Abstract
Toxic epidermal necrolysis (TEN) is a rare life-threatening adverse drug reaction characterized by a massive destruction of the epidermis. Immunohistological studies of skin biopsies of TEN showed infiltrates of predominantly CD8+ T lymphocytes even though other authors reported a prominent involvement of cells of the monocyte-macrophage lineage. The aim of this study was to characterize phenotypically and functionally the cells present in the cutaneous blister fluid of four patients with TEN. We first determined that lymphocytes were predominant in blister fluid obtained early, while monocytes/macrophages later became the most important population. We then showed that this lymphocyte population, mainly CD3+CD8+, corresponded to a peculiar cell subset as they expressed cutaneous leucocyte antigen, killer inhibitory receptors KIR/KAR and failed to express CD28 molecule. Functionally, we determined that blister T lymphocytes had a cytotoxic T lymphocyte (CTL)- and NK-like cytotoxicity. The role of this cytotoxic lymphocyte population present at the site of lesions during TEN remains to be understood.
Keywords: cutaneous drug adverse reaction, toxic epidermal necrolysis, killer inhibitory receptors cytotoxic T lymphocytes
INTRODUCTION
Toxic epidermal necrolysis (TEN) is a rare, potentially fatal disease, most often related to an adverse drug reaction. Clinical findings consist of painful erosions involving the mucous membranes and a generalized cutaneous eruption that progresses in a few hours to a few days to flaccid blisters with detachment of large sheets of epidermis on pressure areas [1]. Looking like a second degree burn, the cutaneous pattern is due to widespread death of epidermal cells through the full thickness of the epidermis. Epithelial layers of the gut and of the bronchi may also be involved and this contributes to the severity of the process. It has been recently demonstrated that the destruction of the epidermis results from disseminated apoptosis of keratinocytes [2] and that expression of Fas-ligand on keratinocytes has a key role in inducing apoptosis [3].
The initial mechanisms leading to apoptosis of epidermal cells of patients with TEN remain unknown. Several pieces of evidence suggest that an immunological reaction to drugs may initiate the process. First, drug-specific T lymphocytes have been isolated from the blood or from the skin lesions of patients with drug eruptions of other clinical types [4–7]. Second, while TEN usually occurs 7–21 days after the initiation of treatment, the rare recurrent cases usually begin within 48 h of rechallenge, suggesting an immunological memory [1]. Third, several immunohistological studies of early skin biopsies of TEN showed infiltrates of predominantly CD4+ T lymphocytes in the dermis and of CD8+ T lymphocytes along the dermoepidermal junction and into the epidermis [8,9]. Even though other authors reported a prominent involvement of cells of the monocyte-macrophage lineage [10], the hypothesis most commonly proposed is that the epidermal cells are the targets of cytotoxic CD8+ lymphocytes. However, this hypothesis remains to be proved.
While in the majority of cutaneous adverse drug reactions it is difficult to isolate sufficient numbers of T lymphocytes from the skin lesions, the blister fluid from patients with TEN contains many mononuclear cells, providing an opportunity for ex vivo analysis of cells that can be suspected of being the immunological effectors of the reaction. A single study of these blister fluid cells from patients with TEN has been made by cytofluorometric analysis, showing a large predominance of activated CD8+ T lymphocytes [11].
In the present study we report further phenotypic and functional characteristics of these CD8+ cells present in the cutaneous lesions of patients with TEN, showing that these lymphocytes are cytotoxic lymphocytes which express receptors that are usually present on NK cells and on a minor population of circulating CD8+ lymphocytes.
PATIENTS AND METHODS
Patients
Four patients with TEN were studied. They were two males and two females, aged 23–57 years. All four had negative serology for HIV or mycoplasma infection. In all cases, histological study of cutaneous biopsy specimens showed a full-thickness epidermal necrosis. Direct immunofluorescence study was negative in all cases. The detachment of epidermis involved 40% (patient 2), 49% (patient 4), 52% (patient 3) and 80% (patient 1) of the body surface area. Sulfasalazine was the inducing drug in patient 2 and carbocisteine was suspected in patient 4. For the two other patients, several drugs were taken before the onset of TEN (patient 3, tiaprofenic acid, quinapril and hydrochlorothiazide; patient 1, ibuprofen, paracetamol and phloroglucinol).
