RA synoviocytes stimulated with TNF-α and IL-1β in the presence of IFN-γ, TGF-β and IL-4. TGF-β and IFN-γ down-regulated the stimulated and, to a greater extent, the constitutive expression of PTX3 mRNA in RA synoviocytes, leading in the latter case to a basal value similar to that observed in quiescent OA synovial cells (Fig. 3a). IL-4 caused only a slight decrease of the PTX3 mRNA. In contrast, TGF-β but not IFN-γ partially inhibited PTX3 message in OA synoviocytes stimulated with TNF-α (Fig. 3b).
PTX3 protein levels in synoviocyte cell cultures, and synovial fluid
It was important to investigate whether PTX3 protein production paralleled gene expression in RA. As shown in Fig. 4a, RA synoviocytes produced higher levels of PTX3 than OA cells in the absence of deliberate stimulation and production was inhibited by TGF-β, IFN-γ and, to a lesser extent, IL-4. TNF-α, unlike IL-1β, induced the production of PTX3 protein by OA synoviocytes, and TGF-β but not IFN-γ inhibited its production (Fig. 4b).
Fig. 4.
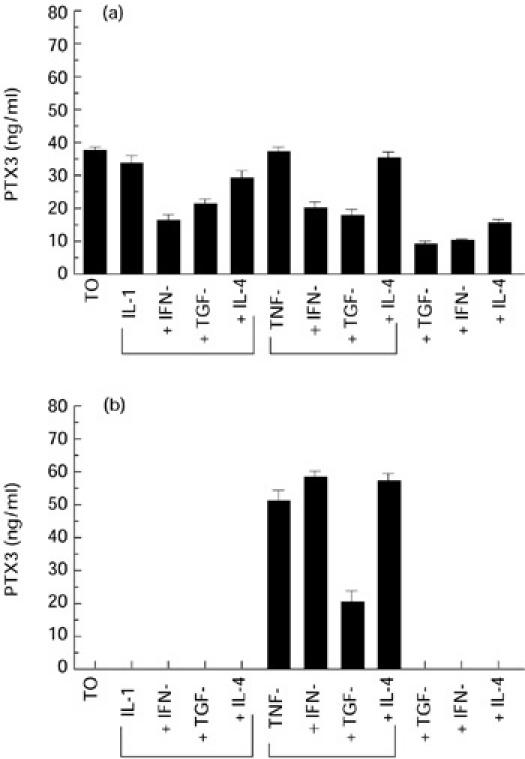
PTX3 protein levels in supernatants from RA (a) and osteoarthritis (OA) (b) synoviocyte cultures after stimulation with different cytokines and growth factors. For details see Patients and Methods. PTX3 protein level was assessed by ELISA.
In an effort to obtain preliminary indications as to the significance of PTX3 expression by RA synoviocytes, protein levels were measured in synovial fluid, in a limited series of RA patients. A greater increase was observed in 11 RA synovial fluids when they were compared with nine traumatic, non-inflammatory joint effusions (9.3 ± 1.9 ng/ml versus 0.5 ± 0.2 ng/ml; P < 0.0002) (Fig. 5).
Fig. 5.
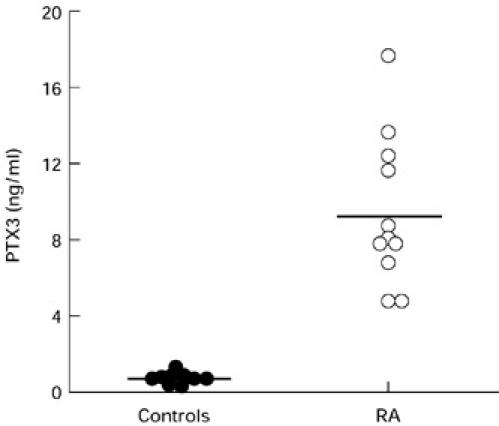
PTX3 protein levels in synovial fluids (11 RA patients and nine controls). PTX3 level was determined by ELISA. Data are reported as mean ± s.d. The horizontal line represents the upper limit of the normal.
