Abstract
Data are limited regarding serum concentrations of soluble CD14 (sCD14), a marker of macrophage activation, in patients with active tuberculosis (TB) and during drug treatment. In this study, concentrations of sCD14 were measured in serum samples obtained from 105 African subjects who were categorized into one of four groups: persons with pulmonary TB alone (TB+HIV−, n = 30), pulmonary TB and HIV co-infection (TB+HIV+, n = 20), or HIV infection alone (TB−HIV+, n = 25), and healthy controls (TB−HIV−, n = 30). Mean total sCD14 was significantly increased in serum of patients with newly diagnosed pulmonary TB (mean = 6·6 g/ml, s.d. = 1·6 g/ml) compared with healthy controls (mean = 3·1 g/ml, s.d. = 0·6 g/ml; P < 0·0001), and this elevation comprised proportionate increases in the α (2·1-fold greater, P < 0·0001) and β (2·0-fold greater, P < 0·0001) forms of sCD14. Total sCD14 was also increased in serum of HIV-infected patients (mean = 4·1 g/ml, s.d. = 1·9 g/ml; P < 0·01), but the highest concentrations were observed in patients with pulmonary TB and HIV co-infection (mean = 8·7 g/ml, s.d. = 3·1 g/ml; P < 0·0001). Analysis of serum samples prospectively collected from TB+HIV− patients during the first 3 months of successful anti-TB treatment demonstrated steep reductions in mean concentrations of the acute-phase protein, C-reactive protein, and the soluble lymphocyte activation marker, sCD25. In contrast, levels of sCD14 increased during the first month of treatment and slowly declined thereafter. These data indicate that the serum concentration of sCD14 is not a sensitive index of response to anti-TB treatment and suggest that cellular activation resolves more slowly in the macrophage pool compared with the lymphocyte pool during anti-TB treatment.
Keywords: CD14, macrophage, tuberculosis, HIV, immune activation
Introduction
CD14 is expressed principally by monocytes and macrophages and plays an important role in mounting the host response to bacterial pathogens (reviewed in [1]). Membrane-associated CD14 (mCD14) acts as a high-affinity cell surface receptor for lipopolysaccharide (LPS) from Gram-negative bacteria and also for cell wall components of Gram-positive organisms. Engagement of this receptor leads to potent cellular activation and release of immune and proinflammatory mediators [2–4]. Membrane-associated CD14 is also shed from cells as soluble CD14 (sCD14), which binds LPS and mediates activation of both mCD14+ and mCD14− cells [5–8]. Thus, in addition to mCD14, sCD14 may have an important regulatory role in the host response to bacterial endotoxins. Soluble CD14 is shed in two forms—a low molecular weight (48 kD) α form (sCD14α) and a high molecular weight (56 kD) β form (sCD14β) [6,7]. No functional distinction between the two forms has been identified, and the effects of different diseases on their relative expression have not been described.
Increasing evidence suggests that mCD14 and sCD14 are important in the host response to Mycobacterium tuberculosis, serving as receptors for lipoarabinomannan (LAM), a major mycobacterial cell wall component [4,9]. In the same way as LPS, LAM interacts with both mCD14 and sCD14, leading to activation of macrophages, release of proinflammatory cytokines, and up-regulation of cell adhesion molecules in vitro[10]. Furthermore, in vivo, concentrations of sCD14 are increased in both bronchoalveolar lavage fluid (BALF) [11] and serum samples [12] obtained from patients with pulmonary tuberculosis (TB) prior to treatment.
Previous work has explored the prognostic value of serum concentrations of sCD14 in various disease states, including leukaemia, sarcoidosis, extrinsic allergic alveolitis, septicaemia, burns, polytrauma, and HIV infection [13–18]. In patients with pulmonary sarcoidosis, serum concentrations of sCD14 correlate with disease activity, lung functional impairment, and response to therapy, suggesting a possible role as a prognostic marker in patients with this disease [15]. Serum sCD14 concentrations correlate with the acute-phase response in patients with trauma, burns, and septicaemia [16] and with disease progression in HIV-infected patients [18]. However, data regarding concentrations of sCD14 levels in serum of patients with TB prior to and during treatment are limited, and the potential use of serum sCD14 as a prognostic marker during anti-TB treatment has not been investigated.
