Abstract
Recent studies have revealed the existence of a distinct type of NK cell leukaemia of the juvenile type, which presents with hypersensitivity to mosquito bites (HMB) as an essential clinical manifestation and is infected with clonal Epstein–Barr virus (EBV). This disorder is thus called HMB-EBV-NK disease and has been reported in Orientals, mostly from Japan. We investigated the profile of cytokine production and the expression of both types of NK inhibitory receptors, i.e. CD94 lectin-like dimers and killer-cell immunoglobulin-like receptors, in NK leukaemic cells from three patients with HMB-EBV-NK disease. It was found that freshly isolated NK leukaemic cells expressed mRNA for interferon-gamma (IFN-γ) and additionally produced IL-10 upon stimulation with IL-2, indicating that the NK cells were of NK1 type. More than 98% of NK cells from the patients bore CD94 at a higher level than did normal NK cells, whereas p70 or NKAT2, belonging to immunoglobulin-like receptor, was not expressed in those NK cells. Freshly isolated leukaemic NK cells transcribed mRNA for CD94-associated molecule NKG2C at an abnormally high level, and upon stimulation with IL-2 and/or IL-12 they expressed NKG2A as well. The disordered expression of these inhibitory receptors not only provides some insights into the pathogenesis of HMB-EBV-NK disease but also can be used as phenotypic markers for the diagnosis of this type of NK cell leukaemia.
Keywords: NK cell, hypersensitivity to mosquito bites, CD94, NKG2, HLA-E
Introduction
Hypersensitivity to mosquito bites (HMB) is a disorder characterized by necrotic skin reactions at bite sites and the associated general symptoms such as high fever, lymphadenopathy, and hepatosplenomegaly [1–3]. This enigmatic disease has been reported in Orientals, mostly Japanese patients in the first two decades of life. The previous case reports and our inquiries of Japanese haematologists and dermatologists showed that there have been at least 59 Japanese patients with HMB. Accumulated clinical and laboratory studies in these cases have revealed a surprising finding that HMB occurs in close association with NK cell leukaemia whose malignant cells are infected with monoclonal (or oligoclonal) Epstein–Barr virus (EBV) [2,4]. We have proposed that this disorder is a distinct clinical entity, which is called HMB-EBV-NK disease in this study. NK leukaemic cells in most cases are large granular lymphocytes (LGL), express surface molecules of normal activated NK cells including CD2, CD11b, CD16, CD38, CD56 and HLA-DR, and exhibit cytotoxicity against NK-sensitive target cells and antibody-dependent cell-mediated cytotoxicity [1,3]. Although these NK cells infiltrate the skin lesions of mosquito bites [1], it remains unclear how HMB is mediated by these NK leukaemic cells.
NK cells participate in the elimination of microorganism-infected and malignant cells by their cytotoxicity during early immunological events [5]. In addition, cytokines secreted by them play a crucial role in various immunoregulations, such as the subsequent generation of adaptive T cell responses [6–8]. Similar to differentiation of T helper cells into Th1 or Th2 subsets by IL-12 or IL-4, it has recently been reported that human NK cells cultured in the presence of IL-12 or IL-4 differentiate into two NK1 and NK2 populations, respectively [9]. IL-12-induced NK1 cells possess vigorous cytotoxicity and secrete interferon-gamma (IFN-γ), IL-10, and tumour necrosis factor-alpha (TNF-α), but not IL-2 or IL-4, whereas NK2 cells selectively produce IL-5 and IL-13 upon stimulation with IL-4.
NK cells can discriminate normal cells from abnormal cells such as virally infected and tumour cells via their killer cell inhibitory receptors (KIR) [10,11]. Normal cells evade NK cell attack by stimulating KIR of NK cells with MHC class I molecules abundantly expressed on them. Therefore, when the expression of MHC class I molecules is down-regulated in vivo in tumour cells, they are unable to induce the inhibitory signal transduction of NK cells, and thereby the tumour cells are effectively lysed by NK cells [10–13]. In human NK cells, several KIR have been identified and termed p70, NKAT2 and CD94. The p70 and NKAT2 KIR belong to the immunoglobulin superfamily and recognize HLA-B and C molecules, respectively [14,15]. On the other hand, CD94 molecules belong to C-type lectin, recognize non-classical HLA-E molecules, and transmit inhibitory signals when they closely bind with NKG2A molecules [16,17]. In contrast, signalling via NKG2C in the context of CD94 is known to activate NK cells [18].
To characterize the function of NK leukaemic cells in HMB-EBV-NK disease and to address the pathogenesis of HMB, we further investigated the NK cells with regard to the cytokine profile and the expression of KIR. This study demonstrates that the NK cells secrete NK1-type cytokines and express a high level of surface CD94 molecules and mRNAs for NKG2A and C, whereas p70 or NKAT2 was not expressed. Since the levels of NKG2A and C molecules of freshly isolated NK leukaemic cells were comparable to those of IL-2- and IL-12-stimulated normal NK cells, it is suggested that the NK cells are in an activated state.
