Abstract
Immunoglobulin gene rearrangements in patients treated with BMT have restricted repertoire diversity. Clonal variability remains low for 3 months and reconstitution of the humoral immune system appears to follow a wave-like pattern. In the present study we analysed serum IgM and IgG repertoires in 44 patients from 1 week to 3 years after transplantation. We applied a quantitative immunoblot technique in combination with a newly developed method for estimation of repertoire diversity in complex mixtures of antibodies. Our results demonstrate that 60% of BMT patients have severely reduced diversity in the IgM repertoire during and after the first year post-BMT, compared with healthy controls. In contrast, the majority of patients have a polyclonal IgG repertoire, similar to that of healthy controls. Serum IgM repertoires remain oligoclonal even though the serum concentration of total IgM is within normal range around 6 months post-BMT. During the first years after transplantation IgM as well as IgG repertoires are less diverse in patients receiving a BM graft from a sibling donor compared with those receiving a graft from an HLA-matched unrelated donor. Patients in the latter group show a higher incidence of infections and minor antigen mismatches which may promote the development of a diverse immunoglobulin repertoire post-BMT.
Keywords: human, generation of diversity, repertoire development, transplantation, antibodies
INTRODUCTION
We have previously shown that immunoglobulin repertoire development in patients treated with BMT is oligoclonal in rearranged DNA gene segments as well as among transcribed variable regions [1,2]. We analysed immunoglobulin heavy chain (VH) rearrangements by polymerase chain reaction (PCR) using VH gene family-specific primers, DNA sequencing and hybridization with complementarity determining region 3 (CDR3)-specific oligonucleotide probes. The VH6-containing heavy chain rearrangements remained oligoclonal up to 3 months after transplantation [1] and VH3 rearrangements up to 6 months post-BMT [2]. In addition, the development of the immunoglobulin repertoire appeared to follow a wave-like pattern with consecutive appearance and disappearance of individual immunoglobulin clones. Based upon these results we suggested that the oligoclonal immunoglobulin repertoire in BMT patients might contribute to the impaired humoral immunity from which these patients suffer. The immune system in BMT patients is characterized by reduced immune responses both in vivo and in vitro [3,4]. Normal levels of circulating IgM and IgG antibodies are achieved during the first year after BMT [5], but specific humoral immunity often remains impaired more than 1 year post-BMT [5,6].
In this study we have expanded our investigation to immunoglobulin repertoires on protein level, i.e. circulating antibodies. This analysis was made possible by application of a new technique, based on quantitative immunoblot (QIB) developed by Nobrega et al. [7,8], that permits the estimation of functional repertoire diversity in complex mixtures of antibodies, e.g. supernatants and sera. Further development of the technique has demonstrated that repertoire diversity in a sample can be related to the discrepancy of its immunoreactivity profile, as obtained in QIB, from the average reactivity profile of a very diverse random mixture of antibodies [9]. By using supernatants of murine spleen B cells stimulated with lipopolysaccharide (LPS) and cultured under limiting dilution conditions, Brissac et al. [9] demonstrated that the reactivity profiles converge towards the average profile when repertoire diversity (number of random cultured clones) increases. By composing a measure of distance between two profiles, in order to quantify the discrepancy between any sample profile and the reference profile, they established a calibration curve that strictly correlates that measure of distance to sample diversity. The reference profile can be obtained by taking the average of a sufficient number of profiles either from random sets of immunoreactivities or from the profile of a very diverse sample.
Using this technique we here demonstrate that immunoglobulin repertoires in healthy controls are highly polyclonal and that BMT patients have severely reduced diversity in the IgM repertoire. More than 1 year post-BMT 39% of the patients still have a repertoire very deviant from that of healthy controls. In contrast, the majority of patients have a polyclonal IgG repertoire, similar to that of healthy controls. We also show that both IgM and IgG repertoires are less diverse in patients receiving a BM graft from a sibling donor compared with those receiving a graft from an HLA-matched unrelated donor (MUD). This difference remains in serum samples taken more than 1 year after transplantation.