In conclusion, synovial fluids from RA patients displayed levels of the PTX3 protein significantly higher than controls.
Immunohistochemical findings
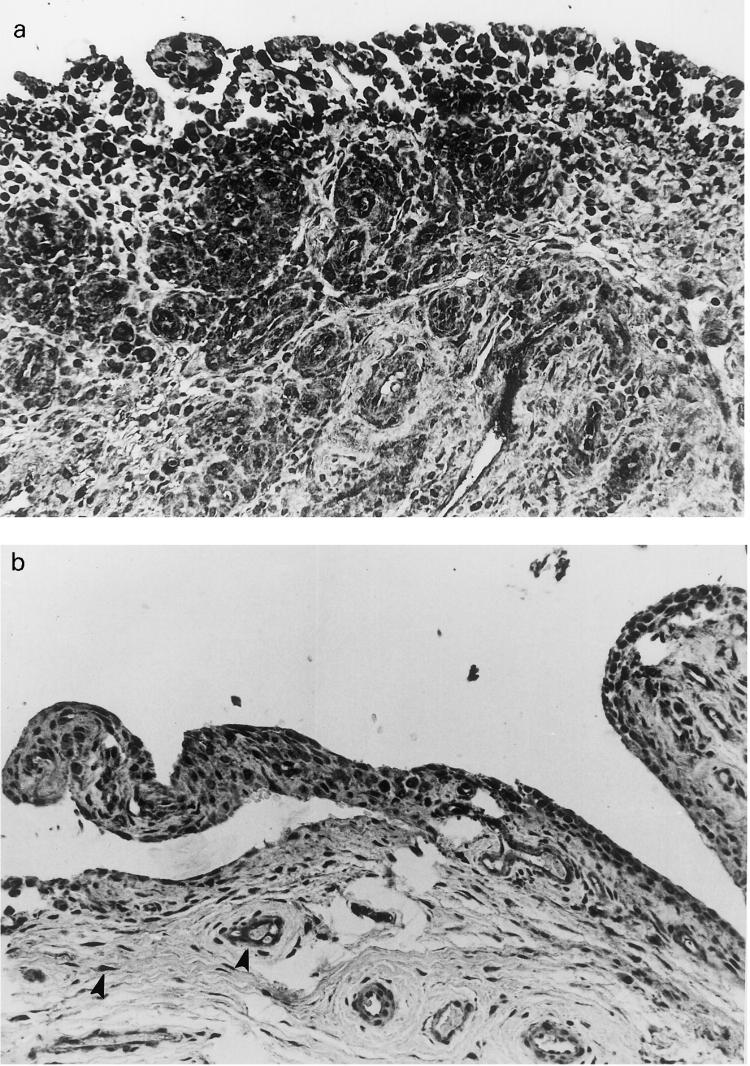
Synovial biopsies from three patients with OA and from three patients with RA were studied by immunohistochemical techniques. In RA specimens intense staining was present in endothelial cells, in vessel walls and in all synovial cells (Fig. 6a). In OA biopsies staining was sometimes evident in some sections, although less intense than in RA biopsies (Fig. 6b).
Fig. 6.
(a) Synovial tissue from a patient with RA. A strong immunostaining for PTX3 is detected in the cytoplasm of hyperplastic synovial lining cells. Stromal and endothelial cells are also labelled. (Immunoperoxidase–haematoxylin, × 250.) (b) Tissue from a patient with osteoarthritis. Synovial stromal and endothelial cells (arrowheads) show a faint staining for PTX3 (Immunoperoxidase–haematoxylin, × 250).
DISCUSSION
PTX3 is the first cloned long pentraxin, an emerging family of molecules consisting of a C-terminal pentraxin-like domain coupled with an N-terminal portion [14,17–22]. Plasma pentraxins (CRP and SAP component) are acute-phase proteins made in the liver. There is evidence that PTX3 is made in mice in response to LPS [14,16], but no data are available regarding its induction and regulation in human diseases.