Cells
Fluid obtained from several cutaneous blisters by direct aspiration was centrifuged and the cells were harvested and frozen in liquid nitrogen. Peripheral blood lymphocytes (PBL) were isolated from blood (obtained at the same time as blister fluid) by Ficoll–Hypaque (Nycomed Pharma AS, Oslo, Norway) density gradient centrifugation. Cutaneous blister fluid was collected once for patients 1, 2 and 3 and on two different days for patient 4 (Table 1).
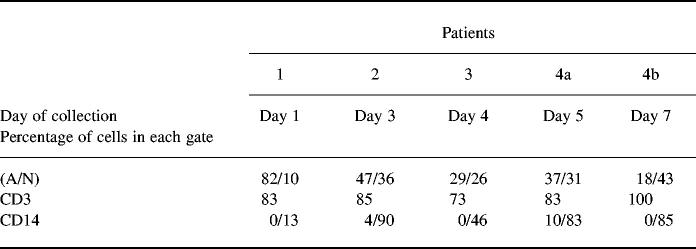
Table 1.
Percentage of blister fluid cells in gates A and N corresponding, respectively, to lymphocyte and monocyte-macrophage gate
Antibodies and reagents
Monoclonal antibodies used were: S4-1 (IgG2a anti-CD3, Tricolor; Caltag Labs, Burlingame, CA), 3B5 (IgG2a anti-CD8, FITC; Caltag), 2ST8.5H7 (IgG1 anti-CD8, PE; Coulter-Immunotech, Marseille, France), S3.5 (IgG1 anti-CD4, RD1; Caltag), NKH-1 (IgG1 anti-CD56, PE; Coulter-Immunotech), TÜK4 (IgG2a anti-CD14, Tri; Caltag), CD28.2 (IgG1 anti-CD28, FITC; Coulter-Immunotech), BB27 (IgG1 anti-CD101, PE; Coulter-Immunotech), IMMU 357 (IgG1 anti-HLA-DR, CyPE; Coulter-Immunnotech), HP-3B1 (IgG2a anti-CD94, PE; Coulter-Immunotech), EB6 (IgG1 anti-CD158a, PE; Coulter-Immunotech), GL183 (IgG1 anti-CD158b, PE; Coulter-Immunotech), DX9 (IgG1 anti-NKB1, PE; Becton Dickinson), IgM anti-cutaneous leucocyte antigen (CLA) (HECA 452) were a generous gift from Dr L. Picker (University of Texas, South-western Medical Center, Dallas, TX). All isotypic controls were purchased from Coulter-Immunotech. The culture medium was RPMI 1640 supplemented with 10% fetal calf serum (FCS), l-glutamine 2 mm, penicillin 100 μg/ml, streptomycin 100 μg/ml and pyruvate 1 mm (all purchased from Life Technologies SARL, Cergy-Pontoise, France).
Flow cytometric analysis
One- or two-colour direct or indirect immunofluorescence staining was performed as previously described [11]. Briefly, aliquots of 2 × 105 cells were incubated for 15 min at 4°C with the corresponding antibodies. For indirect labelling, two steps were required. Control aliquots were stained with unrelated antibodies of the same isotype. After staining, cells were washed in PBS and fixed in PBS 1% formaldehyde. Analysis was performed on a single argon laser flow cytometer XL analyser Epics (Coulter, Hialeah, FL).
Cytotoxicity assay
This was performed as previously described [12]. Briefly, exponentially growing P815 cells (murine mastocytoma cell line) or K562 cells (NK-sensitive human erythroleukaemic cell line) were harvested and labelled for 1.5 h at 37°C with 2.5 μCi/ml of sodium 51chromate in culture medium. Labelled target cells were washed and plated at 1000 cells/well using 96-well V-bottomed microtitre plates (Costar, Cambridge, MA).
For the anti-CD3 induced redirected cytotoxicity against P815 cells, blister fluid cells were resuspended and incubated for 15 min at room temperature with anti-CD3 MoAb (OKT3 ascites fluid diluted at 1:500). Assays at various effector to target cell (E:T) ratios were carried out in triplicate. The final culture volume was 200 μl/well. After 4 h of culture, plates were spun and 100 μl of supernatant were removed from each well and counted in a gamma-counter for determination of 51Cr release. The percentage of specific lysis was calculated with the following formula: (experimental release (ct/min) − spontaneous release (ct/min))/(maximum release (ct/min) − spontaneous release (ct/min)) × 100. The s.d. was always < 10% for each triplicate. A cytotoxic CD4+ clone (TC7) was used at the same time as a positive control in the anti-CD3-induced redirected cytotoxic assays [13].