PATIENTS AND METHODS
Patient characteristics
We included 44 patients treated with allogeneic BMT and 18 healthy controls in this study (ethical permission Dnr 167/98 from the Ethical committee at Huddinge University Hospital). Before transplantation the patients were conditioned with a combination of cyclophosphamide (120 mg/kg) and 10 Gy total body irradiation. Patients were given a combination of methotrexate and cyclosporin A (CsA) as prophylaxis against graft-versus-host disease (GVHD). Details regarding the treatment have been published previously [10,11]. Thirteen of the patients were treated with 0.5 g/kg body weight intravenous immunoglobulin (IVIG; Gammagard; Baxter Medical AB, Kista, Sweden) once a week from 1 week before to 3 months after transplantation (total 13 doses). Major patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics

ALL, Acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CML, chronic myeloid leukaemia; CMV, cytomegalovirus; IVIG, intravenous immunoglobulin; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease.
Collection of serum samples and determination of immunoglobulin levels
Sera were collected from the patients before and at several times after transplantation as well as from healthy controls. The patient samples pre-BMT were collected before conditioning of the patients. The first serum sample post-BMT was collected as early as possible after transplantation, i.e. 3 days to 1.5 weeks, in most patients. The last serum sample was collected 1–3 years post-BMT in 60% of the patients and at 3 months to 1 year after transplantation in the remaining group. IgM and IgG concentrations were determined with nephelometry and ELISA. The samples were diluted in PBS with 0.2% Tween 20 to 60 μg/ml IgM or 250 μg/ml IgG in all samples before analysis in QIB.
Highly polyclonal IgG and IgM samples
As a reference preparation of very diverse human IgG, IVIG was used (Sandoglobin; Sandoz, Basel, Switzerland). For IgM reference IgM was used purified from pooled plasma from > 2500 healthy donors (pooled IgM) using a modified Deutsch–Kistler–Nitschmann ethanol fractionation procedure followed by octanoic acid precipitation and two successive ion-exchange chromatography steps [12]. Both IVIG and pooled IgM were a kind gift from Dr S. Kaveri (INSERM U340, Paris, France).
Quantitative immunoblot
QIB is based on Western blot analysis and makes it possible to analyse immunoreactivities of any antibody mixture (e.g. serum) against a large sample of proteins used as reactivity probes. A detailed description of the technique has been published previously [7,8]. In brief, a human liver protein extract was separated on a 10% SDS–PAGE and transferred to nitrocellulose membranes by electroblotting. After blocking with 0.2% Tween 20 in PBS, individual serum samples were incubated on the membranes and the immunoreactivities were revealed by incubation with secondary antibody conjugated with alkaline phosphatase. An immunoreactivity profile was obtained for each sample by scanning the membrane with a high resolution CCD camera system. The profiles were divided into sections corresponding to the antigenic sections revealed by the procedure and the reactivity within each section was expressed as optical density (OD). By Protogold (Biocell, Cardiff, UK) staining of blotted proteins we could correct for migration distortions in the gel, which allowed comparison of reactivity levels of different antibody mixtures against a given section. A profile thus consisted of a list of reactivity values, one for each section. Using this technique sera from BMT patients were tested taken at different time points before and after transplantation. Sera from 18 healthy controls were also analysed. For all patients and controls IgM as well as IgG reactivities were tested. All samples from one patient were tested on a single membrane and each patient was tested for IgM and IgG on separate membranes. On each membrane a reference sample was included: IVIG for IgG reactivities and pooled IgM for IgM reactivities.
Estimation of repertoire diversity in serum samples
The distance of any sample's immunoreactivity profile obtained in QIB to a reference profile correlates to functional diversity of the sample's immunoglobulin repertoire. Ideally the reference profile should contain a very diverse, random mixture of antibodies produced by an infinite number of immunoglobulin-secreting clones. The most appropriate source containing such a diverse representation of antibody reactivities is human IVIG and pooled human IgM. These preparations are very close to random mixtures, since antibody repertoires in single individuals are very different from each other [13], and are expected to be very diverse since they are selected on a very large number of genetic backgrounds and shaped by individual immune experiences.
Using this measure of distance we followed repertoire diversity fluctuations in sera from BMT patients after transplantation and in healthy controls. Each sample was tested both for IgM and IgG reactivity and the distance to the corresponding reference profile was calculated. For each section the squared difference was measured between the sample profile and the reference profile, normalized by the reference profile value in order to obtain non-dimensional units, and this measurement was averaged over all sections for each sample. The final formula is: distance to RP = Σi(xi − rpi)2/rpi2, where xi is the observed OD for section i, and rpi is the corresponding value for the reference profile (RP). In mice the study of functional repertoire diversity was based on a calibration curve that strictly relates discrepancy of any profile towards the RP, to numbers of immunoglobulin-secreting clones that contributed to the sample tested. In applying this technique to human samples it was necessary to do without any reference to number of clones, and use the distance to the RP as a relative measure of diversity compared with other samples. We thus compared the distance to the RP between control values and patient values at the different time points in order to follow the development of functional repertoire diversity over time. Note that the two quantities behave in inverse relation, i.e. a higher number in distance to the RP corresponds to less diversity.
