Abstract
Although it has been shown that γδ T lymphocytes are able to react with different cell-associated or soluble antigens, the immune repertoire of these cells appears to be skewed to the recognition of mycobacterial antigens. We have studied the number and reactivity of γδ T cells towards several mycobacterial antigens in patients with tuberculosis and leprosy, as well as their healthy contacts and control individuals. We found an increased number of Vδ2+ cells in healthy contacts (PPD+ and lepromin+) and tuberculoid leprosy patients. The γδ T cells from lepromatous leprosy showed a decreased response to all antigens tested, but some of these patients exhibited a significant response to the 30-kD glycoprotein of Mycobacterium tuberculosis. Interestingly, the reactivity of γδ T cells against mycobacterial antigens was significantly increased by costimulatory signals generated through CD7, LFA-1, CD50 and CD69 in all groups. However, signalling through CD69 did not enhance the responsiveness of γδ lymphocytes from lepromatous patients. On the other hand, the in vitro blockade of IL-10 with a specific antibody enhanced the cell proliferation of γδ lymphocytes from lepromatous leprosy patients, whereas exogenous IL-10 had an opposite effect in most individuals studied. These results suggest the potential role of different cell membrane receptors in the regulation of γδ T cell proliferation induced by mycobacteria, as well as the possible involvement of IL-10 in this phenomenon.
Keywords: γδ T cells, T lymphocytes, Mycobacteria, tuberculosis, leprosy
Introduction
Mycobacterial infections, mainly tuberculosis (TB), are widely disseminated diseases that cause about 3 millions of deaths per year [1]. The increase in TB prevalence is mainly associated with the emergence of HIV infection and multidrug-resistant strains of Mycobacterium tuberculosis[2,3]. At present, it is very important to elucidate the precise mechanisms of defence against mycobacteria as well as to identify their antigenic determinants that are able to confer protection, in order to develop more efficient vaccines [4].
The physiological role of TCR1+, γδ T cells has not been fully elucidated [5]. Although a preferential reactivity of these cells towards mycobacterial antigens has been previously reported, their precise role in mycobacterial immunity remains to be determined [6,7]. However, several findings have suggested that γδ T cells have a protective role against mycobacteria, mainly as an early mechanism of immunity [8,9]. Experimental M. tuberculosis infection in TCR-δ-/-mutant mice has further supported this point [10,11]. On the other hand, it has been recently suggested that these cells could exert an anti-inflammatory rather than a protective effect in M. tuberculosis infection [12].
Early reports on the reactivity of γδ T cells suggested that heat-shock proteins (hsp) were the main stimulatory fraction of mycobacteria [13,14]. However, recent reports have demonstrated that stimulation of γδ T cells by M. tuberculosis appears to occur mainly through the recognition of the phosphorylated nucleotides TUBag1–4 [15,16]. Since similar stimulatory molecules have been found in Plasmodium falciparum, it is feasible that γδ T cells are able recognize antigens that are common to different intracellular parasites [17].
Different cell surface molecules appear to be involved in the induction and modulation of γδ T cell activation. As in other T cell subsets, CD28 and CD40L act as costimulatory molecules in TCR1+ lymphocytes [18,19]. In addition, it has been described that LFA-1, CD7, CD50 as well as the early cell activation antigen CD69 participate in the induction of activation of these cells [20–23]. Finally, IL-10 seems to be involved in down-regulation of γδ T cell activity [24,25], a phenomenon that may be relevant in anergic patients with mycobacterial infections.
Most studies of γδ T cells have been carried out with T cell lines or with prestimulated lymphoid cell cultures [26,27]. In order to assess adequately the reactivity of these cells in vivo, we considered it very important to use fresh isolated cells. Here, we have studied the reactivity of peripheral blood γδ T cells from patients with TB and leprosy against different mycobacterial antigens. We found a significant reactivity of fresh isolated γδ T cells from individuals sensitized against mycobacteria towards mycobacterial antigens, including the 30-kD molecule of M. tuberculosis. Interestingly, this reactivity was significantly increased by costimulatory signals generated through CD7, LFA-1, CD50 and CD69. The blockade of IL-10 with a specific MoAb also enhanced this reactivity, mainly in anergic lepromatous leprosy patients.
Materials and methods
Patients
We studied 12 patients with active pulmonary TB and nine healthy contacts, all of them with a positive intradermal test to PPD. Eight healthy individuals with no evidence of immune sensitization against M. tuberculosis (without history of bacille Calmette–Guérin (BCG) vaccination and with a non-reactive PPD intradermal test) were also studied. Thirteen lepromatous leprosy and seven tuberculoid leprosy patients, as well as seven healthy lepromin+ household contacts, were additionally studied. All patients were receiving polychemotherapy at the time of study, but none of them was receiving thalidomide or any other immunomodulatory/suppressive drug.
Reagents and antigens
The BMA031 anti-pan-TCR αβ, CgM1 and TCRd1 anti-pan-TCR γδ as well as δ V1 anti-Vδ1, δ V2 anti-Vδ2 and TigA anti-Vγ2 (or Vγ9 in the alternative nomenclature) MoAbs were purchased from T Cell Sciences (Cambridge, MA). The TP1/55 anti-CD69, HP2/19 anti-CD50, TS1/11 anti-CD11a (LFA-1) as well as an anti-CD7 MoAb were kindly provided by Dr F. Sánchez-Madrid (Hospital de la Princesa, Madrid, Spain). The x63 immunoglobulin (IgG1) and an IgG2a irrelevant MoAb (Sigma Chemical Co., St Louis, MO) were used as isotype-matched controls. Recombinant human IL-10 (rhIL-10) and a blocking anti-human IL-10 MoAb were purchased from R&D Systems (Minneapolis, MN). Mycobacterium leprae was obtained from the National Hansen Disease Center (Carville, LA). Other M. tuberculosis antigens (unfractionated culture filtrate, PPD and the 30–31-kD fibronectin-binding protein) were obtained in our own laboratory [28].