In this study we measured concentrations of total sCD14, sCD14α and sCD14β in serum samples obtained from HIV− patients with newly diagnosed pulmonary TB in West Africa. Also, in view of the importance of macrophage activation and proinflammatory cytokine secretion in the co-pathogenesis of TB and HIV infection, we measured serum levels of sCD14 in patients with pulmonary TB who were co-infected with HIV [19–21]. Further analysis of serum samples prospectively collected from patients during anti-TB treatment enabled us to investigate in detail whether concentrations of sCD14, a marker of macrophage activation, correlate with response to drug treatment and with the changes in activation of the lymphocyte cell pool.
PATIENTS and METHODS
Patients
Fifty adult patients with newly diagnosed smear-positive pulmonary TB were recruited at the Chest Clinic at the Komfo Anokye Teaching Hospital in Kumasi, Ghana, West Africa. Suspected diagnoses of pulmonary TB were confirmed by positive Ziehl–Neelson staining of two or more sputum samples. Details of age, sex, height, and weight were recorded. Chest radiographs were performed on all patients and the extent of their disease was assessed. HIV-1/HIV-2 serology was performed with Wellcozyme ELISA (Murex Diagnostics Ltd, Dartford, UK). Thirty consecutive HIV− patients (TB+HIV−) and 20 consecutive HIV+ patients (TB+HIV+) with TB were enrolled.
A group of 25 HIV+ patients who did not have TB (TB−HIV+) wa also recruited. Fifteen of these patients were asymptomatic women (CDC stage A) attending an antenatal clinic, and 10 were patients attending a sexually transmitted diseases clinic who had HIV-related symptoms but had no current opportunistic infection nor a history of previous AIDS-defining illness (CDC stage B). Thirty HIV− blood donors (TB−HIV−) were also enrolled as healthy controls following medical review.
At the time of enrolment in the study, blood samples were collected from all study participants in pyrogen-free collection tubes and sera were quickly separated and stored at −80°C. Subsequent serum samples were obtained after 1, 2 and 3 months of anti-TB treatment in TB+HIV+ (n = 10) and TB+HIV− (n = 10) groups of patients. Treatment included an intensive phase of daily streptomycin, rifampicin, pyrazinamide and isoniazid for 2 months, followed by a continuation phase of daily isoniazid combined with either thiaocetazone (HIV− patients) or ethambutol (HIV+ patients). Sputum smears of all patients were assessed at 2 and 3 months of treatment by technicians blinded to the clinical status of the patients.
The study was approved by the Committee on Human Research, Publications and Ethics of the School of Medical Sciences, the University of Science & Technology, Kumasi, Ghana, West Africa, and all subjects gave informed consent.
Measurement of sCD14, sCD25 and C-reactive protein
The concentrations of total sCD14 in serum samples were measured by a commercially available enzyme immunoassay (EIA; Medgenix, Fleurus, Belgium) following the manufacturer's protocol. Alpha and β forms of sCD14 were determined as described previously [7]. In brief, each serum sample was independently analysed three times by Western blot using the CD14-specific MoAb MY4 (Coulter, Luton, UK) and then visualized by enhanced chemiluminescence with horseradish peroxidase (HRP)-conjugated sheep anti-mouse immunoglobulin (Amersham, Aylesbury, UK). Luminescence was detected by short exposure of the blots on Hyperfilm MP (Amersham) films. The sCD14 α and β polypeptide bands were quantified by laser densitometric scanning (BioRad Labs, Hemel Hempstead, UK) and the average of two analyses was taken. All readings fell within the linear range of the assay established in preliminary experiments. The absolute levels of the α and β forms were then calculated from the total sCD14 serum measurements.
C-reactive protein (CRP), an acute-phase protein, was measured by EIA using Virgo CRP 150 Kit (Hemagen Diagnostics Inc., Waltham, MA). Soluble CD25 (sCD25), the α subunit of the IL-2 receptor, was measured by ELISA (R&D Systems Inc., Minneapolis, MN).
Statistical analysis
Prism version 2.0 software (GraphPad Software Inc., San Diego, CA) was used for the analysis. All variables were found to approximate to a normal distribution. Changes in variables between two time points was analysed using t-tests. Results were regarded as statistically significant if P ≤ 0·05.