RESULTS
Comparison of healthy controls' reactivity profiles to the reference profiles
In Fig. 1 is shown the mean OD in each section of the antigenic profile for the 18 healthy controls and the OD for the reference profiles of pooled IgM and IVIG. The mean reactivity profile for the controls is very similar to the reference profiles for IgM and IgG reactivities. This was expected, since pooled IgM and IVIG represent a biological mean of individual sera. Furthermore, the distances of individual control reactivity profiles to the RP are relatively low (Fig. 2). The distances for IgM reactivities range from 0.007 to 0.14, with a mean (± s.d.) of 0.06 ± 0.04 and IgG distances range from 0.03 to 0.22 (0.09 ± 0.06). The immunoglobulin repertoire of healthy individuals is considered to be very diverse [13] and we conclude that the distances obtained for healthy adults represent a polyclonal repertoire.
Fig. 1.

Mean IgM (a) and IgG (b) immunoreactivity values for 18 healthy controls (C1–C18; •), expressed as optical density (OD) compared with the immunoreactivity values for pooled human IgM or intravenous immunoglobulin (IVIG), respectively, in the different antigenic sections defined on the immunoblot membrane.
Fig. 2.

Distance to the reference profile (RP) for the individual healthy controls' IgM and IgG reactivity profiles, respectively. As RPs pooled human IgM and intravenous immunoglobulin (IVIG) were used. Sample mean is indicated by a horizontal line. The distance of any sample's immunoreactivity profile to the RP inversely correlates with the samples intravenous immunoglobulin repertoire diversity, so that a higher value of distance corresponds to less diversity.
Individual development of IgM and IgG repertoires among BM recipients
Analysis of immunoglobulin repertoire diversity at the level of functional polyclonality reveals that there was a great variation in patients' behaviour over time. Based on the mean and s.d. of the distance between immunoglobulin profiles from healthy controls compared with the RP, we have defined three categories of repertoire diversity. Normal immunoglobulin repertoire diversity is defined as a distance to the RP below the mean + 1 s.d. obtained for healthy controls (category 1). A distance between the mean + 1 s.d. and the mean + 3 s.d. is defined as deviant immunoglobulin repertoire diversity (category 2). When the distance is above the mean + 3 s.d. the immunoglobulin repertoire diversity is considered very deviant (category 3). In Fig. 3 the BMT patients are divided into three groups according to their IgM repertoire diversity. The first group includes patients that at all time points after transplantation have a normal repertoire (Fig. 3a). The second patient group has at least one time point that is deviant from the healthy controls (Fig. 3b). Patients with a very deviant immunoglobulin repertoire diversity constitute the third group (Fig. 3c). We demonstrate that for IgM reactivities the majority of patients were very deviant from healthy controls at at least one time point after BMT. It is also obvious that development of repertoire diversity varied extensively between patients. Some patients showed an increasing degree of polyclonality over time, whereas others became less polyclonal or showed a fluctuating pattern. In Fig. 4 immunoreactivity values in each section are shown for the individual patient samples expressed as OD. The samples are grouped according to IgM repertoire diversity as defined above. It is demonstrated that the majority of samples fall into the categories of deviant and very deviant IgM repertoire diversity. Individual IgM reactivities increase the distance to the RP, i.e. pooled IgM, with decreasing IgM repertoire diversity (Fig. 4c).
Fig. 3.

Distance to the IgM reference profile (RP) at different time points after transplantation for sera from 41 patients. Three categories of intravenous immunoglobulin repertoire diversity (see Results for details) are defined based on the values obtained for healthy controls: normal, deviant and very deviant. (a) Patients that have a normal IgM repertoire diversity at all time points analysed. (b) Patients with a deviant IgM repertoire during at least one time point post-BMT. (c) Patients with a very deviant IgM repertoire diversity during at least one time point. A vertical bar to the right in each panel indicates the range of distances for the controls.
Fig. 4.