Cell isolation
Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll–Hypaque centrifugation. T cells were isolated by rosetting PBMC with sheep erythrocytes and Ficoll–Hypaque centrifugation. γδ and αβ T lymphocyte-enriched cell populations were obtained using the Cγ M1/TCRδ1 anti-pan γδ T cell and the BMA031 anti-pan αβ T cell MoAbs (T Cell Sciences) plus goat anti-mouse IgG-coated magnetic beads (Bio Mag, Advanced Magnetics, Inc., Cambridge, MA), as described [27]. The usual yield for γδ T cells ranged approximately from 1% to 4% of the initial number of PBMC and their purity was always > 80%. Monocytes were obtained by plastic adherence [7].
Cell proliferation assays
Fresh isolated γδ T cells (1 × 105/well) were cultured in duplicate in round-bottomed 96-well tissue culture plates (Costar Corp., Cambridge, MA) in the presence of M. tuberculosis culture filtrate (5 μg/ml), M. leprae (3 × 106 bacilli/ml), PPD of M. tuberculosis (5 μg/ml), recombinant 65-kD hsp from M. tuberculosis (2 μg/ml) and the 30-kD fibronectin-binding protein of M. tuberculosis (1 μg/ml). Autologous adherent cells (1 × 104/well) were added to cell cultures. Costimulation of γδ T cells was carried out using anti-CD69, -CD7, -CD11a, -CD50 MoAbs (5–10 μg/ml), or an irrelevant isotype-matched MoAb plus a rabbit anti-mouse IgG cross-linker polyclonal antibody (Sigma). In other experiments, the effect of IL-10 on the proliferative response of γδ T cells to mycobacterial antigens was assessed by adding recombinant human IL-10 (5–50 ng/ml) or a blocking anti-IL-10 MoAb (2 μg/ml). Cells were cultured for 5 days at 37°C, 5% CO2, and 18 h before this time 2 μCi of 3H-thymidine (3H-TdR, specific activity 6·7 Ci/mm; New England Nuclear, Boston, MA) were added to each well. Then cells were harvested using a semiautomated device (MH-12 Cell Harvester; Brandel, Gaithersburg, MD) and the ct/min of 3H-TdR incorporated were estimated with a liquid scintillation β-counter (Packard, Ames, IA). Results were expressed as the arithmetic mean of the stimulation index (SI), which was calculated according to the following formula: SI = (ct/min of the duplicate wells with antigen)/(ct/min of the duplicate wells without antigen).
Flow cytometry analysis
To analyse the expression of cell surface markers, PBMC or isolated cell subsets were incubated with the indicated primary MoAb for 30 min, or the proper isotype-matched MoAb, followed by washing and incubation with an FITC-labelled rabbit anti-mouse IgG (Coulter Corp., Hialeah, FL). For the study of CD69 expression, fresh isolated PBMC were stimulated with M. tuberculosis or M. leprae for 5 days and then incubated with PE-labelled MoAb specific for TCR αβ or γδ (Coulter), followed by staining with an FITC-labelled anti-CD69 MoAb. Cells were analysed using a Coulter Profile II flow cytometer and the results were expressed as the percent of positive cells.
To assess whether the cell proliferation observed in γδ T lymphocyte-enriched cell cultures was indeed due to activation/proliferation of this cell subset and not of contaminating αβ lymphocytes, double-labelling experiments were performed. Cells were pulsed with bromodeoxyuridine (BrdU) 18 h before the end of culture, as described [29]. Then cells were washed, fixed and the incorporated BrdU was detected with a mouse anti-BrdU MoAb (Dako Corp., Carpinteria, CA), followed by an FITC-labelled rabbit anti-mouse IgG (Coulter) [29]. Cells were then labelled for γδ and αβ TCR using specific, PE-conjugated mouse anti-human TCR MoAbs (Coulter). Cells were analysed by flow cytometry using as a negative control cells labelled with both the p3x63 cell culture supernatant and a PE-labelled isotype-matched control antibody (Coulter). At least two assays of BrdU labelling were run in parallel with each of the different conditions tested in 3H-TdR cell proliferation assays.
Statistical analysis
Statistical significance of data was determined by Student’s t-test, Mann–Whitney U-test, and anova. Paired samples were analysed using the non-parametrical Wilcoxon sum rank test. P < 0·05 was considered significant.
Results
As expected, similar percentages of CD3+ T cells were found in the peripheral blood from all groups studied (Table 1). However, the absolute number of T cells was significantly increased in TB patients (P < 0·05 compared with PPD− healthy individuals). Healthy contacts (PPD+) of TB patients as well as patients with tuberculoid leprosy and their lepromin+ healthy contacts showed an increased percentage of γδ T cells (P < 0·05 in all cases, Table 1). Similar results were observed when data were expressed as the absolute number of cells per ml of blood (Table 1). In these patients, the Vδ2+ cell subset accounted for the majority of γδ cells, whereas in lepromatous leprosy patients Vδ1+ T cells were predominant (Table 1). The Vδ1+ cells were almost undetectable in most TB patients and in PPD− and lepromin+ healthy individuals.
Table 1.