PATIENTS and METHODS
Patients
Three patients with HMB-EBV-NK disease, 13-, 17- and 31-year-old Japanese women, were studied. The clinical information and the haematologic data of the patients were described previously [1,2]. Briefly, peripheral blood mononuclear cells (PBMC) from three patients contained 40–60% of CD3−4−8−16+56+ NK cells, which were infected with monoclonal or biclonal EBV as determined by Southern blotting with probes for viral terminal repeat. All patients were studied for CD94/NKG2 expression and the 13-year-old girl was further analysed in other studies. PBMC were taken from these patients when they were free from local and systemic symptoms evoked by mosquito bites. As control NK-lineage leukaemic cells, CD3−CD56+ LGL leukaemic cells in a patient with EBV-non-infected, NK-LGL leukaemia (YS) [19], were used. Normal NK cells were obtained from peripheral blood of three healthy age- and sex-matched individuals (13-,16- and 17-year-old Japanese girls).
Monoclonal antibodies
FITC-conjugated MoAbs specific for pan-HLA class I molecules (G46-2.6), CD94 (HP-3D9), p70 KIR (DX9) and NKAT2 (DX27), and purified, non-conjugated form of CD94 (HP-3D9) MoAb were purchased from PharMingen (Hamburg, Germany). PE-conjugated CD56-specific MoAb (YM31) was obtained from Becton Dickinson (San Jose, CA). Biotin- or non-conjugated MoAbs specific for IL-2, IL-4, IL-5, IL-10 and IFN-γ were purchased from PharMingen. Oligonucleotide primers specific for IL-2, IL-4, IL-5, IL-10 and IFN-γ were described previously [20]. Nucleotide sequences of primers specific for NKG2A, NKG2C [21] and HLA-E [22,23] used in this study are indicated in Table 1.
Table 1.
NKG2A, NKG2C, and HLA-E primers used in this study
| Primers* | 5′→3′ | Ref. |
|---|---|---|
| NKG2A-S | GCAGAGATGGATAACCAAGG | [21] |
| NKG2C-S | GAGGAACCTTCTCAGAAGTG | [21] |
| NKG2-AS | GGCCAGCAAACTCTCTTCCC | [21] |
| HLA-E-E1-S | GATCATGGTAGATGGAACCCTCCTT | [23] |
| HLA-E-E3-S | ATCTCCGAGCAAAAGTCAAATGATGCCTCTGAG | [23] |
| HLA-E-E3-AS | CGCCTCAGAGGCATCATTTGACTTTTGCTCGGA | [23] |
| HLA-E-E8-AS | AGACACAGAGGTGGACTGTTTCTCT | [22] |
NKG2-AS primer was used for polymerase chain reaction (PCR) as a common antisense primer for NKG2A-S (444 bp) or NKG2C-S (419 bp) sense primers. For DNA sequencing, PCR product (1276 bp) amplified by HLA-E-E1-S and HLA-E-E8-AS coding all coding region of HLA-E cDNA was subjected to nucleotide sequencing by HLA-E1-S, E3-S, E3-AS, or E8-AS primer.
Preparation of NK cells
RPMI 1640 (Nissui Pharmaceutical Co., Tokyo, Japan) medium supplemented with 25 mm HEPES, 2 mm l-glutamine, 1 mm non-essential amino acid, 5 × 10−2 mm 2-mercaptoethanol, 1 mm sodium pyruvate, 100 μg/l gentamycin (all from Gibco Labs, Grand Island, NY) and 10% fetal calf serum (FCS) was used in this study and designated complete medium.
PBMC were prepared from heparinized venous blood from patients with HMB-EBV-NK disease and normal individuals by centrifugation with Ficoll–Paque (Amersham Pharmacia Biotech AB, Uppsala, Sweden). The cells at the interface were collected and washed three times with complete medium. The NK cell population was separated from PBMC with CD56-specific MoAb and anti-mouse IgG-conjugated magnetic beads (Dynal Inc., Oslo, Norway). PBMC were incubated in complete medium containing 1 μg/ml anti-CD56 MoAb for 30 min at 4°C and washed three times with complete medium. The cells were mixed with anti-mouse IgG-conjugated beads at a ratio of five beads per cell, and incubated for 1 h at 4°C. The cells bound to magnetic beads were collected with a magnet. After washing three times the cells were separated from beads by 6 h cultivation with complete medium at 37°C in 5% CO2 and used as freshly isolated NK cell population.
Flow cytometric analysis
Freshly isolated PBMC were double-stained with FITC-conjugated and PE-conjugated MoAbs for 30 min at 4°C. After washing three times, the cells were analysed with a flow cytometer (FACScan; Becton Dickinson, Oxnard, CA). Lymphocyte fraction was gated to exclude monocytes.