The present study, showing in vitro expression and production as well as high levels in synovial fluids in RA, provides the first demonstration of involvement of the prototypic long pentraxin PTX3 in a human inflammatory disease.
Recent studies have reported that PTX3 mRNA can be induced in several cell lines [14,16,25], usually after exposure to inflammatory stimuli such as LPS, IL-1β and TNF-α. At variance with what has been found in some cell systems [16], OA synoviocytes exhibit a unique response pattern similar to that observed in certain mononuclear cell lines [25], since only TNF-α, but not IL-1β, is capable of inducing the gene.
When PTX3 induction occurs in type B RA synoviocytes is not clear, although IL-1β and TNF-α, together with other macrophage products, such as GM-CSF, IL-6, IL-8, are abundant in rheumatoid joints at both the mRNA and protein level [1,3,28,29], and could mediate the induction of PTX3 in type B synovial cells. However, IL-1Ra and anti-TNF-α antibodies did not abrogate PTX3 over-expression, ruling out the presence of minimal amounts of IL-1β and TNF-α proteins in culture medium. Furthermore, we have been unable to show an autocrine loop which could maintain synoviocyte PTX3 expression, since IL-1β and TNF-α mRNAs were not detectable.
PTX3 expression is still detectable after several subpassages of RA synoviocytes, when contaminating monocytes/macrophages and their products are absent, as demonstrated here and by others [27]. In fact, after three or more passages the cells that were obtained from synovial tissue are almost exclusively type B fibroblast-like synoviocytes, and thus, macrophage cytokine production cannot account for PTX3 expression. Consequently, PTX3 expression is to be considered part of a persistent activation of RA synoviocytes, as already shown by the demonstration of the autonomous and sustained production of proinflammatory cytokines, such as GM-CSF [27], MCP-1, IL-8 [30,31], of anti-inflammatory agents, such as IL-1Ra [32], TGF-β [33] and of IL-10 [34], and of proteolytic enzymes [35].
The function of PTX-3 has not been completely defined. Recently, it was found that the capacity of PTX-3 to recognize ligands differs considerably from that of the classical pentraxins CRP and SAP and that PTX-3 binds C1q and activates the C cascade [36]. Therefore, it is tempting to speculate that in part PTX3 may amplify complement-mediated mechanisms responsible for inflammation and tissue damage in RA [37–39].
As for inhibition of PTX3 expression, here and elsewhere [40] we report that certain cytokines (e.g. TGF-β and IFN-γ) down-regulate PTX3 expression. However, in OA synoviocytes only TGF-β was able partially to inhibit PTX3 induction by TNF-α.
In this respect, it will be interesting to define the molecular basis underlying the differential responsiveness of RA versus OA synoviocytes to TNF-α, IL-1β, IFN-γ and TGF-β.
Employing an immunoenzymatic assay, we have detected increased levels of PTX3 protein in synovial fluids from patients with RA. Since it has been shown that, in contrast to other acute-phase reactants, a major site of PTX3 synthesis is extrahepatic, our findings lead to the conclusion that the main source of PTX3 in RA is the synovial pannus, which is rich in monocytes/macrophages, endothelial cells as well as type A and B synoviocytes, all of which, as shown by the immunohistological findings, can be a potential source of the protein in the synovial fluid.
Studies are in progress to estimate the expression of PTX3 in synovial tissue of other inflammatory arthritides and the role of this molecule in the pathogenesis of rheumatoid arthritis.
Acknowledgments
This work was partly supported by Grants MPI 40% 1997 and 1998 and by the Italian Association for Cancer Research (AIRC).