Quantification of αβ and γδ T cell subsets in the peripheral blood from patients with tuberculosis (TB) and leprosy, and healthy contacts
| CD3 | αβ | γδ | Vδ1 | Vδ2 | |
|---|---|---|---|---|---|
| TB (n = 5) | 66·5 ± 10·0 | 63·1 ± 6·8 | 3·1 ± 0·6 | 0·5 ± 0·3 | 2·8 ± 1·0 |
| (18·9 ± 2·8) | (17·9 ± 1·9) | (0·9 ± 0·2) | (0·1 ± 0·1) | (0·8 ± 0·2) | |
| PPD+ (n = 5) | 64·2 ± 9·3 | 55·8 ± 9·9 | 6·8 ± 2·2* | 1·4 ± 0·7 | 5·5 ± 2·2* |
| (13·5 ± 2·0) | (11·7 ± 2·1) | (1·4 ± 0·5)* | (0·3 ± 0·1) | (1·2 ± 0·5)* | |
| PPD− (n = 5) | 65·9 ± 11·2 | 62·9 ± 9·1 | 3·1 ± 1·1 | 0·9 ± 1·0 | 2·1 ± 0·6 |
| (13·2 ± 2·2) | (12·5 ± 1·8) | (0·6 ± 0·2) | (0·2 ± 0·2) | (0·4 ± 0·1) | |
| TT (n = 5) | 63·0 ± 9·0 | 56·9 ± 8·3 | 6·9 ± 2·0* | 1·1 ± 0·6 | 4·9 ± 1·7* |
| (16·9 ± 2·4) | (15·3 ± 2·2) | (1·9 ± 0·5)* | (0·3 ± 0·1) | (1·3 ± 0·5)* | |
| LL (n = 5) | 61·4 ± 7·1 | 57·1 ± 9·1 | 4·8 ± 2·0 | 2·6 ± 0·8 | 1·9 ± 0·9 |
| (14·0 ± 1·6) | (13·0 ± 2·0) | (1·1 ± 0·5) | (0·6 ± 0·2) | (0·4 ± 0·2) | |
| Lep(+) (n = 5) | 71·0 ± 13·8 | 64·2 ± 10·4 | 7·2 ± 3·1* | 0·8 ± 0·9 | 6·2 ± 2·6* |
| (14·2 ± 2·8) | (12·8 ± 2·1) | (1·4 ± 0·6)* | (0·2 ± 0·2) | (1·2 ± 0·5)* |
Fresh isolated peripheral blood mononuclear cells were labelled with specific MoAbs and analysed by flow cytometry as described in Materials and methods.
TT, Tuberculoid leprosy patients; LL, lepromatous leprosy patients; Lep(+), lepromin healthy contacts.
Data correspond to the arithmetic mean ± 1 s.d. of the percent of positive cells of five individuals in each group. Data between parentheses correspond to the arithmetic mean ± 1 s.d. of the number of cells/mm3 × 10−2.
P < 0·05 compared with PPD− healthy individuals.
To study the responsiveness of αβ and γδ T cells towards mycobacterial antigens in unfractionated mononuclear cell cultures, we stimulated in vitro PBMC from leprosy and TB patients (and their healthy contacts) with M. leprae and M. tuberculosis, respectively. Cell activation was determined through the detection of the early activation antigen AIM/CD69. We found that most γδ T cells became CD69+ in PBMC cultures from healthy PPD+ individuals and tuberculoid leprosy patients (77·3% and 82·1% positive cells, respectively; Fig. 1). A similar phenomenon, but at lower degree, was observed in TB and lepromin+ healthy contacts (53·5% and 38·0% positive cells, respectively). In contrast, a significantly smaller fraction of αβ T cells became activated when stimulated with mycobacterial antigens (< 21·6% in all groups, Fig. 1). As expected, the anergic lepromatous leprosy patients showed a very low level of cell activation, a phenomenon that was more evident in αβ than in γδ T cells (2·9% and 6·6% of CD69+ cells, respectively). These data indicate that the previously reported high reactivity of γδ T cells towards mycobacterial antigens seems to be further increased in PPD+ healthy contacts and tuberculoid leprosy patients, whereas is deeply diminished in lepromatous leprosy patients.
Fig. 1.
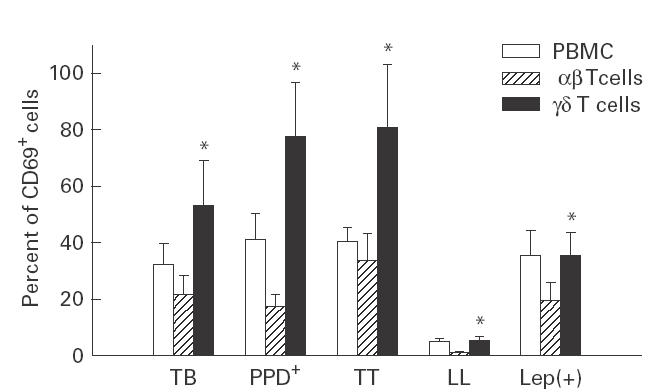
Expression of the early cell activation antigen CD69 by αβ and γδ T cells from patients with tuberculosis (TB), leprosy and their healthy contacts after stimulation with mycobacteria. Fresh isolated peripheral blood mononuclear cells (PBMC) were stimulated with Mycobacterium tuberculosis or M. leprae for 5 days and then double-labelled with MoAb against TCR αβ or γδ, and CD69 and analysed by flow cytometry, as described in Materials and methods. TT, Tuberculoid leprosy patients; LL, lepromatous leprosy patients; Lep(+), lepromin+ healthy contacts. Data correspond to the arithmetic mean ± 1 s.d. of the percent of CD69+ cells of four independent experiments. *P < 0·05 compared with αβ T cells.