RESULTS
To characterize the cells present in the blister fluid, flow cytometric analysis was performed with cells obtained from four patients with TEN. As a control, the peripheral blood lymphocytes, isolated at the same time as blister fluid from three patients out of four, were simultaneously stained and analysed.
In blister fluid, the ratio lymphocytes/monocytes decreased with time
On the basis of size and granularity of cells, we could clearly define two gates (A and N on Fig. 1). In gate A the cells presented the size and granularity characteristics of lymphocytes and were as expected stained with anti-CD3 MoAb (mean of positive cells in the four patients = 81 ± 5.4%). In gate N, the cells were of greater size and granularity, characteristic of monocytes and were CD14+ (Table 1). The ratio lymphocytes/monocytes decreased with time: for patient 4, who was sampled twice, there were 37% lymphocytes and 31% monocytes on day 5 after the eruption commenced, whereas on day 7 there were only 18% lymphocytes and 43% monocytes. The three other patients were sampled only once. Patient 1 was sampled 1 day after the eruption commenced, patient 2 3 days after the eruption commenced, and patient 3 4 days after the eruption commenced. The lymphocyte/monocyte ratio decreased with time, depending on the day of collection of cells in these three patients (Table 1).
Fig. 1.
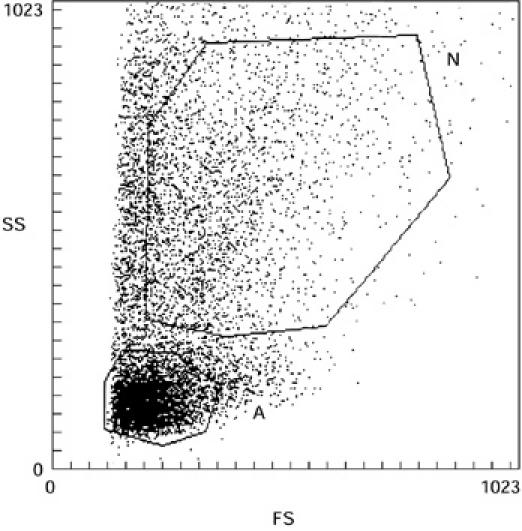
Flow cytometric analysis of toxic epidermal necrolysis (TEN) blister fluid cells on the basis of size and granulosity. A, morphologic characteristics of lymphocytes; N, morphologic characteristics of monocytes-macrophages.
T lymphocytes of blister fluid were mainly CD8+
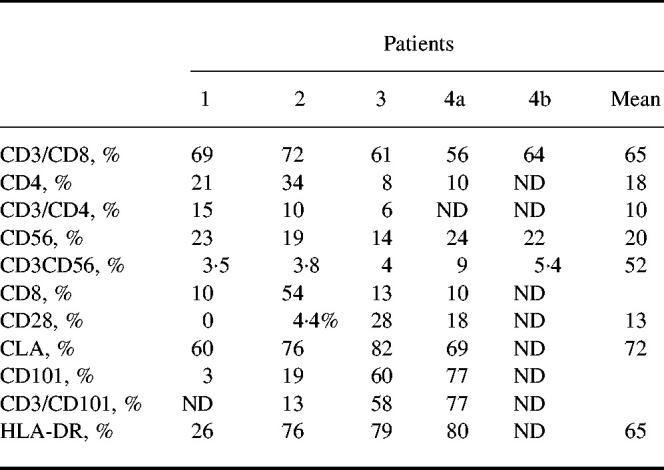
Further phenotypic analysis of T lymphocytes (gate A) of the four patients showed a mean of 81% (± 5.4%) CD3+ lymphocytes, a mean of 64% (± 6.3%) were CD3+CD8+ cells. Only a mean of 10% (± 4.5%) were CD3+CD4+. Interestingly, we found that for three patients (patients 1, 3 and 4) most of the CD8+ lymphocytes expressed the heterodimeric form of the CD8 molecule, whereas in one patient (patient 2) most of the CD8+ cells expressed the homodimeric CD8. Most T lymphocytes were CD28−, as only a mean of 13% (± 12.7%) of cells in gate A expressed CD28. A low percentage of CD3− cells were NK lymphocytes, as 15% (± 3.7%) were stained by anti-CD56 MoAb (Table 2). Blister fluid lymphocytes were significantly more often CD3−CD8+ than the blood lymphocytes of the same patients (65% versus 19%; P = 0.0035, Student's t-test).