Reverse transcriptase-polymerase chain reaction
Freshly isolated NK cells from patients with HMB-EBV-NK disease were analysed for expression of mRNAs for IL-4, IL-5, IL-10, IFN-γ, NKG2A and C, and β-actin. In some experiments NK cells from the patients and normal individuals were cultured for 5 days in complete medium supplemented with rIL-2 (50 U/ml; Genzyme, Cambridge, MA) and/or rIL-12 (10 ng/ml; PharMingen) and analysed for NKG2A and C. Total RNAs of these cells were extracted with an RNA extraction kit (RNeasy; Qiagen, Hilden, Germany). First-strand cDNA was reverse transcribed using each RNA sample and was amplified by polymerase chain reaction (PCR) using specific primers (Table 1) with an RNA PCR kit (GeneAmp RNA PCR kit; Takara Biomedicals, Osaka, Japan) according to the manufacturer's directions. PCR was run for 40 cycles with a thermal cycler (DNA amplifier; Sanyo Co., Osaka, Japan) as follows: 1·5 min at 94°C, 2·5 min at 57°C, 30 s at 72°C. The PCR products and DNA mol. wt marker VI (Boehringer Mannheim, Mannheim, Germany) were loaded in 2% agarose gels and visualized with UV exposure of 1 μg/ml ethidium bromide-staining agarose gel.
Enzyme-linked immunospot assay
Cytokine profiles of NK cells from patients with HMB-EBV-NK disease and normal subjects were examined by enzyme-linked immunospot (ELISPOT) assay as described previously [6]. One hundred microlitres of the purified form of IL-2-, IL-4-, IL-5-, IL-10- or IFN-γ-specific MoAbs dissolved at a concentration of 1 μg/ml in 0·1 m carbonate buffer (pH 9·0) were added to each well of 96-well plates (MultiScreen-HA; Millipore, Bedford, MA) and incubated at 4°C for 12 h. After incubation, plates were washed twice with PBS, blocked with PBS containing 10% FCS at 37°C for 1 h, and washed twice with PBS. Freshly isolated NK cells from the patients and normal subjects were cultured for 24 h in RPMI 1640 supplemented with 1 μg/ml concanavalin A (Con A). The cells (5 × 103/well) were further cultured overnight in antibody-coated plates at 37°C in a 5% CO2 condition. Plates were then vigorously washed 10 times with PBS and incubated with 0·5 μg/ml of biotin-conjugated MoAb (anti-IL-2, -IL-4, -IL-5, -IL-10 or -IFN-γ MoAb) in 100 μl of PBS containing 10% FCS at 37°C for 2 h. After washing five times with PBS, the plates were incubated with streptavidin-peroxidase (Boehringer Mannheim; 1:1000 in PBS containing 10% FCS) at 37°C for 1 h. After extensive washing, 100 μl of substrate (1 μg/ml of 3,3′-diaminobenzidine tetrahydrochloride containing 0·003% H2O2; Sigma Chemical Co., St Louis, MO) were added to each well and incubated at 37°C for 15 min. Developed spots were counted using a dissecting microscope.
Proliferation assay
NK cells isolated from a patient with HMB-EBV-NK disease and a normal individual by using immunomagnetic beads were precultured with rIL-2 (50 U/ml) for 5 days. The cultured NK cells were incubated in triplicate for 24 h in 96-well plates (Corning, Cambridge, MA) in 100 μl of complete medium. 3H-TdR (Amersham, Arlington Heights, IL; 1 μCi/well) was added to the culture 8 h before harvesting. The cells were harvested on glassfibre filters using a cell harvester (Cambridge Technologies, Watertown, MA) and their radio uptake was measured in a scintillation counter. Anti-CD94 or isotype-matched control MoAb was added in this assay at varying concentrations.
Cytotoxicity assay
Varying numbers of NK cells that were cultured with rIL-2 (50 U/ml) for 5 days were assayed by incubating with 1 × 104 51Cr-labelled K562 cells for 10 h at 37°C. Target K562 cells were radiolabelled by suspension at a concentration of 1 × 106 cells/ml in RPMI 1640 medium containing 200 μCi/ml Na51Cr (DuPont-New England Nuclear, Boston, MA) at 37°C for 60 min and washed three times. After incubation the radioactivity in the medium and cells was counted by a gamma counter. The percent lysis was calculated as described previously [24]. To examine the function of CD94 molecules on NK cells, 1 μg/ml anti-CD94 or isotype-matched control MoAb was added to the cytotoxicity assay.
Results
NK1-type cytokine profile of NK leukaemic cells of HMB-EBV-NK disease
CD56+ cells purified with immunomagnetic beads from PBMC of three patients with HMB-EBV-NK disease and those of three age- and sex-matched normal subjects were expanded by culturing in IL-2-supplemented medium and subjected to ELISPOT assay for IL-4, IL-5, IL-10 and IFN-γ. Representative data from a patient and a normal individual are shown in Fig. 1a. CD56+ cells apparently secreted IL-10 and IFN-γ, but not IL-4 or IL-5, suggesting that the leukaemic NK cells can be classified into NK1-type [9]. A RT-PCR study of cytokines of freshly isolated NK cells from the patient revealed that they expressed mRNA only for IFN-γ, but not for IL-4, IL-5 or IL-10 (Fig. 1b). NK cells from the other two patients also exhibited similar results. These results suggest that the NK cells may become IL-10-producing NK1 cells upon stimulation with IL-2.
Fig. 1.