To assess further the reactivity of γδ T cells, we performed cell proliferation assays using different antigenic fractions of mycobacteria. No significant differences were detected in the reactivity of freshly isolated γδ T cells from TB patients and their PPD+ healthy contacts against the M. tuberculosis antigens tested (P > 0·05 in all cases, Table 2). As expected, fresh isolated γδ T cells from PPD− control individuals showed a significant weak response against all antigens tested in our short-time culture assay (P < 0·05 in all cases, compared with PPD+ individuals). On the other hand, tuberculoid leprosy patients and lepromin+ healthy contacts showed a similar reactivity against M. leprae and the antigens of M. tuberculosis tested (Table 2). These individuals showed a significant weak response to the 65-kD hsp of M. tuberculosis compared with either M. leprae or the 30-kD antigen (P < 0·05 in both cases). As expected, the proliferative response of γδ T cells from anergic lepromatous leprosy patients against M. leprae and the 65-kD hsp of M. tuberculosis was very low. However, a moderate but significant response against PPD and the 30-kD antigen from M. tuberculosis was observed in these patients (P < 0·05 in both cases, compared with M. leprae). Interestingly, in some individuals γδ T cell proliferation in response to 30-kD antigen was higher than that induced with M. tuberculosis or M. leprae(Fig. 2). In addition, high and low responders to the 30-kD antigen could be identified in most groups studied, mainly among TB and lepromatous leprosy patients.
Table 2.
Cell proliferation of fresh isolated γδ T cells from tuberculosis (TB) and leprosy patients after stimulation with Mycobacterium tuberculosis or M. leprae
| M. leprae | M. tuberculosis | PPD | hsp 65 kD | 30-kD antigen | ||
|---|---|---|---|---|---|---|
| TB | (7) | ND | 11·9 ± 2·9 | 17·6 ± 3·9 | 6·4 ± 3·3 | 8·9 ± 5·5 |
| PPD+ | (5) | ND | 10·8 ± 2·7 | 14·8 ± 3·2 | 4·1 ± 1·9* | 8·7 ± 3·5 |
| PPD− | (5) | ND | 2·8 ± 0·9 | 3·5 ± 1·1 | 3·1 ± 0·7 | 3·9 ± 1·9 |
| TT | (5) | 9·7 ± 1·3 | ND | 13·2 ± 3·2 | 5·6 ± 1·3* | 10·2 ± 3·4 |
| LL | (9) | 1·9 ± 0·7 | ND | 10·9 ± 2·5 | 2·8 ± 0·8 | 4·1 ± 2·8** |
| Lep(+) | (5) | 12·2 ± 2·8 | ND | 9·6 ± 1·2 | 4·3 ± 0·8* | 13·1 ± 5·2 |
Fresh isolated γδ T cells from patients and controls were incubated in the presence of different stimuli for 5 days; at the end of cell culture, the lymphocyte proliferation was assessed by 3H-TdR incorporation as described in Materials and methods. The number of individuals studied is indicated in parentheses.
TT, Tuberculoid leprosy patients; LL, lepromatous leprosy patients; Lep(+), lepromin healthy contacts; ND, not done.
Data correspond to the arithmetic mean ± 1 s.d. of the stimulation index.
P < 0·05 compared with M. leprae or M. tuberculosis;
P < 0·05 compared with M. leprae.
Fig. 2.
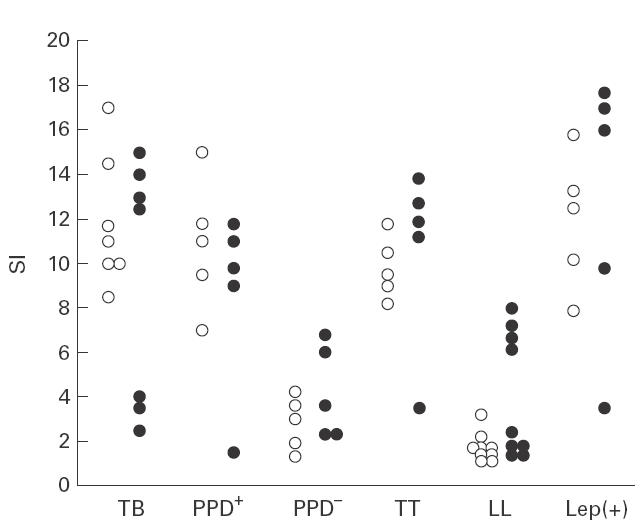
Cell proliferation response of isolated γδ T lymphocytes from tuberculosis (TB) and leprosy patients induced by 30-kD antigen from Mycobacterium tuberculosis. Fresh isolated γδ T cells from patients and controls were incubated in the presence of the 30-kD antigen from M. tuberculosis (•), or whole M. tuberculosis or M. leprae (○) for 5 days. Then lymphocyte proliferation was assessed by 3H-TdR incorporation, as described in Materials and methods. TT, Tuberculoid leprosy patients; LL, lepromatous leprosy patients; Lep(+), lepromin+ healthy contacts; SI, stimulation index.
We then investigated the possible role of IL-10 and different costimulatory signalling molecules in the in vitro proliferative response of fresh isolated γδ T cells to mycobacterial antigens. The addition of IL-10 (5 ng/ml) to γδ T cell cultures from both TB patients and PPD+ healthy individuals stimulated with M. tuberculosis resulted in a moderate but significant diminution of cell proliferation (P < 0·05 in both cases, Wilcoxon sum rank test; Table 3). Similar results were observed in tuberculoid leprosy patients and their healthy contacts. However, no significant effect of IL-10 was detected on the poor reactive cells from lepromatous leprosy patients. In additional separate experiments, using cells from TB patients, no significant differences in the inhibitory effect of IL-10 were observed when higher concentrations of this cytokine were employed (up to 50 ng/ml, data not shown). Interestingly, IL-10 blockade, with a specific MoAb, significantly enhanced the proliferation of γδ T cells from lepromatous leprosy patients (P < 0·05, Table 3). This phenomenon was not observed in other individuals.
