Abstract
Periodontal disease is a bacterial dental plaque-induced destructive inflammatory condition of the tooth-supporting tissues, which is thought to be mediated by T lymphocytes secreting T helper 2 (Th2) cytokines, resulting in recruitment of high numbers of antibody-producing B lymphocytes/plasma cells as well as polymorphonuclear leucocytes (PMN) secreting tissue-destructive components, such at matrix metalloproteinases and reactive oxygen metabolites into the gingival connective tissues. One treatment strategy may be to down-regulate the Th2 response to those dental plaque microorganisms which induce the destructive inflammatory response. In this study we have examined the effects of a potent down-regulator of Th2 responses on ligature-induced periodontal disease in an experimental rat model. A single s.c. injection into Wistar rats of 0·1 or 1 mg of SRL172, a preparation of heat-killed Mycobacterium vaccae (NCTC 11659), 13 days before application of the ligature, significantly reduced the subsequent destruction of the tooth-supporting tissues, as measured by loss of periodontal attachment fibres (P < 0·001) and bone (P < 0·002). This protective effect occurred not only on the experimental (ligatured) side but also on the control unligatured side. SRL172 has undergone extensive toxicological studies and safety assessments in humans, and it is suggested that it may provide a safe and novel therapeutic approach to periodontal disease.
Keywords: periodontal disease, SRL172, vaccination, Th1/Th2 balance
Introduction
Oral microorganisms colonizing tooth surfaces (dental plaque) in close contact with the gingival margin trigger inflammatory processes in the gums. These responses may be non-destructive (gingivitis) or destructive (periodontitis or periodontal disease). Periodontal disease results in various degrees of breakdown of the tooth-supporting tissues (the periodontium). This includes destruction of periodontal attachment fibres and resorption of the alveolar bone, which may progress to periodontal pocket formation, increased tooth mobility and tooth loss [1].
The tissue destruction is associated with a shift from a Th1 cytokine secretion profile in gingivitis to a Th0/Th2-dominated cytokine secretion profile in periodontal disease [2,3]. Moreover, with the development of periodontal disease there is a shift in the dental plaque microflora from a largely aerobic Gram-positive flora associated with gingivitis to a complex and more specific anaerobic subgingival Gram-negative flora [4]. Severe and progressive periodontal disease may be associated with some of these anaerobic species, which are termed periodentopathogens [4].
Risk factors for periodontal disease include genetic background [5] but also depression [6–8], increasing age [9], smoking [10,11], and uncontrolled diabetes mellitus of long duration [12,13]. We have emphasized [14] that all these periodontal disease risk factors, including depressive mood states [15,16], lipopolysaccharides (LPS) from Gram-negative bacteria [17], nicotine [18], increasing age [19,20], and insulin deficiency induce sustained hypothalamo–pituitary–adrenal (HPA) axis activity and thereby increase secretion of glucocorticoids, which are known to suppress Th1 and favour Th2 responses [21], partly by suppressing IL-12 and enhancing IL-10 production by antigen-presenting cells (APC) [22,23].
Recruitment of phagocytic polymorphonuclear leucocytes (PMN) during the local Th2 response is typical of active periodontal disease, and PMN degranulation is one of the major pathogenic mechanisms [24]. Thus, treatment strategies aimed at reducing Th2 and favouring Th1 responses may be useful in this condition.
The concept of preventing periodontitis by immunization has been suggested previously [25], but has been difficult to pursue because of the complexity of the periodontal microflora and multiplicity of species involved. Recent studies in primates have suggested that it is possible to reduce the gingival colonization of some putative pathogens by immunization [26,27]. So far however minimal or no effect has been reported on the progression of periodontal disease [28].
An alternative strategy to that of immunization against a specific microorganism is to use potent immunomodulators that have long-lasting systemic effects on the nature of the immune response to unrelated antigens. Effects of this type have been induced with a mycobacterial preparation which down-regulated the Th2 response to ovalbumin even in mice that had been preimmunized with this potently Th2-inducing antigen [29]. Such a strategy would be expected to reduce the influx of PMN, releasing tissue-destructive components in response to the load of anaerobic pathogens.