Table 2.
Flow cytometric analysis of gated blister fluid lymphocytes
CLA, Cutaneous leucocyte antigen; ND, not done.
Blister fluid T cells heterogeneously expressed activation molecules
To determine whether T lymphocytes in blister fluid were resting or activated cells, further analysis was performed using antibodies against activation cell surface structures. HLA-DR, a classical activation molecule in T lymphocytes, was highly expressed (65 ± 26.2%) on blister fluid cells from three patients of four. Moreover, CD101, a cell surface molecule known to be induced during T cell activation [12], was also highly expressed in the case of two patients (58% and 77%). However, it was very low in one patient (3%), who also had a low percentage (26%) HLA-DR+ cells (Table 2).
These results seemed to correlate with those obtained with PBL of these patients, although we observed a great heterogeneity of HLA-DR and CD101 expression among the blood samples (data not shown). However, this heterogeneity has already been described for PBL obtained from healthy donors [12].
CD3+ blister fluid cells expressed cell surface structures restricted to cutaneous lymphocytes
To delineate further the CD3+CD8+CD28− population of blister fluid, additional phenotypic analysis was performed. We first focused on CLA. It was found that a great majority of the cells from blister fluid were CLA+ (72 ± 5.9%) (Table 2).
Blister fluid T lymphocytes were cytotoxic
A CD3-induced redirected cytotoxic assay was performed with blister fluid cells from patient 1 to determine whether or not these cells exhibited cytotoxic T lymphocyte (CTL) activity. As shown in Fig. 2, it was found that blister fluid cells labelled with anti-CD3 MoAbs were able to lyse the murine mastocytoma target cells (P815). This effect was specific, as the percentage of specific 51Cr release decreased with the E:T ratio (it ranged from 29% for 100:1 to 9% specific 51Cr release for 1:1 ratio, as shown in Fig. 2). It should be mentioned that in the absence of anti-CD3 MoAbs the cells were unable to lyse P815 (data not shown).
Fig. 2.
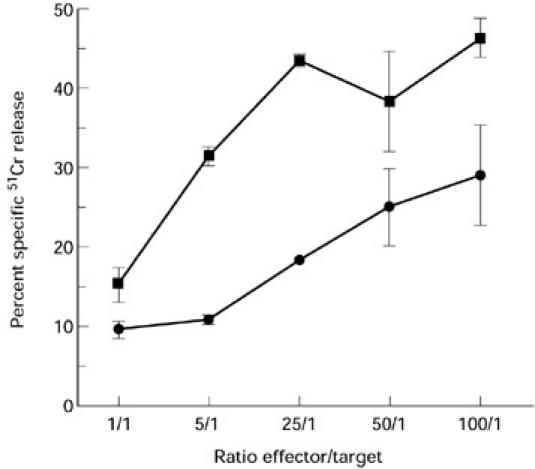
Cytotoxicity of blister fluid cells (▪) and of a positive control T cell clone (TC7) (•) evaluated in a 4-h 51Cr release assay. Assays were done in triplicate. The percentage specific lysis was calculated as described in Patients and Methods.
Blister T lymphocytes had a NK-like cytotoxicity
We performed a cytotoxic assay against the NK-sensitive target cell line K562 with blister fluid from two different patients to determine whether these cells exhibited an NK-like cytotoxic activity. Results presented in Fig. 3 indicate that blister fluid cells were able to lyse K562. The percentage of specific 51Cr release decreased with the E:T ratio for the two patients.
Fig. 3.
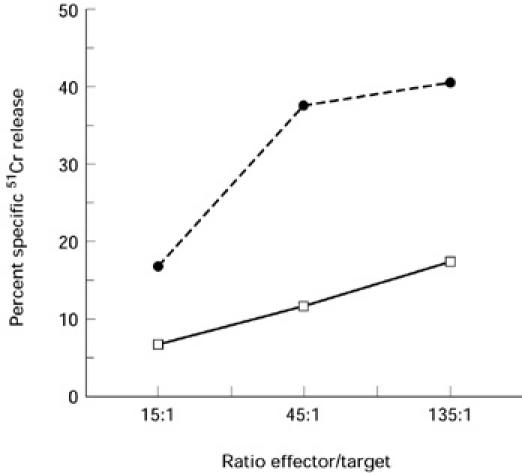
Cytotoxicity of two different patient blister fluid cells against the NK-sensitive cell line K562 evaluated in a 4-h 51Cr release assay. Assays were done in triplicate. The percentage specific lysis was calculated as described in Patients and Methods.