Table 3.
Effect of IL-10 and stimulation by CD7, CD69, and LFA-1 on cell proliferation of isolated γδ T cells from tuberculosis (TB) and leprosy patients stimulated with Mycobacterium tuberculosis or M. leprae
| – | IL-10 | a-IL-10 | a-CD69 | a-CD7 | a-LFA-1 | x63 | |
|---|---|---|---|---|---|---|---|
| TB | 13·6 | 9·9 | 13·9 | 17·1 | 15·6 | 16·9 | 14·0 |
| PPD+ | 15·8 | 10·1 | 15·1 | 18·6 | 19·0 | 20·9 | 16·3 |
| TT | 8·6 | 5·9 | 9·7 | 14·7 | 15·8 | 14·6 | 9·0 |
| LL | 1·0 | 1·0 | 4·8 | 1·3 | 4·3 | 3·9 | 1·6 |
| Lep(+) | 10·4 | 6·6 | 11·0 | 15·9 | 16·9 | 19·9 | 10·9 |
Fresh isolated γδ T cells from patients and controls were cultured for 5 days in the presence of M. tuberculosis or M. leprae plus IL-10, or MoAbs against IL-10, CD69, CD7 or LFA-1; at the end of cell culture, lymphocyte proliferation was assessed by 3H-TdR incorporation, as described in Materials and methods. The irrelevant x63 MoAb was employed as an isotype-matched negative control.
TT, Tuberculoid leprosy patients; LL, lepromatous leprosy patients; Lep(+), healthy contacts lepromin+.
Data correspond to the stimulation index of a representative experiment out of five.
Co-stimulation of γδ T cells from TB patients and their healthy contacts through CD69, CD7 and LFA-1 induced a moderate but significant enhancement of cell proliferation against M. tuberculosis (P < 0·05 in all cases, Table 3). A similar phenomenon was observed in cells from tuberculoid leprosy patients and their lepromin+ healthy contacts when they were stimulated with M. leprae (P < 0·01 in all cases). Interestingly, γδ T cells from anergic lepromatous leprosy patients also significantly increased their reactivity against M. leprae when they were costimulated by CD7 and LFA-1 (P < 0·01 in both cases), but not by CD69 (P > 0·05). No significant effect of anti-CD7, anti-LFA-1 and anti-CD69 MoAbs was observed when they were added in absence of mycobacteria (data not shown).
Since it has been reported that CD50/intercellular adhesion molecule-3 (ICAM-3) is able to induce γδ T cell activation [22], we tested the effect of a stimulatory anti-CD50 MoAb on the cell proliferation of these lymphocytes towards mycobacterial antigens. We found that the HP2/19 anti-CD50 MoAb was able to exert a moderate but significant mitogenic effect by itself, in absence of mycobacteria in all groups studied (P < 0·01 in all cases, compared with control MoAb; Fig. 3). Co-stimulation by CD50 also significantly increased the cell proliferation of γδ T lymphocytes induced by mycobacteria (P < 0·05 in all cases).
Fig. 3.
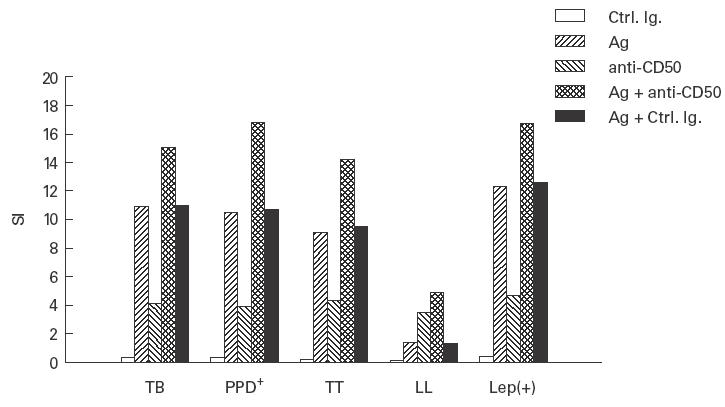
Effect of stimulation through CD50 on the cell proliferation of isolated γδ T lymphocytes induced by mycobacteria. Fresh isolated γδ T cells from patients and controls were stimulated or not with mycobacteria (Mycobacterium tuberculosis for tuberculosis (TB) and PPD+ individuals, and M. leprae for tuberculoid leprosy patients (TT), lepromatous leprosy patients (LL) and lepromin+ healthy contacts (Lep(+)) for 5 days in the presence of the HP2/19 anti-CD50 MoAb or an irrelevant isotype-matched MoAb (Ctrl. Ig.). At the end of cell culture, lymphocyte proliferation was assessed by 3H-TdR incorporation as described in Materials and methods. Data correspond to the stimulation index (SI) of a representative experiment out of four.
To assess whether the cell proliferation observed in cultures of isolated γδ T lymphocytes was indeed due to the activation/proliferation of these cells and not of contaminating αβ T lymphocytes, we performed in parallel flow cytometry double-labelling experiments for the simultaneous detection of γδ TCR and DNA synthesis (BrdU incorporation). As shown by a representative experiment in Fig. 4, in γδ T lymphocyte-enriched cell cultures (> 80% purity) stimulated with mycobacterial antigens, most γδ TCR-bearing cells were induced to DNA synthesis, whereas the contribution of αβ T cells to the overall response was usually mild (< 10% in all experiments).