The purpose of this study was to examine whether SRL172, a preparation of heat-killed Mycobacterium vaccae, which potently down-regulates Th2 responses [29], can reduce the tissue destruction in a well-established experimental model of periodontal disease in Wistar rats.
Materials and methods
Animals
Twenty-five female Wistar rats, weighing 200–230 g, were obtained from Möllegaard Breeding Centre (Ejby, Denmark), and used after 2 weeks of acclimatization to their housing conditions. At that time the average weight was 227 g [25]. During the experiment all rats gained weight to reach an average of 257 g [28]. Standard rat chow pellets and tap water were available ad libitum. The animals were maintained in individual cages under a 12–24 h light/dark cycle (light on 7·00 am to 7·00 pm) with temperature and humidity at 22°C and 40–60%, respectively.
SRL172 and immunization of rats
SRL172 was provided by SR Pharma (London, UK) at a concentration of 10 mg/ml in borate buffered (pH 8·0) saline and stored at 4°C. SRL172 was administrated as 0·1 or 1·0 mg in 100 μl saline given subcutaneously in the neck skin 13 days before periodontal disease induction. On the same day controls received saline only. Local inflammation in the SRL172 injection sites had resolved before the ligatures were put in place. There were eight rats in each of the vaccinated groups and nine control animals.
Experimental periodontitis model
Twenty-five animals (eight vaccinated with 0·1 mg, eight with 1·0 mg, and nine controls) were anaesthetized by subcutaneous injection in the neck with Hypnorm-Dormicum (fenatyl/fluanizone, medazolam), 0·2 ml/100 g body weight. A sterile silk ligature (Ethicon Perma-hand seide, Norderstedt, Germany) was tied around the neck of the maxillary right 2nd molar tooth in the gingival sulcus. The ligatures were left in the same position for the period of 8 weeks and served as a retention device for oral microorganisms. The left 2nd molar served as non-ligated internal control. At the end of the experiment all animals were killed by decapitation. The maxillae were excised and fixed in 4% formaldehyde.
Radiographic examination
The specimens were stabilized with dental wax on a Trophy digital x-ray sensor, orientated with the axis of the teeth parallel to the sensor surface by using ×4 magnification loop glasses (Zeiss, Aalen, Germany). The distances between the cemento–enamel junction (CEJ) and bone (B) on mesial and distal surfaces of the 2nd molars were displayed digitally. The examiner was unaware of whether the specimens came from experimental or control animals. Reliability was assessed by reading the x-rays twice with an interval of > 1 month. The standard error of the mean difference between readings was 0·163.
Histological examination
After the radiographic examinations the specimens were decalcified in 10% EDTA for approximately 2 weeks, until complete decalcification could be confirmed radiographically. The specimens were dehydrated in graded alcohol, carefully orientated and embedded in paraffin with the axis of the teeth parallel to the cutting direction. The blocks were cut in serial sections of 5 μm thickness in a mesio-distal direction. The most central section from each tooth, i.e. the one that comprised the centre of the dental pulp, was selected for analysis, stained with haematoxylin and eosin and mounted.
The sections were placed in a Nikon microscope with a camera and a TV monitor (Sony, Barcelona, Spain). Sections from experimental sites were magnified × 120 in order to measure CEJ to periodontal attachment fibres (F) and CEJ–B distance reliably on drawings made from the monitor screen. Reliability was assessed by reading 10 randomly selected specimens twice with an interval of > 1 month. The standard error of the mean difference between the two was 0·003 and 0·005 for CEJ–F and CEJ–B, respectively.
Results
All results are shown in Table 1, and illustrated in the photomicrograph (Fig. 1) of representative teeth.
Table 1.