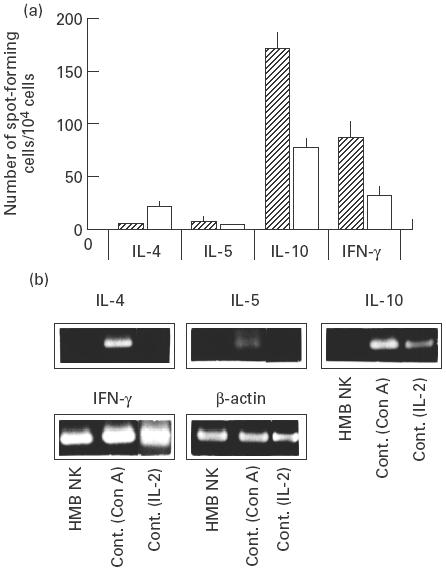
Cytokine expression of NK leukaemic cells of HMB-EBV-NK disease. (a) Purified NK cells from a patient with HMB-EBV-NK disease and a normal subject were cultured in the presence of rIL-2 (50 U/ml). The IL-4-, IL-5-, IL-10- and IFN-γ-producing capacity of each NK cell was examined by ELISPOT assay. Data are expressed as the mean ± s.e.m. of results in duplicate experiments. (b) Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed with specific primers for IL-4, IL-5, IL-10, IFN-γ, and β-actin in NK cells freshly isolated from the patient. Normal peripheral blood mononuclear cells activated with rIL-2 (50 U/ml) or concanavalin A (2 μg/ml) were used as control.
High expression of CD94 and lack of expression of p70 and NKAT2 on NK leukaemic cells of HMB-EBV-NK disease
PBMC freshly isolated from three patients with HMB-EBV-NK disease were dual-stained with PE-conjugated CD56 and FITC-conjugated CD94-, p70 KIR- or NKAT2 KIR-specific MoAb for flow cytometric analysis. As represented by the control shown in Fig. 2a, in healthy individuals approx. 40%, approx. 35% and approx. 40% of CD56+ NK cells were positive for CD94, p70 KIR and NKAT2 KIR, respectively. On the other hand, in a patient with HMB, > 98% of CD56+ NK cells were positive for CD94 and negative for p70 KIR and NKAT2 KIR, suggesting that almost all CD56+ cells were CD94+ p70− NKAT2− NK leukaemic cells and normal NK cells were scarcely present. Virtually the same data were obtained from CD56+ cells from the other two patients with HMB-EBV-NK disease, as > 98% of CD56+ cells showed high expression of CD94 and lack of expression of p70 and NKAT2 (data not shown). As summarized in Table 2, the level of CD94 expression in NK leukaemic cells of HMB-EBV-NK disease was significantly higher than that in normal NK cells as assessed by fluorescence intensity. Another type of NK LGL leukaemic cell (YS) [19] expressed CD94 at a comparable level to normal NK cells (Fig. 2b, Table 2). In contrast to CD94, p70 and NKAT2 were not expressed on HMB NK cells, which presented a striking contrast to normal NK cells but a similarity to YS cells.
Fig. 2.
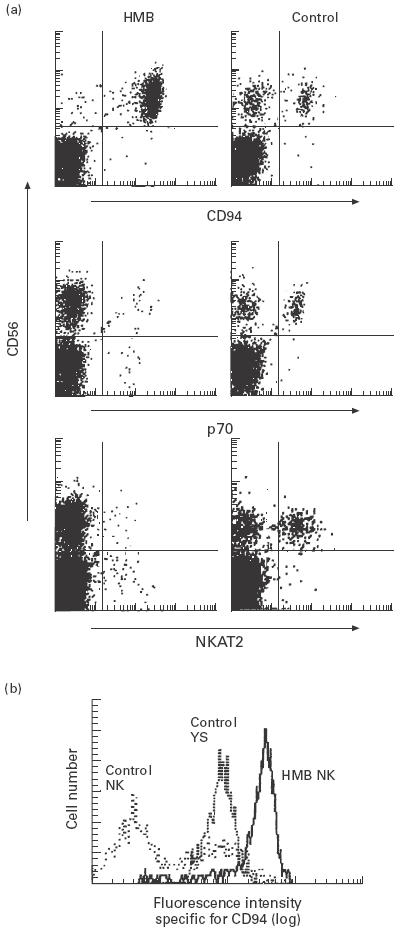
Expression of inhibitory receptors on NK leukaemic cells of HMB-EBV-NK disease. (a) Peripheral blood mononuclear cells from a patient with HMB-EBV-NK disease and a healthy individual were stained with PE-conjugated anti-CD56 MoAb and FITC-conjugated anti-CD94 MoAb, anti-p70 MoAb or anti-NKAT2 MoAb, and analysed with a flow cytometer. (b) Histogram of CD94 expression in CD56+ cell populations from a patient with HMB-EBV-NK disease (HMB), a patient with NK-large granular lymphocyte (LGL) leukaemia whose leukaemic cells were not infected with Epstein–Barr virus (EBV) (YS), and a healthy individual (control).
Table 2.