Individual IgM (a–c) and IgG (d–e) (see next page) immunoreactivity values in patient sera expressed as optical density (OD). (a,d) Patient samples with normal IgM repertoire diversity. (b,e) Patient samples with deviant IgM repertoire diversity. (c,f) Patient samples with very deviant IgM repertoire diversity. Individual reactivities in antigenic sections are represented by (+). Pooled human IgM (a–c) and intravenous immunoglobulin (IVIG) (d,e) are represented by solid lines.
In contrast to the IgM repertoire, IgG repertoire diversity was within normal range at all time points tested in the majority of patients (31/44). Thirteen patients showed an oligoclonal IgG repertoire on at least one time point post-BMT, six patients were deviant and seven patients very deviant from healthy controls. Individual IgG reactivities were mostly very close to the IVIG reference profile (Fig. 4d–f), supporting the finding that most patients had a normal IgG repertoire diversity. Even samples from patients with a very deviant IgM repertoire diversity demonstrated in most cases a normal IgG repertoire diversity (Fig. 4f).
General development of IgM and IgG repertoire variability among BM recipients
To obtain an overview of the general behaviour of patient repertoires post-BMT the time after BMT was divided into four intervals and the distribution of the different samples between the three categories was analysed in these intervals: normal (category 1), deviant (category 2) and very deviant (category 3) repertoire. The percentage of patients having an oligoclonal IgM repertoire increased after transplantation, with a peak at 3 weeks to 3 months post-BMT when 47% of the patient samples were very deviant from healthy controls (Fig. 5a). More than 1 year after transplantation 39% of the patient samples still had an IgM repertoire very deviant from the healthy controls.
Fig. 5.
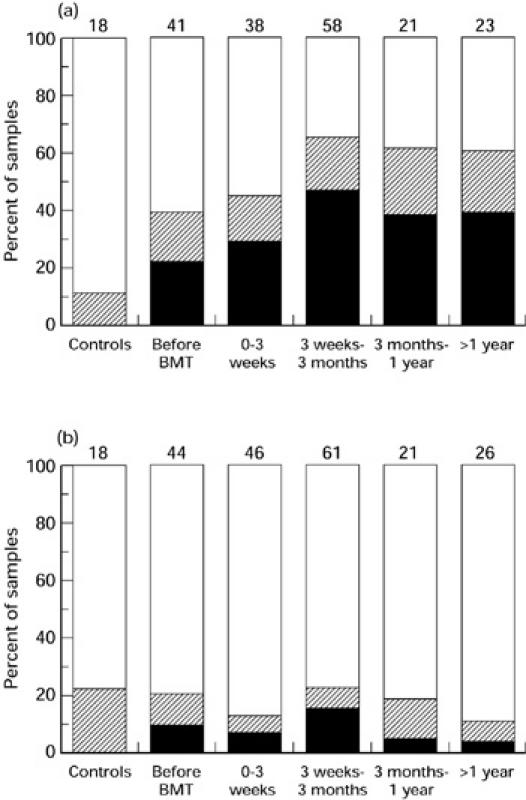
General development of IgM and IgG repertoire diversity at different time intervals after transplantation. The percentage of samples that fall into category 1 (□), 2 (hatched) and 3 (▪), as defined in Results, is shown. The number of individual samples is indicated at the top of each bar. (a) IgM reactivities for all patients. (b) IgG reactivities for all patients.
The pattern of IgG reactivities is clearly different from IgM. Only a minority of BMT patients had an oligoclonal IgG repertoire very deviant from the healthy controls. Similar to IgM the oligoclonality peak appeared at 3 weeks to 3 months post-BMT (Fig. 5b). In this time interval 15% of the patient samples fell into the very deviant category. More than 1 year after transplantation, however, only 4% of the patient samples had a very deviant IgG repertoire compared with healthy controls. To exclude the possibility that the IVIG-treated patient group contributed significantly to the diverse IgG repertoire, we analysed the distribution of samples from only non-IVIG-treated patients. At more than 1 year post-BMT, 80% of the samples showed a normal diversity, 13% a deviant diversity and 7% a very deviant diversity compared with healthy controls. These results are very similar to the patient group as a whole.