The great majority of CD3+ lymphocytes were KIR/KAR+
As we had shown that blister fluid cells had a NK-like cytotoxic activity despite a low percentage of NK lymphocytes among the blister fluid cells, we investigated whether CD3+ lymphocytes corresponded to a peculiar subset by studying KIR/KAR molecule expression. KIR antibodies were first used in simple staining and showed that there were KIR/KAR+ cells among lymphocytes. Fifteen per cent (± 8.5%) were CD158a+, 28% (± 11.7%) were CD158b+ and 58% (± 10.9%) were CD94+, whereas only 1% (± 1.5%) were positively stained with NKB1. Next, a double staining was performed using a CD3 antibody and a mixture of four anti-KIR/KAR antibodies: anti-CD158a, CD158b, CD94 and anti-NKB1 (all of them directly labelled with the same fluorochrome). The great majority of CD3+ cells were double-stained by the mixture of antibodies (6.5 ± 12% double-positive cells) (see Table 3 and a representative experiment obtained with patient 1 in Fig. 4). Thus, these results indicate that most CD3+ lymphocytes in blister fluid expressed at least one of the KIR/KAR molecules tested. The percentage of CD3+ KIR/KAR+ cells was significantly lower in the PBL of these patients (8 ± 6%, P < 0.001, Student's t-test), as usually reported in PBL of healthy donors, thus providing evidence for a specific enrichment in blister fluid. This was especially marked for CD3−CD94+ cells (58% of blister fluid cells versus 11% of PBL; P = 0.0012).
Table 3.
Flow cytometric analysis after labelling by anti-KIR/KAR MoAbs of gate A blister fluid cells
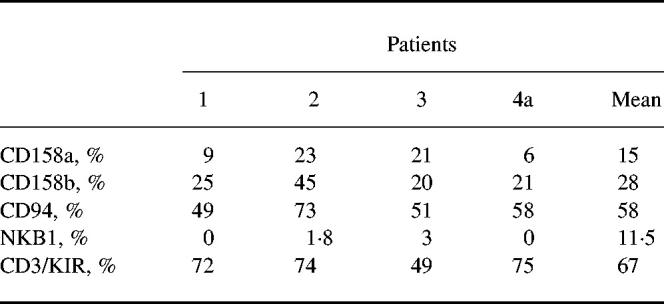
Fig. 4.
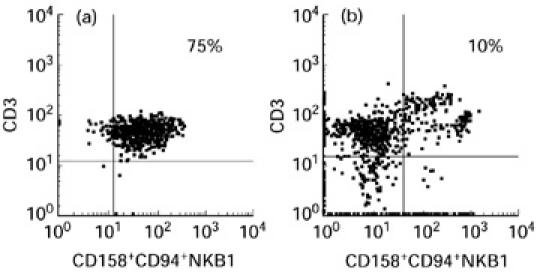
Flow cytometric analysis results after double staining of blister fluid cells (a) and peripheral blood lymphocytes (PBL) (b) of patient 1, using a CD3 antibody directly coated and a mixture of the four anti-KIR/KAR antibodies (all of them directly labelled with the same fluorochrome).
DISCUSSION
This study has analysed the different cell populations present in the blister fluid of patients with TEN. Prior reports that used immunolabelling to characterize the mononuclear cells present at the site of lesions in TEN obtained discordant results. While most authors reported that the prenecrotic epidermis contained nearly exclusively CD8+ T lymphocytes [8,9,11], others suggested that cells of monocyte-macrophage lineage were predominant and might be responsible for apoptosis of epidermal cells through the local production of tumour necrosis factor-alpha (TNF-α) [10]. These discrepancies probably depended on how soon the skin samples had been obtained.
Because of the small number of patients investigated our study is descriptive rather than definitive, but it provides several original findings. Our results show that both lymphocytes and monocyte-macrophages were present in the blister fluid that accumulated at the site of separation of the epidermis from the dermis. Early samples contained mainly CD8+ lymphocytes, while the relative amount of monocyte-macrophages increased with time. These results suggest that lymphocytes were probably more important than cells of monocyte-macrophage lineage in initiating the death of keratinocytes at the early stage of TEN.