Expression of CD94, p70 and NKAT2 inhibitory molecules on hypersensitivity to mosquito bites (HMB) and control NK cells
| Mean fluorescence intensity (log)* | ||||
|---|---|---|---|---|
| NK cells | CD94 | p70 | NKAT2 | Control† |
| HMB patient 1 | 214.8 | 4.9 | 4.5 | 4.5 |
| HMB patient 2 | 120.1 | 3.7 | 4.5 | 4.1 |
| HMB patient 3 | 156.9 | 4.6 | 6.2 | 4.9 |
| YS (LGL control) | 78.8 | 4.6 | 4.8 | 4.2 |
| Healthy control 1 | 65.0 | 54.0 | 30.1 | 4.2 |
| Healthy control 2 | 46.5 | 57.9 | 48.4 | 4.7 |
| Healthy control 3 | 75.4 | 57.7 | 41.3 | 4.5 |
The expression levels of CD97, p70 and NKAT2 molecule on CD56+ cells were measured with CELLQuest software (Becton Dickinson). NK large granular lymphocyte (LGL) line (YS cells) and CD56+ cells of healthy peripheral blood mononuclear cells were used as controls.
Rat IgG MoAb was used as a control MoAb.
Vigorous expansion of NK cells from the patient's PBMC by in vitro cultivation with IL-2
After 5-day cultivation with rIL-2, PBMC from a patient with HMB-EBV-NK disease expanded more vigorously (six to seven-fold) than those from age- and sex-matched healthy individuals. It was noteworthy that 98% of the propagating cells from PBMC of the patient expressed CD56, whereas approximately 40% of CD56+ cells were present in the cultured control PBMC (Table 3). The expanded patient's CD56+ cells exhibited the same CD94+ p70− NKAT2− phenotype as freshly isolated ones (data not shown).
Table 3.
Proliferation of NK cells in the patient's peripheral blood mononuclear cells (PBMC) stimulated with rIL-2
| PBMC* | 3H-TdR incorporation (103 ct/min ± s.d.) | Percent of CD56+ cells in cultured PBMC |
|---|---|---|
| HMB patient | 72·2 ± 3·1 | 98 |
| Healthy control 1 | 13·8 ± 0·8 | 42 |
| Healthy control 2 | 14·3 ± 1·1 | 35 |
PBMC from a hypersensitivity to mosquito bites (HMB) patient or healthy individuals were cultured in complete medium supplemented with 50 U/ml rIL-2 for 5 days, and subjected to proliferation assay and flow cytometric analysis with PE-conjugated anti-CD56 MoAb.
High expression of NKG2 mRNA in NK leukaemic cells
CD94 molecules strictly bind with NKG2 molecules, which are subdivided into A to F, and CD94/NKG2A and CD94/NKG2C complexes transmit inhibitory and activation signals, respectively, into NK cells [16,18]. Since NK cells of HMB-EBV-NK disease bore high amounts of CD94 on their surface, RT-PCR was performed to examine the expression of NKG2A and C molecules in these cells. Freshly isolated NK leukaemic cells already expressed both mRNAs for NKG2A and C molecules, whereas mRNAs for these NKG2 molecules were undetectable in fresh NK cells from healthy controls (Fig. 3). When cultured in the presence of IL-2, normal NK cells could produce mRNA for NKG2C and a barely perceptible amount of mRNA for NKG2A. Moreover, the combined addition of IL-2 and IL-12 stimulated NK cells to remarkably express NKG2A as well as NKG2C. In the NK leukaemic cells, NKG2A expression was augmented by IL-2 and more markedly by a combination of IL-2 and IL-12. The expression of NKG2C in the NK leukaemic cells seemed to be already at maximal level even before stimulation with these cytokines. CD94 expression on either NK cells of HMB-EBV-NK disease or normal individuals was not affected by stimulation with IL-2 and/or IL-12 or Con A (data not shown). Taken together these data suggest that NK leukaemic cells of HMB-EBV-NK disease were in an activated state as estimated by the expression of NKG2 molecules.
Fig. 3.
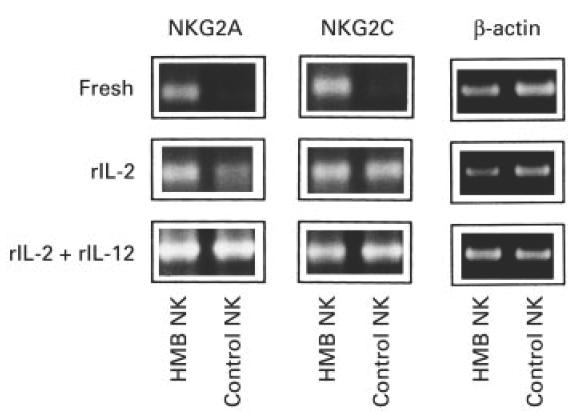
Expression of NKG2A and C molecules in NK leukaemic cells of HMB-EBV-NK disease. Freshly isolated, rIL-2-cultured, or rIL-2 + rIL-12-cultured NK cells from a patient with HMB-EBV-NK disease or a healthy control were subjected to reverse transcriptase-polymerase chain reaction with primers specific for NKG2A or C.