Fig. 4.
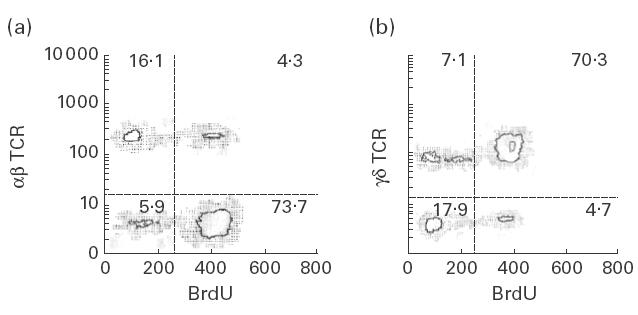
Cell proliferation induced by mycobacteria in γδ T cell-enriched cultures. Fresh isolated γδ T cells from a tuberculosis patient were stimulated with Mycobacterium tuberculosis for 5 days and then double-labelling experiments for simultaneous detection of αβ (a) or γδ (b) TCR and DNA synthesis (bromodeoxyuridine (BrdU) incorporation) were performed as described in Materials and methods. Data from a representative experiment out of four are shown. Numbers correspond to the percent of cells in each quadrant. At the onset of this cell culture, the percentages of γδ and αβ T cells were 82·3% and 14·7%, respectively.
Discussion
Due to its scarcity in human tissues, including peripheral blood, most studies on the reactivity of human γδ T cells have been performed using cell lines or prestimulated cell cultures [26,27]. These studies have provided valuable information on their specificity, but not on the in vivo reactivity of these cells. In this study, we analysed the role of γδ T cells in mycobacterial diseases using fresh isolated cells from TB and leprosy patients and their healthy contacts. We found that PPD+ and lepromin+ healthy contacts have an increased number of γδ T cells in their peripheral blood. A similar finding was made in tuberculoid leprosy patients, but not in lepromatous leprosy or TB patients. This increased proportion of γδ T lymphocytes may be a consequence of the T cell clonal expansion induced by mycobacterial antigens in vivo and might be related to resistance against mycobacteria. The Vδ2+ cell subset accounted for most of the γδ T lymphocytes found in individuals with an increased number of these cells in the peripheral blood. This is not an unexpected finding, since it has been reported that Vδ2+, but not Vδ1+ lymphocytes, preferentially respond to mycobacterial antigens [30,31].
We assessed the reactivity of γδ T cells towards mycobacterial antigens by detecting the early activation marker AIM/CD69 [32]. In this regard, it has been described that stimuli different from those that act through the T cell receptor, including cytokines such as IL-15, are able to induce the expression of CD69 by lymphocytes. Thus, not all γδ T cells that became CD69+ in our assays were necessarily recognizing mycobacterial antigens, and a significant proportion of them were probably activated as bystander cells. Furthermore, it has been described that the expression of CD69 by γδ T cells induced by antigen is not followed by cell proliferation in all cases [33]. However, it is well known that in vivo, bystander cells are activated and recruited into inflammatory cell infiltrates, participating in cell-mediated immune reactions. Thus, we think that the expression of CD69 by γδ T cells in PBMC stimulated with mycobacteria is a good indicator of the overall reactivity of this cell subset towards M. tuberculosis or M. leprae. In this context, the higher reactivity of γδ T cells from PPD+ healthy contacts and tuberculoid leprosy patients is in agreement with the increased number of γδ T cells found in the peripheral blood from these individuals. However, as shown in Fig. 1, in lepromin+ healthy contacts no apparent agreement was observed between the number of γδ T cells in peripheral blood and the degree of induction of CD69 by M. leprae in vitro. It is feasible that in lepromin+ healthy contacts, the small increase in the number of CD69+γδ T lymphocytes after their stimulation with mycobacteria may be the result of a cell activation that is not followed by cell proliferation. However, the 3H-TdR cell proliferation assays indicate that γδ T cells from lepromin+ healthy contacts are indeed induced to proliferate by mycobacterial antigens. Thus, the cause of the diminished induction of CD69 seen in γδ T cells from these individuals remains to be determined
We further assessed the reactivity of fresh isolated γδ T cells towards mycobacterial antigens using a cell proliferation 3H-TdR incorporation assay. As previously described, the γδ T cells from TB patients were efficiently stimulated in vitro by M. tuberculosis (unfractionated culture filtrate), PPD, and, to a less extent, the hsp 65 kD from M. tuberculosis. A similar trend of reactivity, including that induced by the 30-kD antigen from M. tuberculosis, was observed in the PPD+ healthy contacts. Thus, no evident differences in γδ T cell reactivity towards M. tuberculosis were observed in patients with an ongoing infection compared with apparently immune individuals. Therefore, this pattern of γδ T cell reactivity seems to be consequence of immune sensitization and is not indicative of resistance against M. tuberculosis. A similar conclusion could be reached analysing the pattern of reactivity of the γδ T cells in patients with M. leprae infection and their healthy contacts. However, it is worth mentioning that the behaviour of M. leprae infection is not exactly the same as than in the case of M. tuberculosis. Tuberculoid leprosy patients have a non-progressive infection and very few bacilli with a strong cellular immune response against M. leprae, that is accompanied by tissue damage due to hypersensitivity immune-mediated phenomena. On the other hand, lepromatous leprosy patients exhibit a progressive disease with a heavy bacilli load and no cellular immune response against M. leprae (anergy). Therefore, the lack of in vitro reactivity of γδ T cells from lepromatous leprosy patients towards M. leprae, as has been reported for whole PBMC or unfractionated T cells, is an expected but novel finding. On the other hand, the mild to moderate reactivity of these patients with the secretory 30-kD antigen of M. tuberculosis is of interest, since this molecule seems to be synthesized by the majority of mycobacteria and confers resistance in experimental models of mycobacterial infection [34]. In addition, this antigen seems to be important in the physiology of mycobacteria and is recognized by sera from lepromatous leprosy patients [28]. It is also of interest that low and high responders to the 30-kD could be identified in most groups studied. It is very likely that the genetic background (mainly MHC haplotypes) has a strong influence on the reactivity towards this antigen, and it is feasible that the resistance against mycobacteria may be influenced by this reactivity. Further studies on the role of immune recognition of 30-kD antigen in both leprosy and TB will disclose the potential importance of this molecule in mycobacterial immunity.