Temporal correlations in development of immunoglobulin repertoire diversity
To estimate whether immunoglobulin repertoire diversity at a given time point after BMT is influenced by the degree of diversity earlier during immune reconstitution, we calculated the correlation between the set of individual distances to the RP obtained in one time interval and the corresponding values in the other time intervals. Although all patients were not represented by a sample in every time interval analysed, sufficient numbers of patients were obtained in each pair of time intervals considered, except one, to perform the analysis. The exception concerned the correlation between repertoire diversity in samples taken later than 1 year after BMT and samples taken in the interval between 3 months and 1 year post-BMT. Both for IgM and IgG repertoire diversity a strong correlation was found between all time intervals analysed except for the latest time point. The correlation coefficient is 0.9 and P < 0.001 when comparing IgM repertoire diversity in the first time interval with the result before transplantation. Repertoire diversity in samples taken later than 1 year after transplantation showed no significant correlation with repertoire diversity during the other time intervals. The correlation coefficient is 0.37 and P = 0.08 when comparing the latest time point with the situation before transplantation.
Difference in repertoire diversity between BMT patients grafted with marrow from a MUD or a sibling donor
We compared repertoire diversity among patients receiving a BM graft from a sibling donor or a MUD in all time intervals, for IgM (Fig. 6) and IgG (Fig. 7) repertoires, respectively. IgM repertoire diversity in patients receiving a BM graft from a sibling donor was significantly reduced, compared with repertoire diversity in patients receiving BM from a MUD, at more than 1 year after BMT (P = 0.02) as well as between 3 weeks and 3 months post-BMT (P = 0.02). For IgG repertoire diversity there was a larger proportion of the BM recipients with a sibling-derived graft that had a reduced repertoire diversity, more than 1 year post-BMT, compared with patients that had received a BM graft from a MUD (NS).
Fig. 6.

Development of IgM repertoire diversity at different time intervals after transplantation for patients receiving BM from a sibling (a) or from an HLA-matched unrelated donor (MUD) (b). The percentage of samples that fall into category 1 (□), 2 (hatched) and 3 (▪), as defined in Results, is shown. The number of individual samples is indicated at the top of each bar.
Fig. 7.

Development of IgG repertoire diversity at different time intervals after transplantation for patients receiving BM from a sibling (a) or from an HLA-matched unrelated donor (MUD) (b). The percentage of samples that fall into category 1 (□), 2 (hatched) and 3 (▪), as defined in Results, is shown. The number of individual samples is indicated at the top of each bar.
Comparison of patient characteristics
In Table 1 the characteristics are summarized of patients that received a BM graft from a sibling donor or a MUD. There are significant differences in recipient age (P = 0.012), 3 year relapse probability (P = 0.01) and 3 year relapse-free survival (P = 0.016). Three year overall patient survival was 81% in the sibling group compared with 60% in the MUD group (P = 0.08). To perform a detailed analysis of clinical parameters in relation to IgM repertoire diversity the patients were divided into two groups. The first group (n = 12) contained patients that had a normal (as defined in Results, second paragraph) repertoire diversity at all time intervals or had a deviant repertoire diversity at one time interval only. The second group (n = 29) contained patients that had a deviant repertoire diversity at more than one time interval or a very deviant repertoire at one or more time intervals. All characteristics presented in Table 1 were compared between the two groups and one of these clinical findings was found to differ. Thus, 3 year overall survival was 92% in the group with a more diverse IgM repertoire compared with 62% in the group with a less diverse immunoglobulin repertoire (P = 0.06) (Fig. 8). This difference was mainly contributed by the patients receiving BM from a MUD. Among those patients the 3 year survival was 86% in the group with a more diverse IgM repertoire and 38% in the group with a less diverse IgM repertoire (P = 0.06).
Fig. 8.
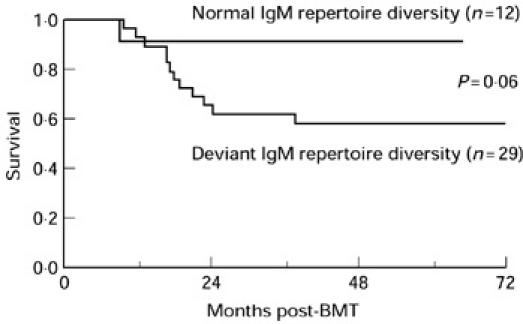
Survival analysis demonstrating the cumulative proportion of surviving patients having either normal or deviant IgM repertoire diversity. The first group (n = 12) contained patients that had a normal repertoire diversity during all time intervals or had a deviant repertoire diversity at one time interval only. The second group (n = 29) contained patients that had a deviant repertoire diversity at more than one time interval or a very deviant repertoire at one or more time intervals. Graphs were calculated using the Kaplan–Meier product limit method. Differences between the two groups were compared using the log rank test.