Periodontal tissue destruction
| Ligatured side | ||||
|---|---|---|---|---|
| Bone loss | Control side | |||
| X-ray | Histometry | Fibre loss | Fibre loss | |
| SRL172, 0·1 mg | 0·73 ± 0·095 | 0·78 ± 0·120 | 0·465 ± 0·082 | 0·042 ± 0·020 |
| SRL172, 1·0 mg | 0·70 ± 0·141 | 0·76 ± 0·137 | 0·472 ± 0·135 | 0·028 ± 0·039 |
| Saline control | 1·07 ± 0·122 | 0·99 ± 0·124 | 0·790 ± 0·171 | 0·105 ± 0·058 |
| P values (t-test) | ||||
| 0·1s mg/control | < 0·001 | 0·002 | < 0·001 | 0·006 |
| 1·0 mg/control | < 0·001 | 0·001 | < 0·001 | 0·004 |
Fig. 1.
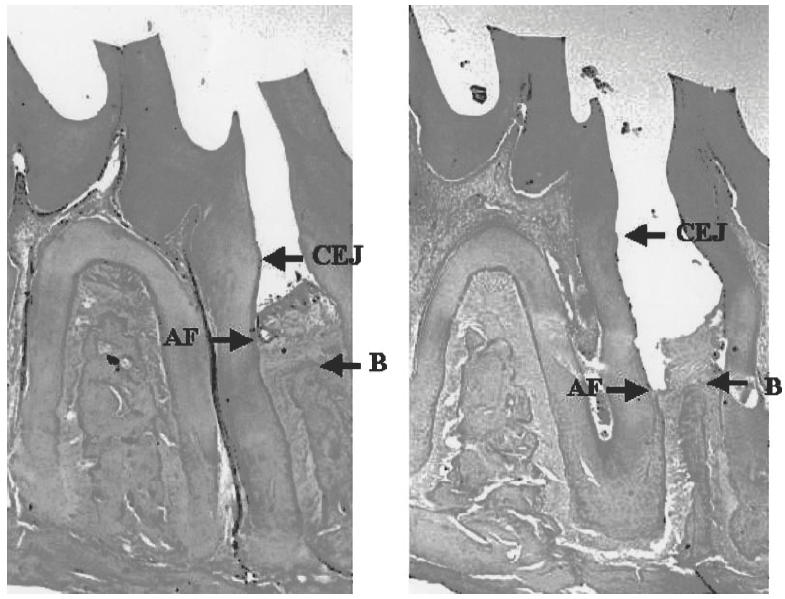
Photomicrographs showing histological sections of an experimental tooth in a control Wistar rat injected with 100 μl saline (right), and in a rat prevaccinated with 0·1 mg of SRL172 in 100 μl saline (left). A difference in loss of periodontal attachment fibres (AF) and bone (B) can be observed. CEJ, Cemento–enamel junction; AF, the most coronal attachment fibres; B, alveolar bone crest.
Radiographically the bone loss, measured as the distance from the cemento–enamel junction to the most coronal bone (CEJ–B) in the experimental sites in Wistar rats vaccinated with 0·1 mg of SRL172 in 100 μl saline given 13 days before disease induction, was 0·725 ± 0·095 mm (mean ± s.d.). In the other vaccinated group, where SRL172 was administered as 1·0 mg in 100 μl saline, also given 13 days before disease induction, the CEJ–B was 0·70 ± 0·141 mm. In the control animals given saline only, the CEJ–B distance was 1·07 ± 0·12 mm. The bone loss was significantly reduced (P < 0·001) in both vaccinated groups relative to that seen in the non-vaccinated controls.
The reduction in bone loss in the vaccinated groups, when compared with controls, was also highly significant when measured histometrically (P = 0·002 and P = 0·001 for the recipients of 0·1 mg and 1·0 mg SRL172, respectively).