Several studies argued for a role of cytotoxic T lymphocytes during cutaneous drug reactions. Several lymphocyte clones were obtained after restimulation by the culprit drug from the blood of patients after cutaneous drug reactions. These clones derived from the blood and expanded ex vivo with IL-2 and feeder cells had a specific cytotoxic activity against autologous presenting cells and autologous keratinocytes in the presence of the culprit drug [4–7]. These results were compatible with the hypothesis of lymphocyte-mediated cytotoxic reaction against keratinocytes during cutaneous drug reactions. We found that freshly isolated blister T lymphocytes exhibit both CTL activities and a NK-like cytotoxic activity, as these cells were able to kill the mastocytoma cell line P815 in the presence of anti-CD3 MoAb and the cell line K562. We already reported with T cell clones that the same lymphocytes were able to exhibit both CTL- and NK-like activity [15].
Blister fluid lymphocytes from patients with TEN were T cells, mostly CD8+CD28−, and the majority expressed markers of NK cells. NK cells are a subset of lymphocytes that constitutively exhibit cytolytic functions against susceptible tumour or virally infected cells. Recently several classes of receptors were described on human NK cells. These receptors are MHC class I-binding proteins that activate (KAR, killer activating receptor) or inhibit (KIR, killer inhibitory receptor) the cytolytic functions of human NK cells [16]. The recognition of MHC class I molecules depends on the extracellular domain of the KIR/KAR receptor, while activation or inhibition are related to the intracytoplasmic domains of these receptors. The cytometric analysis does not allow to distinguish between KIR (inhibitor) and KAR (activator), which differ only by the presence of an ITIM in the cytoplasmic domain [16]. Using single MoAbs we found that CD94, recognizing HLA-E [16], and CD158a and CD158b [17], recognizing HLA-C alleles [15], were highly expressed in our four patients, while NKB1, recognizing HLA-Bw4, was not [18]. Interestingly, using simultaneously several MoAbs recognizing the extracellular domains of four different KIRs/KARs, it was observed that 49–75% of the T lymphocytes present in the blister fluid expressed at least one KIR/KAR. This high percentage of NK receptors on the T lymphocytes present in situ contrasted with the low percentage expression of these receptors in the circulating lymphocytes of the same patients, that was in the range previously observed for the blood lymphocytes of normal humans' (1–8%) PBL KIR/KAR+ [19]. We found that TEN blister fluid T cells shared with the KIR/KAR+ T cell subset described in normal blood a CD8+ and CD28− phenotype [19].
These receptors are inducible on the T lymphocyte surface by certain cytokines. IL-15 induces functional expression of CD94 on T lymphocytes responding to superantigen or to alloantigen [17]. The expression of KIR on T lymphocytes contained in the blister fluid during TEN could be a way to down-regulate a massive cytotoxic reaction directed against keratinocytes, as previous reports indicated that KIRs on T lymphocytes provide a negative signal to lymphocyte function [19]. Alternatively, we could also hypothesize that the responsible drug, by linking the HLA class I molecules, blocked the recognition of HLA molecule by KIR and thus up-regulated the cytotoxic activity of T lymphocytes against an autoantigen.
In addition, TEN blister fluid T cells also expressed the CLA antigen, a homing antigen, described in cutaneous T lymphocytes and involved in endothelial adhesion [20]. These findings suggest the involvement of a population of skin-homing T cells in TEN. Finally, these cells seem to share some features with the intraepithelial lymphocytes of the intestinal mucosa. These intraepithelial lymphocytes are mainly CD8+, lack expression of CD28 and exhibit cytolytic activity [21].
The cells contained in the blister during TEN thus provide a very interesting way to study further the immune reaction in cutaneous drug reactions, but also more generally to investigate the epidermal lymphocyte population.