DISCUSSION
In the present work we demonstrate that the serum IgM repertoire in patients transplanted with allogeneic BM is oligoclonal compared with healthy controls. At 3 weeks to more than 1 year post-BMT over 60% of the patient samples had severely reduced diversity compared with samples from healthy controls. For serum IgG antibodies however, only 10–20% of the patients had an IgG repertoire that deviated from that of healthy controls more than 1 year post-BMT. The more diverse IgG repertoire was not a result of IVIG treatment since exclusion of IVIG-treated patients from the analysis gave similar results.
Since IgG-secreting clones are derived from IgM-positive B cells by immunoglobulin class-switching, without changing the specificity of the produced antibody, this difference in diversity between the IgM and the IgG repertoire must be the result of an active selective mechanism. It is possible that the IgG repertoire is diversified by extensive somatic mutations in peripheral lymphoid organs. We have however performed a detailed sequence analysis of more than 250 VH6-containing immunoglobulin rearrangements in two BMT patients and we found < 0.3 mutations per 100 sequenced base pairs, compared with the germ-line VH6 gene, later than 2 months post-BMT [1]. Suzuki et al. reported a general low incidence of somatic mutations up to 1 year post-BMT, equal to the level of mutations in the preimmune repertoire in healthy controls [14]. The low incidence of somatic mutations is supported by the high frequency of B cells with a naive phenotype (> 90% of peripheral B cells are CD19+, IgM+, IgD+) in BMT patients compared with healthy controls and the reduced level of B cells with a memory phenotype [14,15].
Recombination activating genes (RAG-1 and RAG-2) are transcribed in germinal centre B cells from mice [16,17], and it has been demonstrated that secondary VJ recombination of Vκ–Jκ takes place in germinal centre B cells in vivo [18,19]. Assuming that this mechanism is active also in humans, a conceivable explanation for the discrepancy in repertoire diversity between IgM and IgG might be secondary V(D)J recombination events in germinal centres leading to diversification of the IgG repertoire compared with the IgM repertoire.
A high turnover of cells from the IgM+ to the IgG+ B cell pool would also be a possible explanation for the discrepancy in serum IgM and IgG repertoire diversity post-BMT. Such a scenario would lead to rather few IgM-producing clones and thus a less diverse IgM repertoire. In this context it is important to realise that the serum IgM repertoire post-BMT remains oligoclonal although the amount of total serum IgM has reached normal levels. In patients, IgM levels are usually within the normal range at 2–6 months after transplantation [5]. In mice the number of cells in the IgM-secreting B cell compartment, and the level of serum IgM, is regulated independently of the size of the pre-B cell and mature B cell pools [20]. Thus, although fewer mature B cells are induced to progress to IgM-secreting plasma cells in BMT patients after transplantation, they are sufficient to maintain normal serum IgM levels.
The number of CD4+ T cells is lower than normal during 6–12 months post-BMT [21]. It is also demonstrated that the T cell repertoire is oligoclonal up to 9 months post-BMT and that the first appearing T cells are CD45RO+, CD25+ cells. These markers indicate that these cells have been previously activated and that they represent peripheral expansion of mature donor-derived T cells. At 9 months post-BMT a second wave of thymus-derived CD45RA+ naive resting T cells reappear in patients' circulation and the T cell repertoire diversifies [22]. Despite the reduced frequency of CD4+ T cells early after BMT they clearly provide sufficient help for class switching and diversification of the IgG repertoire in transplanted patients, since we demonstrate a normal diverse IgG repertoire in most patients post-BMT.
Our finding that the IgM repertoire post-BMT is oligoclonal is supported by clinical studies. Normal titres of specific anti-tetanus toxoid antibodies can be reached in vaccinated BMT patients as early as 4 months after transplantation, but the response is qualitatively different from that of healthy individuals. The number of tetanus toxoid-specific B cell clones involved in the response in patients is markedly reduced for a prolonged period of time, sometimes for as long as 10 years after BMT [23]. Without reimmunization with tetanus toxoid, all BMT patients lose specific antibodies at 2 years after transplantation regardless of the immune status of the BM donor [24]. In a study of 30 adult recipients of allogeneic BMT and 40 adult recipients of autologous BMT no correlation could be demonstrated between total immunoglobulin levels and levels of specific antibodies against tetanus or pneumococci antigens after transplantation [25].