The periodontal fibre loss measured histometrically as the distance between the cemento–enamel junction and the periodontal attachment fibres (CEJ–F) was 0·465 ± 0·082 mm in the vaccinated group given 0·1 mg SRL172, and 0·472 ± 0·135 mm in the group that had received 1·0 mg SRL172. The equivalent distance was 0·790 ± 0·171 mm in the non-vaccinated control group given saline only. The fibre loss was significantly reduced (P < 0·001) in both vaccinated groups relative to that seen in the non-vaccinated controls.
The measurement of the CEJ–F distance was also applied to the non-ligatured left 2nd molar. As anticipated, the disease was much milder on this side, but nevertheless the CEJ–F was strikingly reduced in both vaccinated groups (P = 0·006 and 0·004 for the 0·1-mg and 1·0-mg doses, respectively).
For all of the measurements the two vaccine doses were equally efficient, with no significant differences between the groups that had received 0·1 or 1·0 mg of SRL172.
Discussion
Ligature-induced periodontal disease in rats is a predictable model system to study the breakdown of tooth-supporting tissues induced by oral subgingival anaerobic microorganisms [30]. The ligature causes abundant bacterial plaque, induction of a subgingival pocket and a subsequent shift from a largely aerobic to the more anaerobic microflora typical of periodontal disease. The advantage of this model is that we do not have to introduce exogenous antigens or pathogens, but merely change the environment to allow growth of a pathogenic microflora, much as in human periodontal disease.
This study was designed to determine whether a single injection of a material which down-regulates Th2 responses can alter disease progression in this model [29]. Recruitment of PMN into inflamed tissues is much higher in active periodontal disease where Th2 dominates and the Th1 cytokine profile is down-regulated [3], compared with gingival inflammation without tissue destruction [31,32]. PMN recruitment during periodontitis may represent a compensatory mechanism to combat invading pathogens during a weak Th1 response where the macrophage phagocytosis may be down-regulated [14]. However, most of the soft tissue destruction in periodontal disease is attributable to matrix metalloproteinases (MMPs), released from PMN [33], together with reactive oxygen and nitrogen intermediates [30,34]. Bone resorption by osteoclasts is regulated by inflammatory cytokines such as IL-1, tumour necrosis factor-alpha and IL-6, as well as NO [35].
We have demonstrated that in spite of the high challenge of anaerobic microorganisms in the gingival sulci of the experimental rats in this model, SRL172 significantly inhibited the breakdown of the tooth-supporting tissues as measured by periodontal attachment fibre and bone loss. This effect is likely to be due mainly to the ability of SRL172 to down-regulate Th2 responses non-specifically [29], recently confirmed in small studies in hay fever [36] and asthma [37] patients. However, SRL172 may also contribute directly to immunity to the pathogens by driving Th1 responses [38] to shared antigens such as heat shock proteins, which are known to be highly conserved, and important targets of the immune system [39].
The use of potent immunomodulators such as SRL172, with long-lasting systemic effects on the immune response, may represent a new strategy for prevention or treatment of periodontal disease. The availability of data in man suggesting that SRL172 has an acceptable safety profile indicates that this agent should be tested as an immunotherapeutic for periodontal disease.
Acknowledgments
We are grateful to the Division of Environmental Toxicology of the Norwegian Defence Research Establishment (Kjeller, Norway) for supporting this work and providing the laboratory facilities. We thank Dr Gareth Bowen of SR Pharma for his advice and critical reading of the manuscript.