Functional expression of CD94 molecules on NK leukaemic cells
To test whether CD94 molecules highly expressed on NK leukaemic cells of HMB-EBV-NK disease are functional, we examined the in vitro effects of addition of anti-CD94 MoAb, which is capable of transducing inhibitory signals [25]. In the cytotoxicity assay against NK-sensitive K562 target cells, both NK cells of HMB-EBV-NK disease and normal NK cells vigorously lysed K562. Their cytotoxicities were abrogated by the addition of anti-CD94 MoAb, but not control MoAb, in a dose-dependent fashion (Fig. 4). Similarly, the proliferation of NK cells of HMB-EBV-NK disease and a normal subject, assessed by the incorporation of 3H-TdR, was also reduced dose-dependently by anti-CD94 MoAb (Fig. 4). Thus, the NK leukaemic cells expressed functional CD94 inhibitory receptors.
Fig. 4.
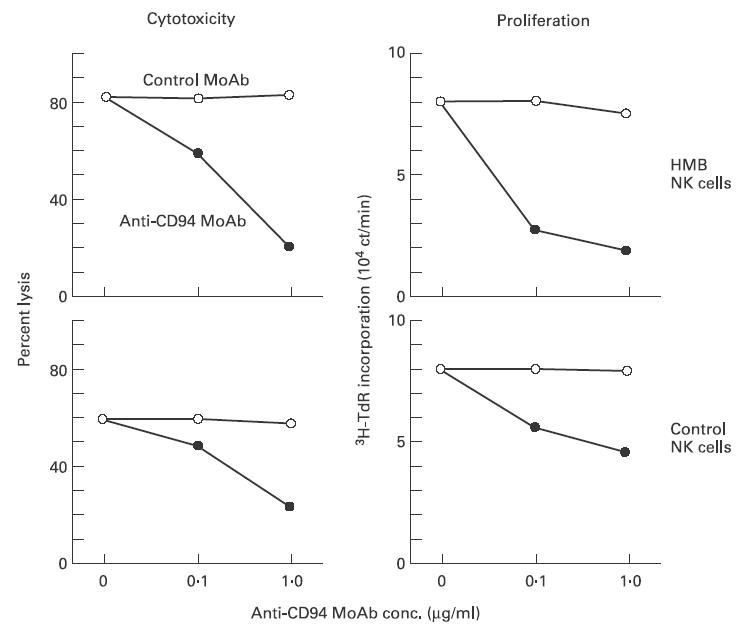
Expression of functional CD94 molecules on NK leukaemic cells of HMB-EBV-NK disease. Anti-CD94 or control MoAb was added at the indicated concentrations in the K562 cytotoxicity assay and the proliferation assay of IL-2-precultured and NK cells from a patient with HMB-EBV-NK disease and a control individual.
Normal expression and absence of mutation of HLA-E molecules in a patient with HMB-EBV-NK disease
CD94/NKG2 complex recognizes non-classical HLA-E molecules [16,17]. The expression level of HLA-E molecules strictly correlates with the total amounts of HLA-A, B, and C proteins, since the stable surface expression of HLA-E depends on the amount of leader peptide derived from classical HLA molecules [26]. Therefore, one possible explanation for the high expression of CD94 in NK cells of patients with HMB-EBV-NK disease is that decreased expression of HLA-E or mutated HLA-E molecules leads to up-regulation of CD94 expression. Flow cytometric and RT-PCR studies however, revealed that PBMC from patients with HMB-EBV-NK disease expressed HLA class I molecules at a comparable level to those from normal individuals, and HLA-E cDNA from the patients could be amplified at the same level as that from healthy controls (data not shown). In addition, nucleotide sequences of total length of the patient's HLA-E cDNAs were identical to normal healthy individuals and previously identified HLA-E cDNA clones, JTW-15 [27,28]. Therefore, these results demonstrate that the HLA-E molecule of patients with HMB-EBV-NK disease is normal in terms of its expression and sequence, suggesting that abnormality of HLA-E is not a mechanism of the high expression of CD94 molecules.
Discussion
In this study we further characterized NK leukaemic cells of HMB-EBV-NK disease in two respects: the profile of cytokine production, and the expression pattern of inhibitory receptors. It has been reported that NK cells differentiate into IFN-γ- and IL-10-producing NK1-type cells after cultivation with IL-12 [9]. In our patients with HMB-EBV-NK disease, freshly isolated leukaemic cells spontaneously produced IFN-γ. After cultivation with IL-2, the leukaemic cells produced both IFN-γ and IL-10. Therefore, the leukaemic cells of HMB-EBV-NK disease can be classified into NK1-type cells. The NK leukaemic cells were functional, because they had a cytotoxic activity against NK cell-sensitive target cells. It is speculated that the NK leukaemic cells may secrete NK1 cytokines when patients are bitten by a mosquito, and after resolution of symptoms the cells may again produce IFN-γ alone.
The expression of CD94 lectin-like dimers and that of KIR on the leukaemic NK cells was abnormal in the following three respects. First, CD94 was highly expressed on the NK cells. The level of CD94 expression in the NK cells of HMB-EBV-NK disease was greatly higher than that of the EBV-non-infected NK-LGL leukaemic cells. We found that this expression is functional in transducing inhibitory signals, because their cytotoxicity and proliferation were inhibited by anti-CD94 MoAb. This suggests that the cells can be down-modulated when HLA-E binds to CD94.