REFERENCES
- 1.Roujeau JC, Stern R. Severe adverse cutaneous reaction to drugs. N Engl J Med. 1994;331:1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 2.Paul C, Wolkenstein P, Adle H, Wechsler J, Garchon HJ, Revuz J, Roujeau JC. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol. 1996;134:710–4. doi: 10.1111/j.1365-2133.1996.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 3.Viard I, Wehrli P, Bullani R, et al. Inhibition of toxic epidermal necrolysis by blockage of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–3. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 4.Hertl M, Geisel J, Boecker C, Merk HF. Selective generation of CD8+ T-cell clones from the peripheral blood of patients with cutaneous reactions to beta-lactam antibiotics. Br J Dermatol. 1993;128:619–26. doi: 10.1111/j.1365-2133.1993.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 5.Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Huntziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phentytoin, and carbamazepine. J Immunol. 1995;155:462–72. [PubMed] [Google Scholar]
- 6.Schnyder B, Mauri-Hellweg D, Zanni M, Bettens F, Pichler WJ. Direct MHC-dependent presentation of the drug sulfamethoxazole to human T cell clones. J Clin Invest. 1997;100:136–41. doi: 10.1172/JCI119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnyder B, Frutig K, Mauri-Hellweg D, Limat A, Yawalkar N, Pichler WJ. T-cell-mediated cytotoxicity against keratinocytes in sulfamethoxazol-induced skin reaction. Clin Exp Allergy. 1998;28:1412–7. doi: 10.1046/j.1365-2222.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 8.Miyauchi H, Hosokawa H, Akaeda T, Iba H, Asada Y. T-cell subsets in drug-induced toxic epidermal necrolysis. Arch Dermatol. 1199;1:851–5. [PubMed] [Google Scholar]
- 9.Villada G, Roujeau JC, Clérici T, Bourgault I, Revuz J. Immunopathology of toxic epidermal necrolysis. Arch Dermatol. 1992;128:50–3. doi: 10.1001/archderm.128.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Paquet P, Nikkels A, Arrese JE, Vanderkelen A, Piérard GE. Macrophage and tumor necrosis factor α in toxic epidermal necrolysis. Arch Dermatol. 1994;130:605–8. [PubMed] [Google Scholar]
- 11.Correia O, Delgado L, Ramos JP, Resende C, Fleming, Torrinha JA. Cutaneous T-cell recruitment in toxic epidermal necrolysis. Arch Dermatol. 1993;129:466–8. [PubMed] [Google Scholar]
- 12.Schiavon V, Roth P, Bolton WE, Farcet JP, Bensussan A, Boumsell L. Lymphocyte subsets in normal individuals: analysis by four color immunofluorescence and flow cytometry on whole blood. Tissue Antigens. 1996;48:312–8. doi: 10.1111/j.1399-0039.1996.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 13.Bensussan A, Gluckman E, El Marsafy S, et al. BY55 monoclonal antibody delineates within human cord blood and bone marrow lymphocytes distinct cell subsets mediating cytotoxic activity. Proc Natl Acad Sci USA. 1994;91:9136–40. doi: 10.1073/pnas.91.19.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagot M, Echakir H, Mami-Chouaib F, et al. Isolation of tumor specific cytotoxic CD4+ and CD4+CD8+ dim+ T cell clones infiltrating a cutaneous T cell lymphoma. Blood. 1998;91:433–41. [PubMed] [Google Scholar]
- 15.David V, Bourge JF, Guglielmi P, Mathieu-Mahul D, Degos L, Bensussan A. Human T cell clones used a CD3-associated surface antigen recognition structure to exhibit both NK-like and allogeneic cytotoxic reactivity. J Immunol. 1987;138:2831–6. [PubMed] [Google Scholar]
- 16.Moretta A, Sivori S, Vitale M, et al. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182:875–84. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mingari MC, Ponte M, Bertone S, et al. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci USA. 1998;95:1172–7. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin V, Gumpertz JE, Parham P, Phillips JH, Lanier LL. NKB1: an NK cell receptor involved in the recognition of polymorphic HLA-B molecules. J Exp Med. 1994;180:537–43. doi: 10.1084/jem.180.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingari MC, Moretta A, Moretta L. Regulation of KIR expression in human T cells: a safety mechanism that may impair protective T-cell responses. Immunol Today. 1998;19:153–7. doi: 10.1016/s0167-5699(97)01236-x. [DOI] [PubMed] [Google Scholar]
- 20.De Boer OJ, Hors E, Pals ST, Bos JD, Das PK. Functional evidence that the HECA-452 antigen is involved in the adhesion of human neutrophils and lymphocytes to tumor necosis factor-alpha-stimulated endothelial cells. Immunology. 1994;81:359–65. [PMC free article] [PubMed] [Google Scholar]
- 21.Mowat AMcI, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–66. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]