REFERENCES
- 1.Lindhe J. Textbook of clinical periodontology. 2. Copenhagen: Munksgaard; 1995. [Google Scholar]
- 2.Seymour GJ, Gemell E, Kjeldsen M, Yamazaki K, Nakajima T, Hara K. Cellular immunity and hypersensitivity as components of periodontal destruction. Oral Dis. 1996;2:96–101. doi: 10.1111/j.1601-0825.1996.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 3.Tokoro Y, Matsuki Y, Yamamoto T, Suzuki T, Hara K. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue, to periodontal diseases. Clin Exp Immunol. 1997;107:166–74. doi: 10.1046/j.1365-2249.1997.d01-880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socransky SS, Haffajee AD. The bacterial aetiology of destructive periodontal disease. J Periodontol. 1992;63:322–31. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 5.Michalowicz B. Genetic and heritable risk factors in periodontal disease. J Periodontol. 1994;65:479–88. doi: 10.1902/jop.1994.65.5s.479. [DOI] [PubMed] [Google Scholar]
- 6.Genco RJ, Ho AW, Kopman J, Grossi SG, Dunford RG, Tedesco LA. Models to evaluate the role of stress in periodontal disease. Ann Periodontol. 1998;3:288–302. doi: 10.1902/annals.1998.3.1.288. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro da Silva AM, Oakly DA, Newman HN, Nohl FS, Lloyd HM. Psychosocial factors and adult onset rapidly progressive periodontitis. J Clin Periodontol. 1996;23:789–94. doi: 10.1111/j.1600-051x.1996.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 8.Moss ME, Beck JD, Kaplan BH, et al. Exploratory case-control analysis of psychosocial factors and adult periodontitis. J Periodontol. 1996;67:1060–9. doi: 10.1902/jop.1996.67.10s.1060. [DOI] [PubMed] [Google Scholar]
- 9.Hugoson A, Jordan T. Frequency distribution of individuals aged 20–70 years according to severity of periodontal disease. Comm Dent Oral Epidemiol. 1982;10:187–92. doi: 10.1111/j.1600-0528.1982.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Canut P, Lorca A, Magan R. Smoking and periodontal disease. J Clin Periodontol. 1996;22:743–9. doi: 10.1111/j.1600-051x.1995.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales YM, De Nardin A, Grossi SC, Machtei EE, Genco RJ, De Nardin E. Serum cotinine levels, smoking and periodontal attachment loss. J Dent Res. 1996;75:796–802. doi: 10.1177/00220345960750021001. [DOI] [PubMed] [Google Scholar]
- 12.Dennison DK, Gottsegen R, Rose LF. Diabetes and periodontal diseases. J Periodontol. 1996;67:166–76. [PubMed] [Google Scholar]
- 13.Thorstensson H, Hugoson A. Periodontal disease experience in adult long-duration insulin-dependent diabetics. J Clin Periodontol. 1993;20:352–8. doi: 10.1111/j.1600-051x.1993.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 14.Breivik T, Thrane PS. Psychoneuroimmune interactions in periodontal disease. In: Ader R, Felten DL, Cohen N, editors. Psychneuroimmunology. San Diego: Academic Press; 2000. [Google Scholar]
- 15.Charlton BG, Ferrier IN. Hypothalamo–pituitary–adrenal axis abnormalities in depression: a review and a model. Psychol Med. 1989;19:331–6. doi: 10.1017/s003329170001237x. [DOI] [PubMed] [Google Scholar]
- 16.Dallman MF, Akana SF, Bradbury MJ, Strack AM, Simon-Hanson E, Scribner KA. Regulation of hypothalamo–pituitary–adrenal axis during stress: feedback, facilitation and feeding. Semin Neurosci. 1994;6:205–13. [Google Scholar]
- 17.Tilders FJH, De Rijk RH, van Dam AM, Vincent VAM, Schotanus K, Persoons JHA. Activation of the hypothalamo–pituitary–adrenal axis by bacterial endotoxins: routes and intermediate signals. Psychoneuroendocrinology. 1994;19:209–32. doi: 10.1016/0306-4530(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.Matta SG, Foster CA, Sharp BM. Nicotine stimulates expression of cFos protein in the parvocellular paraventricular nucleus and brain stem catecholaminergic regions. Endocrinology. 1993;132:2149–56. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- 19.Dodt C, Dittman J, Hruby J, et al. Different regulation of adrenocorticotropin and cortisol secretion in young mentally healthy elderly, and patients with senile dementia of Alzheimer's type. J Clin Endocrinol Metab. 1991;72:272–6. doi: 10.1210/jcem-72-2-272. [DOI] [PubMed] [Google Scholar]