REFERENCES
- 1.Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–89. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 2.Fassbender HG. Histomorphologic basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141–51. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- 3.Firestein GS, Zvaifler NJ. Rheumatoid arthritis: a disease of disordered immunity. In: Gallin JI, Goldstein IM, Willis K, editors. Inflammation: basic principles and clinical correlates. 2. New York: Raven Press; 1992. [Google Scholar]
- 4.Rammers EF, Sano H, Wilder R. Platelet-derived growth factors and heparin-binding (fibroblasts) growth factors in the synovial pathology of rheumatoid arthritis. Sem Arthritis Rheum. 1991;21:191–9. doi: 10.1016/0049-0172(91)90009-o. [DOI] [PubMed] [Google Scholar]
- 5.Lafyatis R, Remmers EF, Roberts AB, et al. Anchorage independent growth by synoviocytes from arthritic and normal joints: stimulation by exogenous platelet growth factor β and retinoids. J Clin Invest. 1989;83:1267–76. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krane SM, Conco W, Stephenson ML, et al. Mechanisms of matrix degradation in rheumatoid arthritis. Ann NY Acad Sci. 1990;580:340–54. doi: 10.1111/j.1749-6632.1990.tb17943.x. [DOI] [PubMed] [Google Scholar]
- 7.Trabandt A, Aicher WK, Gay RE, et al. Spontaneous expression of immediately-early response genes c-fos and egr-1 in collagenase-producing rheumatoid synovial fibroblasts. Rheumatol Int. 1992;12:53–9. doi: 10.1007/BF00300977. [DOI] [PubMed] [Google Scholar]
- 8.Case JP, Lafyatis R, Remmers EF, et al. Transin/stromelysin expression in rheumatoid synovium: a transformation-associated metalloproteinase secreted by phenotypically invasive synoviocytes. Am J Pathol. 1989;135:1055–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Maciewicvz RA, Wotton SF. Degradation of cartilage matrix components by the cysteine proteinases, cathepsin B and L. Biomed Biochim Acta. 1991;50:561–4. [PubMed] [Google Scholar]
- 10.Nguyen Q, Mort JS, Roughley PJ. Cartilage proteoglycan aggregate is degradated more extensively by cathepsin L than by cathepsin B. Biochem J. 1990;286:569–73. [PMC free article] [PubMed] [Google Scholar]
- 11.Keyszser GH, Heer AH, Kriegsman J, et al. Comparative analysis of cathepsin L, cathepsin D, and collagenase messenger RNA expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis, by in situ hybridization. Arthritis Rheum. 1995;38:976–84. doi: 10.1002/art.1780380714. [DOI] [PubMed] [Google Scholar]
- 12.Breviario F, D'aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells: cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;287:22190–7. [PubMed] [Google Scholar]
- 13.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor and IL-1 inducible protein is a novel member of the pentraxin family of acute phase proteins. J Immunol. 1993;150:1804–12. [PubMed] [Google Scholar]
- 14.Introna M, Vidal Alles V, Castellano M, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–72. [PubMed] [Google Scholar]
- 15.Gewurz H, Zhang X, Lint TF. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee GW, Goodman AR, Lee TH, et al. Relationship of TSG-14 protein to the pentraxin family of major acute phase proteins. J Immunol. 1994;153:3700–7. [PubMed] [Google Scholar]
- 17.Noland TD, Friday BB, Maulit MT, et al. The sperm acrosomal matrix contains a novel member of the pentraxin family of calcium-dependent binding proteins. J Biol Chem. 1994;269:32607–14. [PubMed] [Google Scholar]
- 18.Reid MS, Blobel CP. Apexin, an acrosomal pentraxin. J Biol Chem. 1994;269:32615–20. [PubMed] [Google Scholar]
- 19.Schlimgen AK, Helms JA, Vogel H, et al. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–26. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- 20.Seery LT, Schoenberg DR, Barbaux S, et al. Identification of a novel member of the pentraxin family in Xenopus laevis. Proc R Soc Lond Series B Biol Sci. 1993;253:263–70. doi: 10.1098/rspb.1993.0112. [DOI] [PubMed] [Google Scholar]