We were also interested in the possible role of costimulatory signals in the immune response against mycobacteria, mainly in the anergy observed in lepromatous leprosy patients. The key role of IL-10 in the induction of Th2 responses and the inhibition of cell mediated immunity has been previously reported [35,36]. However, the effect of this cytokine on immune phenomena mediated by γδ T cells has not been properly evaluated. Thus, it is of interest that IL-10 also seems to interfere with the proliferative response of γδ T cells and that the blockade of this cytokine increases the reactivity of these cells. This phenomenon is most apparent in lepromatous leprosy patients, indicating the important role of IL-10 in the anergy of γδ T cells towards M. leprae.
The involvement of CD7 and LFA-1 in the costimulation of γδ T cells has been described [37]. In addition, the potential role of ICAM-3/CD50 as a stimulatory molecule for these cells has been reported [22]. Therefore, we explored the possible role of these cell receptors in the activation/proliferation of γδ T lymphocytes induced by mycobacteria. In addition, we studied the possible effect of a molecule involved in activation of lymphoid cells and induction of cytokine synthesis, the early activation antigen CD69 [23,32]. Our findings indicate that cell signalling through CD69, CD7 and LFA-1 is able to induce mild to moderate increase of the γδ T cell proliferation induced by M. tuberculosis in PPD+ healthy contacts and TB patients. The in vivo significance of this finding is unclear, but this phenomenon suggests the potential involvement of these molecules in the regulation of γδ T cell reactivity. In this context, it is worth mentioning the modest but consistent costimulatory effect of CD7 and LFA-1 on the γδ T cells from anergic lepromatous leprosy patients. CD50 may have a similar role, although this adhesion receptor may have additional functions since it was able to induce γδ T cell proliferation in the absence of exogenous antigens.
In conclusion, our data further indicate that human γδ T cells participate in the immune response against mycobacteria and that they are also involved in the lack of response observed in anergic patients. In addition, our findings suggest the potential role of different cell membrane receptors in the regulation of γδ T cell proliferation as well as the involvement of IL-10 in this phenomenon. It will be interesting to study the effect of all these molecules on the reactivity of γδ T lymphocytes towards non-peptidic antigens derived from mycobacterias [38,39].
Acknowledgments
This work was supported by the grant 1173-9202 from CONACYT, México (to R.G.-A.).
REFERENCES
- 1.Snider DE, Raviglione M, Kochi A. Global burden of tuberculosis. In: Bloom BR, editor. Tuberculosis pathogenesis, protection, and control. Washington, DC: ASM Press; 1994. pp. 3–11. [Google Scholar]
- 2.McDyer JF, Hackley MN, Walsh TE, Cook JL, Seder RA. Patients with multidrug-resistant tuberculosis with low CD4+ T cell counts have impaired Th1 responses. J Immunol. 1997;158:492–500. [PubMed] [Google Scholar]
- 3.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 4.Bonato VLD, Lima VMF, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–75. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan G, Freedman VH, Russell D, Colston MJ. Tuberculosis research comes of age. Mol Med Today. 1998;4:330–3. doi: 10.1016/s1357-4310(98)01294-5. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PF, Grisso CL, Abrams JS, Band H, Rea TH, Modlin RL. Gamma delta T lymphocytes in human tuberculosis. J Infect Dis. 1992;165:506–12. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 7.Ueta Ch, Tsuyuguchi I, Kawasumi H, Takashima T, Toba H, Kishimoto S. Increase of γδ T cells in hospital workers who are in close contact with tuberculosis patients. Infect Immun. 1994;62:5434–41. doi: 10.1128/iai.62.12.5434-5441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, Rossman MD, Imir T, Oner-Eyuboglu AF, Lee CW, Biancaniello R, Carding SR. Disease-specific changes in γδ T cell repertoire and function in patients with pulmonary tuberculosis. J Immunol. 1996;157:4222–9. [PubMed] [Google Scholar]
- 9.Ferrick DA, Schrenzel MD, Mulvania T, Hsleh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann SHE. Bacterial and protozoal infections in genetically disrupted mice. Curr Opin Immunol. 1994;6:518–25. doi: 10.1016/0952-7915(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 11.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1995;25:2877–81. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 12.D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–21. [PubMed] [Google Scholar]
- 13.Rajasekar R, Sim G, Augustin A. Self heat shock and γδ T-cell reactivity. Proc Natl Acad Sci USA. 1990;87:1767–71. doi: 10.1073/pnas.87.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Born W, Happ MP, Dallas A, et al. Recognition of heat shock proteins and γδ T cell function. Immunol Today. 1990;11:40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- 15.Wesch D, Marx S, Kabelitz D. Comparative analysis of alpha beta and gamma delta T cell activation by Mycobacterium tuberculosis and isopentenyl pyrophosphate. Eur J Immunol. 1997;27:952–6. doi: 10.1002/eji.1830270422. [DOI] [PubMed] [Google Scholar]