Our results demonstrate that patients who received BM from a sibling donor have a less diverse immunoglobulin repertoire than those receiving BM from a MUD. The difference is significant for IgM during the two last time points tested (Fig. 6), and the difference is also present for the IgG repertoire (Fig. 7). A factor that might be of importance is the higher age of the recipients of a sibling graft (Table 1). It has long been known that immune reconstitution post-BMT is negatively affected by a higher recipient age [26–28]. It was suggested that older patients have a more impaired thymus function. Another possible explanation could be that the higher incidence of minor antigen mismatches in patients with an unrelated donor stimulates the diversification of the immunoglobulin repertoire. Half of the patients in the MUD group has one or two HLA-DP or -DQ mismatches compared with only one patient in the sibling group. The frequency of infections is higher in patients receiving a graft from a MUD compared with a sibling donor [29,30]. Also in our material there was a tendency to more infections in the MUD group (Table 1). This implies that an oligoclonal repertoire, rather than contributing to sensitivity to infections, is a sign of lack of infections and that a polyclonal repertoire is secondary to immune activation by pathogens or antigen mismatches. The higher probability of relapse-free survival in the sibling group compared with the MUD group (Table 1) is probably not related to immunoglobulin repertoire diversity, but is more likely due to the lower relapse probability (Table 1), less immune suppressive treatment, lower incidence of GVHD and lower probability of infections.
A related finding is the better outcome in 3 year patient survival for patients with a more diverse IgM repertoire compared with patients with a less diverse IgM repertoire. This difference was mainly contributed by the patients receiving BM from a MUD. The result was not due to the underlying disease since the frequency of acute leukaemias was the same in the two groups. The small number of patients in the two groups does not allow us to correlate the cause of death to the degree of repertoire diversity. Among MUD patients with a less diverse IgM repertoire four patients died of relapse (one with a complicating infection), one of interstitial pneumonia associated with Aspergillus infection, and one of lung and kidney failure in association with adenovirus pneumonia.
Acknowledgments
We are grateful to H. Svensson for statistical analysis. This work was supported by grants from the Swedish Medical Research Council (grant no K98-06X-11599), the Tobias Foundation, the Åke Wiberg Foundation, Nilsson's Cancer Foundation and the French Embassy in Stockholm. I.N.B. is supported by a fellowship for graduate students from the Swedish Cancer Foundation (grant no. 2995-B97-02UBD). J.S. is the recipient of a Praxis visiting fellowship at the Gulbenkian Institute of Science, Oeiras, Portugal.
REFERENCES
- 1.Näsman I, Lundkvist I. Evidence for oligoclonal diversification of the VH6-containing immunoglobulin repertoire during reconstitution after bone marrow transplantation. Blood. 1996;87:2795–80. [PubMed] [Google Scholar]
- 2.Näsman-Björk I, Lundkvist I. Oligoclonal dominance of immunoglobulin VH3 rearrangements following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;21:1223–3. doi: 10.1038/sj.bmt.1701261. [DOI] [PubMed] [Google Scholar]
- 3.Lum LG. The kinetics of immune reconstitution after human marrow transplantation. Blood. 1987;69:369–8. [PubMed] [Google Scholar]
- 4.Kagan JM, Champlin RE, Saxon A. B-cell dysfunction following human bone marrow transplantation: functional-phenotypic dissociation in the early posttransplant period. Blood. 1989;74:777–8. [PubMed] [Google Scholar]
- 5.Storek J, Saxon A. Reconstitution of B cell immunity following bone marrow transplantation. Bone Marrow Transplant. 1992;9:395–40. [PubMed] [Google Scholar]
- 6.Lum LG. Immune recovery after bone marrow transplantation. Hematol Oncol Clin North Am. 1990;4:659–7. [PubMed] [Google Scholar]
- 7.Nobrega A, Haury M, Grandien A, Malanchère E, Sundblad A, Coutinho A. Global analysis of antibody repertoires. II. Evidence for specificity, self-selection and the immunological ‘homunculus’ of antibodies in normal serum. Eur J Immunol. 1993;23:2851–9. doi: 10.1002/eji.1830231119. [DOI] [PubMed] [Google Scholar]
- 8.Haury M, Grandien A, Sundblad A, Coutinho A, Nobrega A. Global analysis of antibody repertoires. I. An immunoblot method for the quantitative screening of large numbers of reactivities. Scand J Immunol. 1994;39:79–8. doi: 10.1111/j.1365-3083.1994.tb03343.x. [DOI] [PubMed] [Google Scholar]