- 21.Tsui CC, Copeland NG, Gilbert DJ, et al. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;15:2463–78. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu YC, Perin MS. Human neuronal pentraxin II (NPTX2): conservation, genomic structure, and chromosomal localization. Genomics. 1995;28:220–7. doi: 10.1006/geno.1995.1134. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson LS, Pitsillides AA, Worrall JG, et al. Light microscopy characterisation of the fibroblastic synovial lining cells (synoviocytes) Arthritis Rheum. 1992;35:1179–84. doi: 10.1002/art.1780351010. [DOI] [PubMed] [Google Scholar]
- 25.Alles VV, Bottazzi B, Peri G, et al. Inducible expression of PTX-3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–93. [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Alvaro Gracia JM, Zvaifler NJ, Brown CB. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in GM-CSF production and gene expression in rheumatoid arthritis and its regulation by IL-1 and TNF-α. J Immunol. 1991;146:3365–71. [PubMed] [Google Scholar]
- 28.Panay GS, Lanchbury JS, Kingsley GH. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729–35. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- 29.Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37:783–9. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]
- 30.Hirota K, Akahoshi T, Endo H, et al. Production of interleukin-8 by cultured synovial cells in response to interleukin-1 and tumor necrosis factor. Rheumatol Int. 1992;12:13–6. doi: 10.1007/BF00246871. [DOI] [PubMed] [Google Scholar]
- 31.Koch AE, Kunkel AL, Harlow LA, et al. Enhanced production of monocyte chemoattractant protein in rheumatoid arthritis. J Clin Invest. 1992;90:772–9. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seitz M, Loetscher P, Dewald B, et al. Production of interleukin-1 receptor antagonist, inflammatory chemotactic proteins, and prostaglandin E by rheumatoid and osteoarthritic synoviocytes. Regulation by IFN-gamma and IL-4. J Immunol. 1994;152:2060–5. [PubMed] [Google Scholar]
- 33.Brennan FM, Chantry D, Turner M, et al. Detection of transforming growth factor-beta in rheumatoid arthritis synovial tissue: lack of effect on spontaneous cytokine production in joint cell cultures. Clin Exp Immunol. 1990;81:278–85. doi: 10.1111/j.1365-2249.1990.tb03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsikis PD, Chu C, Brennan FM, et al. Immunoregulatory role of interleukin-10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–27. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trabandt A, Aicher WK, Gay RF. Expression of the collagenolytic and Ras-induced cystine proteinase cathepsin L and proliferation-associated oncogenes in synovial cells of MRL/l mice and patients with rheumatoid arthritis. Matrix. 1990;10:349–61. doi: 10.1016/s0934-8832(11)80142-3. [DOI] [PubMed] [Google Scholar]
- 36.Bottazzi B, Vouret-Craviari V, Bastone A, et al. Multimer formation and ligand recognition by the long pentraxin PTX3: similarities and differences with the short pentraxin C reactive proteins and serum amyloid P component. J Biol Chem. 1997;272:32817–23. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 37.Katona IM, Ohura K, Allen JB, et al. Modulation of monocyte chemotactic function in inflammatory lesions. Role of inflammatory mediators. J Immunol. 1991;146:708–14. [PubMed] [Google Scholar]
- 38.Jose PJ, Moss IK, Maini RN, et al. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5A in the acute inflammatory phase. Ann Rheum Dis. 1990;49:747–52. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breitner S, Storkel S, Reikel W, et al. Complement components C1q, C1r/C1s, and C1 INH in rheumatoid arthritis. Correlation of in situ hybridization and northern blot results with function and protein concentration in synovium and primary cell cultures. Arthritis Rheum. 1995;38:492–8. doi: 10.1002/art.1780380406. [DOI] [PubMed] [Google Scholar]
- 40.Polentarutti N, Picardi G, Basile A, et al. Interferon-gamma inhibits expression of the long pentraxin PTX3 in human monocytes. Eur J Immunol. 1998;28:496–501. doi: 10.1002/(SICI)1521-4141(199802)28:02<496::AID-IMMU496>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]