- 16.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 17.Behr C, Poupot R, Peyrat MA, Poquet Y, Constant P, Dubois P, Bonneville M, Fournie JJ. Plasmodium falciparum stimuli for human γδ T cells are related to phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–6. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testi R, Lanier LL. Functional expression of CD28 on T cell antigen receptor γδ-bearing T lymphocytes. Eur J Immunol. 1989;19:185–8. doi: 10.1002/eji.1830190129. [DOI] [PubMed] [Google Scholar]
- 19.Ramsdell F, Seaman MS, Clifford KN, Fanslow WC. CD40 ligand acts as a costimulatory signal for neonatal thymic γδ T cells. J Immunol. 1994;152:2190–7. [PubMed] [Google Scholar]
- 20.Takamizawa M, Fagnoni F, Mehta-Damani A, Rivas A, Engleman EG. Cellular and molecular basis of human γδ T cell activation. J Clin Invest. 1995;95:296–303. doi: 10.1172/JCI117654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrel S, Salvi S, Rafti F, Favrot M, Rapin C, Sekaly RP. Direct involvement of CD7 (gp40) in activation of TcRγ/δ+ T cells. Eur J Immunol. 1991;21:1195–9. doi: 10.1002/eji.1830210515. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Caselles T, Rubio G, Campanero MR, del Pozo MA, Muro M, Sánchez-Madrid F, Aparicio P. ICAM-3, the third LFA-1 counterreceptor, is a co-stimulatory molecule for both resting and activated T lymphocytes. Eur J Immunol. 1993;23:2799–806. doi: 10.1002/eji.1830231112. [DOI] [PubMed] [Google Scholar]
- 23.Moretta A, Poggi A, Pende D, et al. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med. 1991;174:1393–8. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pechhold K, Wesch D, Schondelmaier Kabelitz D. Primary activation of Vγ9-expressing γδ T cells by Mycobacterium tuberculosis. Requirement for Th1-type CD4 T cell help and inhibition by IL-10. J Immunol. 1994;152:4984–92. [PubMed] [Google Scholar]
- 25.Marx S, Wesch D, Kabelitz D. Activation of human γδ T cells by Mycobacterium tuberculosis and Daudi lymphoma cells. Differential regulatory effect of IL-10 and IL-12. J Immunol. 1997;158:2842–8. [PubMed] [Google Scholar]
- 26.Balaji KN, Boom WH. Processing of Mycobacterium tuberculosis bacilli by human monocytes for CD4+αβ and γδ T cells: role of particulate antigen. Infect Immun. 1998;66:98–106. doi: 10.1128/iai.66.1.98-106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukaguchi K, Balaji KN, Boom WH. CD4+αβ T cell and γδ T cell responses to Mycobacterium tuberculosis. Similarities and differences in Ag recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–96. [PubMed] [Google Scholar]
- 28.Espitia C, Sciutto E, Bottasso O, González-Amaro R, Hernández-Pando R, Mancilla R. High antibody levels to the mycobacterial fibronectin-binding antigen of 30–31 kDa in tuberculosis and lepromatous leprosy. Clin Exp Immunol. 1992;87:362–7. doi: 10.1111/j.1365-2249.1992.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esin S, Batoni G, Källenius G, Gaines H, Campa M, Svenson SB, Andersson R, Wigzell H. Proliferation of distinct human T cell subsets in response to live, killed or soluble extracts of Mycobacterium tuberculosis and Mycobacterium avium. Clin Exp Immunol. 1996;104:419–25. doi: 10.1046/j.1365-2249.1996.d01-691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyemura K, Deans RJ, Band H, Ohmen J, Panchamoorthy G, Morita CT, Rea TH, Modlin RL. Evidence for clonal selection of γδ T cells in response to a human pathogen. J Exp Med. 1991;174:683–92. doi: 10.1084/jem.174.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panchamoorthy G, McLean J, Modlin RL, Morita CT, Ishikawa S, Brenner MB, Band H. A predominance of the T cell receptor Vγ2/Vδ2 subset in human mycobacteria-responsive T cells suggests germline gene encoded recognition. J Immunol. 1991;147:3360–9. [PubMed] [Google Scholar]
- 32.Cebrián M, Redondo JM, López-Rivas A, Rodríguez-Tarduchi G, de Ortiz Landázuri MO, Sánchez-Madrid F. Expression and function of AIM, an activation inducer molecule of human lymphocytes is dependent on the activation of protein kinase C. Eur J Immunol. 1989;19:809–15. doi: 10.1002/eji.1830190505. [DOI] [PubMed] [Google Scholar]
- 33.Lahn M, Kalataradi H, Mittelstadt P, et al. Early preferential stimulation of γδ T cells by TNF-α. J Immunol. 1998;160:5221–30. [PubMed] [Google Scholar]
- 34.Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with mayor extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–4. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore KW, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 36.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of Th1 and Th2 responses. Immunol Today. 1991;12:49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi N, Hiromatsu K, Matsuzaki G, Harada M, Matsumoto Y, Nomoto K, Yoshikai Y. A sustained increase of cytosolic Ca2+ in γδ T cells triggered by co-stimulation via TCR/CD3 and LFA-1. Cell Calcium. 1997;22:421–30. doi: 10.1016/s0143-4160(97)90069-5. [DOI] [PubMed] [Google Scholar]
- 38.Porcelli SA, Morita CT, Modlin RL. T-cell recognition of non-peptide antigens. Curr Opin Immunol. 1996;8:510–6. doi: 10.1016/s0952-7915(96)80039-2. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma/delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]