The mechanism underlying the high expression of CD94 is speculative. The NK1 nature of the NK cells raises the possibility that the released cytokines affect the expression of CD94. For example, IL-10 down-regulates classical MHC class I expression [29]. Since the expression levels of classical HLA class I and HLA-E molecules are correlated with each other [26], IL-10 produced by the NK leukaemic cells potentially inhibits HLA-E expression, which may lead to the high expression of CD94 by relaxing possible feedback suppression. Alternatively, the patients might have an abnormality in HLA-E. A nucleotide sequence analysis of HLA-E gene from a patient revealed two nucleotide substitutions in exon 2 coding α1 domain, compared with the previously described cDNA sequence of HLA-E (HLA-6.2 [23]). However, HLA-6.2 was constructed with genomic DNA of B cell line derived from an American [23], and these mutations were found commonly among Japanese controls and a cell line derived from a Japanese patient [27]. Therefore, the contribution of these mutations toward the function of HLA-E is completely neglected, indicating the absence of a pathogenic role of HLA-E in the high expression of CD94. Finally, EBV infection possibly augments CD94 expression via unknown mechanisms.
Second, the NK leukaemic cells expressed high levels of NKG2. mRNA for NKG2C was markedly expressed in freshly isolated NK leukaemic cells even when stimulated with IL-2 or IL-12, while the expression of NKG2A mRNA became more apparent after cultivation with the cytokines. Since CD94/NKG2A or C complex inhibits or activates the cellular kinetics of NK cells, respectively [16,18], one can assume that the NK cells are in an activated state despite the high expression of CD94. The fact that normal NK cells expressed mRNAs for NKG2A and C upon stimulation with IL-2 and/or IL-12 further supports that highly expressed NKG2 is a marker for activated NK cells.
The third feature in the NK leukaemic cells is the lack of expression of p70 and NKAT2. Because inhibitory signalling via p70 and NKAT2 seems not to operate, the cells are considered to be in an activated condition. The absence of p70 and NKAT2, accompanied with high expression of CD94, was not found in NK cells separated from healthy individuals. This polarized expression of the inhibitory receptors provides an intriguing characteristic for the NK leukaemic cells in HMB-EBV-NK disease.
The NK leukaemic cells share functional features with normal NK cells, as they had cytolytic ability, NK1 cytokine profile, and susceptibility to IL-2 and/or IL-12. This implies that HMB is mediated directly or indirectly by NK leukaemic cells. However, they differ from normal cells in the high expression of CD94/NKG2 complex and the lack of p70 and NKAT2. Upon mosquito biting, the disordered regulation of the inhibitory receptors may induce strong NK cell activation and result in exaggerated local and systemic reactions. Our findings are important not only for exploration of the pathomechanism of HMB-EBV-NK disease but also for utilization of CD94, p70 and NKAT2 as phenotypic markers in the diagnosis of this unique NK cell leukaemia.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science, and Culture of Japan. We gratefully acknowledge Dr Yoshitaka Nakao (Department of Hematology, Higashi Osaka City Hospital) for supplying the blood sample.
REFERENCES
- 1.Tokura Y, Tamura Y, Takigawa M, Koide M, Satoh T, Sakamoto T, Horiguchi D, Yamada M. Severe hypersensitivity to mosquito bites associated with natural killer cell lymphocytosis. Arch Dermatol. 1990;126:362–8. [PubMed] [Google Scholar]
- 2.Ishihara S, Ohshima K, Tokura Y, et al. Hypersensitivity to mosquito bites conceals clonal lymphoproliferation of Epstein–Barr viral DNA-positive natural killer cells. Jpn J Cancer Res. 1997;88:82–87. doi: 10.1111/j.1349-7006.1997.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuki M, Ueda S, Tagawa S, et al. Natural killer cell-derived large granular lymphocyte lymphoma of lung developed in a patient with hypersensitivity to mosquito bites and reactivated Epstein–Barr virus infection. Am J Hematol. 1998;59:309–15. doi: 10.1002/(sici)1096-8652(199812)59:4<309::aid-ajh7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Tokura Y, Ishihara S, Ohshima K, Hidano A, Koide M, Seo N, Takigawa M. Severe mosquito bite hypersensitivity, natural killer cell leukaemia, latent or chronic active Epstein–Barr virus infection and hydroa vacciniforme-like eruption. Br J Dermatol. 1998;138:905–6. doi: 10.1046/j.1365-2133.1998.02236.x. [DOI] [PubMed] [Google Scholar]
- 5.Kos FJ, Engleman Eg. Immune regulation: a critical link between NK cells and CTLs. Immunol Today. 1996;17:174–6. doi: 10.1016/0167-5699(96)80616-5. [DOI] [PubMed] [Google Scholar]
- 6.Seo N, Tokura Y, Furukawa F, Takigawa M. Down-regulation of tumoricidal NK and NK T cell activities by MHC Kb molecules expressed on Th2-type γδ T and αβ T cells coinfiltrating in early B16 melanoma lesions. J Immunol. 1998;161:4138–45. [PubMed] [Google Scholar]