- 9.Brissac C, Nobrega A, Carneiro J, Stewart J. Functional diversity of natural IgM. Int Immunol. 1999;11:1501–7. doi: 10.1093/intimm/11.9.1501. [DOI] [PubMed] [Google Scholar]
- 10.Ringdén O, Pihlstedt P, Markling L, et al. Prevention of graft-versus-host disease with T cell depletion or cyclosporin and methotrexate. A randomized trial in adult leukemic marrow recipients. Bone Marrow Transplant. 1991;7:221–6. [PubMed] [Google Scholar]
- 11.Klaesson S, Ringdén O, Ljungman P, Aschan JH, Hägglund H, Winiarski J. Increased mortality in veno-occlusive disease of the liver in bone marrow transplanted recipients treated with intravenous immune globulin. Transplantation. 1995;60:1225–3. [PubMed] [Google Scholar]
- 12.Hurez V, Kazatchkine MD, Vassilev T, et al. Pooled normal human polyspecific IgM contains neutralizing anti-idiotypes to IgG autoantibodies of autoimmune patients and protects from experimental autoimmune disease. Blood. 1997;90:4004–1. [PubMed] [Google Scholar]
- 13.Berek C, Milstein C. The dynamic nature of the antibody repertoire. Immunol Rev. 1988;105:5–2. doi: 10.1111/j.1600-065x.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki I, Milner EC, Glas AM, et al. Immunoglobulin heavy chain variable region gene usage in bone marrow transplant recipients: lack of somatic mutation indicates a maturational arrest. Blood. 1996;87:1873–8. [PubMed] [Google Scholar]
- 15.Storek J, Witherspoon RP, Storb R. Reconstitution of membrane IgD−(mIgD−) B cells after marrow transplantation lags behind the reconstitution of mIgD+ B cells. Blood. 1997;89:350–1. [PubMed] [Google Scholar]
- 16.Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag 1 and Rag 2 expression in germinal center B cells. Science. 1996;274:2094–7. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 17.Hikida M, Mori M, Takai T, Tomochika K, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–4. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 18.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel M, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–5. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 19.Papavasiliou F, Casellas R, Suh H, et al. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–30. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 20.Agenès F, Rosado MM, Freitas AA. Independent homeostatic regulation of B cell compartments. Eur J Immunol. 1997;27:1801–7. doi: 10.1002/eji.1830270731. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson K. Reconstruction of the haemopoietic and immune systems after marrow transplantation. Bone Marrow Transplant. 1990;5:209–2. [PubMed] [Google Scholar]
- 22.Roux E, Helg C, Dumont-Girard F, Chapuis B, Jeannet M, Roosnek E. Analysis of T-cell repopulation after allogeneic bone marrow transplantation: significant differences between recipients of T-cell depleted and unmanipulated grafts. Blood. 1996;87:3984–9. [PubMed] [Google Scholar]
- 23.Gerritsen EJ, Van Tol MJ, Van 't Veer MB, et al. Clonal dysregulation of the antibody response to tetanus-toxoid after bone marrow transplantation. Blood. 1994;84:4374–8. [PubMed] [Google Scholar]
- 24.Ljungman P, Wiklund-Hammarsten M, Duraj V, et al. Response to tetanus toxoid immunization after allogeneic bone marrow transplantation. J Infect Dis. 1990;162:496–50. doi: 10.1093/infdis/162.2.496. [DOI] [PubMed] [Google Scholar]
- 25.Hammarström V, Pauksen K, Svensson H, et al. Serum immunoglobulin levels in relation to levels of specific antibodies in allogeneic and autologous bone marrow transplant recipients. Transplantation. 1998 doi: 10.1097/00007890-200004270-00011. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 26.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–7. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 27.Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–8. doi: 10.1111/j.1600-065x.1997.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 28.Small TN, Avigan D, Dupont B, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biol Blood Marrow Transplant. 1997;3:65–7. [PubMed] [Google Scholar]
- 29.Ochs L, Xiao OS, Miller J, et al. Late infections after allogeneic bone marrow transplantation: comparison of incidence in related and unrelated donor transplant recipients. Blood. 1995;86:3979–8. [PubMed] [Google Scholar]
- 30.Ringdén O. Bone marrow transplantation using unrelated donors for haematological malignancies. Med Oncol. 1997;14:11–2. doi: 10.1007/BF02990940. [DOI] [PubMed] [Google Scholar]