- 20.Friedman M, Green MF, Sharland DE. Assessment of hypothalamic–pituitary–adrenal function in the geriatric age group. J Gerontol. 1969;24:292–7. doi: 10.1093/geronj/24.3.292. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156:2406–12. [PubMed] [Google Scholar]
- 22.Visser J, van Boxel‐dezaire A, Methorst D, Brunt T, de Kloet ER, Nagelkerken L. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood. 1998;91:4255–64. [PubMed] [Google Scholar]
- 23.Vieira PL, Kalinski P, Wierenga EA, Kapsenberg ML, de Jong E. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro- generated human dendritic cells without affecting their T cell stimulatory potential. J Immunol. 1998;161:5245–51. [PubMed] [Google Scholar]
- 24.Ding Y, Haapsalo M, Kerosuo E, Lounatamaa K, Kotiranta A, Sorsa T. Release and activation of human neutrophil matrix metallo- and serine proteinases during phagocytosis of Fusobacterium nucleatum, Porphyromonas gingivalis and Treponema denticola. J Clin Periodontol. 1997;24:237–48. doi: 10.1111/j.1600-051x.1997.tb01837.x. [DOI] [PubMed] [Google Scholar]
- 25.Casto TD. The treatment of periodontoclasia with a polyvalent vaccine (Goldenberg's Inava Endocorps Vaccine) Dent Cosmos. 1925;68:689–91. [Google Scholar]
- 26.McArthur WP, Magnusson I, Marks RG, Clark WB. Modulation of colonisation by black-pigmented Bacteroides species in squirrel monkeys by immunisation with Bacteroides gingivalis. Infect Immun. 1989;57:2313–7. doi: 10.1128/iai.57.8.2313-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nisengard R, Blann D, Zelonis L, McHenry K, Reynolds H, Zambon JJ. Effects of immunisation of B. macacae on induced periodontitis—preliminary findings. Immunol Invest. 1989;18:225–37. doi: 10.3109/08820138909112239. [DOI] [PubMed] [Google Scholar]
- 28.Klausen B, Evans RT, Ramamurthy NS, et al. Periodontal bone level and gingival proteinase activity in gnotobiotic rats immunised with Bacteroides gingivalis. Oral Microbiol Immunol. 1991;6:193–201. doi: 10.1111/j.1399-302x.1991.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang CC, Rook GAW. Inhibition of an established allergic response to ovalbumin in Balb/c mice by killed Mycobacterium vaccae. Immunology. 1998;93:307–13. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohinai Z, Benedek P, Feher E, et al. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Brit J Pharmacol. 1998;123:353–60. doi: 10.1038/sj.bjp.0701604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee W, Aitken S, Sodek J, McCulloch C. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodont Res. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 32.Shapira L, Borinski R, Sela MN, Soskolne A. Superoxide formation and chemiluminescence of peripheral polymorphonuclear leukocytes in rapidly progressive periodontitis patients. J Clin Periodontol. 1991;18:44–48. doi: 10.1111/j.1600-051x.1991.tb01118.x. [DOI] [PubMed] [Google Scholar]
- 33.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–84. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 34.Chapple ILC. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 35.Jilka RL, Hangoc G, Girasole G, et al. Increased osteoblast development after estrogen loss: mediation by interleukin-6. Science. 1992;3:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 36.Hopkin JM, Shaldon S, Ferry B, et al. Mycobacterial immunisation in grass pollen asthma and rhinitis. Thorax. 1998;53(Suppl. 4) Abstr. S63. [Google Scholar]
- 37.Camporota L, Corkhill A, Long H, et al. Effects of intradermal Mycobacterium vaccae on allergen-induced airway responses and IL-5 generation by PBMC in mild to moderate asthma; Proceedings of ATS International Conference, Toronto, May 5–10, 1999; 2000. [Google Scholar]
- 38.Abou-Zeid C, Gares M-P, Inwald J, et al. Induction of a type 1 immune responses to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856–62. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young DB. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992;4:396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]