- 7.Seo N, Tokura Y. Down-regulation of innate and acquired anti-tumor immunity by bystander γδ and αβ T lymphocytes with Th2 or Tr1 cytokine profile. J Interferon Cytokine Res. 1999;19:555–61. doi: 10.1089/107999099313686. [DOI] [PubMed] [Google Scholar]
- 8.Seo N, Tokura Y, Takigawa M, Egawa K. Depletion of IL-10- and TGF-β-producing regulatory γδ T cells by administering a daunomycin-conjugated specific mAb in early tumor lesions augments the activity of CTLs and NK cells. J Immunol. 1999;163:242–9. [PubMed] [Google Scholar]
- 9.Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–4. [PubMed] [Google Scholar]
- 10.Long EO, Burshtyn DN, Clark WP, Peruzzi M, Rajagopalan S, Rojo S, Wagtmann N, Winter Cc. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135–44. doi: 10.1111/j.1600-065x.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 11.Lanier Ll. NK cell receptors. Ann Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 12.Vetvicka V, Hanikyrova M, Vetvickova J, Ross Gd. Regulation of CR3 (CD11b/CD18)-dependent natural killer (NK) cell cytotoxicity by tumour target cell MHC class I molecules. Clin Exp Immunol. 1999;115:229–35. doi: 10.1046/j.1365-2249.1999.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakker AB, Phillips JH, Figdor CG, Lanier Ll. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, γδ T cells, and antigen-specific CTL. J Immunol. 1998;160:5239–45. [PubMed] [Google Scholar]
- 14.Kim J, Chwae YJ, Kim MY, Choi IH, Park JH, Kim Sj. Molecular basis of HLA-C recognition by p58 natural killer cell inhibitory receptors. J Immunol. 1997;159:3875–82. [PubMed] [Google Scholar]
- 15.Peruzzi M, Wagtmann N, Long Eo. A p70 killer cell inhibitory receptor specific for several HLA-B allotypes discriminates among peptides bound to HLA-B*2705. J Exp Med. 1996;184:1585–90. doi: 10.1084/jem.184.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyington JC, Riaz AN, Patamawenu A, Coligan JE, Brooks AG, Sun Pd. Structure of CD94 reveals a novel C-type lectin fold: implications for the NK cell-associated CD94/NKG2 receptors. Immunity. 1999;10:75–82. doi: 10.1016/s1074-7613(00)80008-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty De. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95:5199–204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier LL, Corliss B, Wu J, Phillips Jh. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 19.Tagawa S, Hatakeyama M, Shibano M, Taniguchi T, Kitani T. The expression of the p75 subunit of interleukin 2 receptor in Tac negative leukemic cells of two patients with large granular lymphocytic leukemia. Blood. 1988;71:1161–4. [PubMed] [Google Scholar]
- 20.Seo N, Tokura Y, Matsumoto K, Furukawa F, Takigawa M. Tumor-specific cytotoxic T lymphocyte activity in Th2–type Sezary syndrome: its enhancement by interferon-gamma (IFN-γ) and IL-12 and fluctuations in association with disease activity. Clin Exp Immunol. 1998;112:403–9. doi: 10.1046/j.1365-2249.1998.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houchins JP, Yabe T, McSherry C, Bach Fh. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173:1017–20. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiszer D, Ulbrecht M, Fernandez N, Johnson JP, Weiss EH, Kurpisz M. Analysis of HLA class Ib gene expression in male gametogenic cells. Eur J Immunol. 1997;27:1691–5. doi: 10.1002/eji.1830270715. [DOI] [PubMed] [Google Scholar]
- 23.Koller BH, Geraghty DE, Shimizu Y, DeMars R, Orr Ht. HLA-E. A novel HLA class I gene expressed in resting T lymphocytes. J Immunol. 1988;141:897–904. [PubMed] [Google Scholar]
- 24.Seo N, Egawa K. Suppression of cytotoxic T lymphocyte activity by γ/δ T cells in tumor-bearing mice. Cancer Immunol Immunother. 1995;40:358–66. doi: 10.1007/BF01525386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Villar J, Melero I, Rodriguez A, et al. Functional ambivalence of the Kp43 (CD94) NK cell-associated surface antigen. J Immunol. 1995;154:5779–88. [PubMed] [Google Scholar]
- 26.O'callaghan CA, Bell Ji. Structure and function of the human MHC class Ib molecules HLA-E, HLA-F and HLA-G. Immunol Rev. 1998;163:129–38. doi: 10.1111/j.1600-065x.1998.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno S, Trapani JA, Koller BH, Dupont B, Yang Sy. Isolation and nucleotide sequence of a cDNA clone encoding a novel HLA class I gene. J Immunol. 1988;140:4024–30. [PubMed] [Google Scholar]
- 28.Boyson JE, McAdam SN, Gallimore A, Golos TG, Liu X, Gotch FM, Hughes AL, Watkins Di. The MHC E locus in macaques is polymorphic and is conserved between macaques and humans. Immunogenet. 1995;41:59–68. doi: 10.1007/BF00182314. [DOI] [PubMed] [Google Scholar]
- 29.Kundu N, Fulton Am. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I, and enhances NK lysis. Cell Immunol. 1997;180:55–61. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]