Results
Patient characteristics
Of the 50 patients with smear-positive pulmonary TB, 30 were male and 20 were female; their mean age was 31·6 years (s.d. = 8·6 years). Patients typically had advanced disease with consolidation involving a median of four out of six radiographic zones (range 2–6), and cavitation was seen in 37 (74%). The mean body mass indices of the TB+HIV− and TB+HIV+ patients were 16·1 kg/m2 (s.d. = 1·41) and 15·4 kg/m2 (s.d. = 2·3), respectively.
Increased serum concentrations of sCD14 in patients with TB, HIV and TB/HIV co-infection
There was a 2·1-fold greater mean serum concentration of sCD14 in the TB+HIV− group (mean = 6·6 μg/ml, s.d. = 1·6) compared with healthy controls (mean = 3·1 μg/ml, s.d. = 0·6; P < 0·0001) (Fig. 1). Although the mean serum sCD14 concentration in the TB−HIV+ group was also significantly elevated (mean = 4·1 μg/ml, s.d. = 1·9) compared with healthy controls (P < 0·01), the patients with CDC stage A disease (n = 15) had a mean level comparable to that of healthy controls (mean = 2·9 μg/ml, s.d. = 0·8), while symptomatic patients (CDC stage B, n = 10) had increased levels (mean = 5·6 μg/ml, s.d. = 1·8; P < 0·0001). However, the most highly elevated mean serum sCD14 concentration was seen in the TB+HIV+ group (mean = 8·7 μg/ml, s.d. = 3·1), a level 2·8-fold higher than that in the healthy controls (P < 0·0001).
Fig. 1.
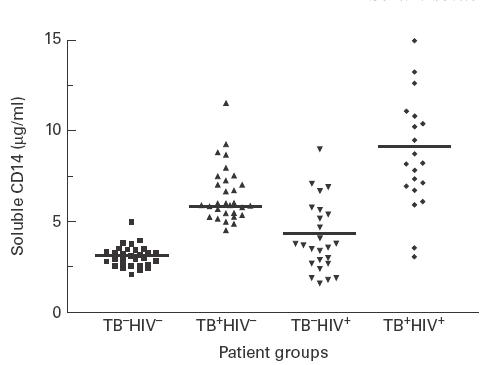
Serum concentrations of sCD14 (μg/ml) in healthy controls (TB−HIV−, n = 30) and in patients with pulmonary TB alone (TB+HIV−, n = 30), HIV infection alone (TB−HIV+, n = 25), or pulmonary TB and HIV co-infection (TB+HIV+, n = 20) at diagnosis. Mean values for each patient group are indicated by a bar.
TB enhances expression of both the α and β forms of sCD14
To determine whether the α and β forms of sCD14 are differentially up-regulated in patients with active TB, we compared concentrations of both molecules in serum samples of patients with TB and healthy controls (Table 1). Of the two species, sCD14α was present in higher concentrations (> 2-fold) in healthy controls as well as in patients with TB. More importantly, higher mean concentrations of sCD14α (2·1-fold greater, P < 0·0001) and sCD14β (2·0-fold greater, P < 0·0001) were present in patients with TB compared with healthy controls. However, the ratio of sCD14α:sCD14β was the same in the two groups, showing that the two forms of sCD14 are up-regulated proportionately in persons with TB.
Table 1.
Mean ± s.d. serum concentrations of total sCD14, CD14α and CD14β (μg/ml) in patients with pulmonary tuberculosis (TB+HIV−) and healthy controls (TB−HIV−)
| TB+HIV− (n = 30) mean (s.d.) | TB−HIV− (n = 30) Mean (s.d.) | P | |
|---|---|---|---|
| Total sCD14 | 6·57 (1·56) | 3·11 (0·65) | < 0·00001 |
| sCD14α | 4·62 (1·17) | 2·15 (0·48) | < 0·00001 |
| sCD14β | 1·95 (0·46) | 0·96 (0·22) | < 0·00001 |
| CD14α:CD14β | 2·4 | 2·2 | NS |
Prolonged elevation of serum sCD14 levels during anti-TB treatment
All patients with TB who were studied prospectively during drug treatment (n = 20) responded to therapy, resulting in negative staining of all sputum smears for acid-fast organisms after 3 months of therapy. Mean ± s.d. serum sCD14 concentrations in these 20 patients rose significantly during the first month of treatment (6·9 ± 0·7 μg/ml versus 7·8 ± 0·6 μg/ml; P = 0·01) but subsequently declined during the second and third months of treatment (7·8 ± 0·6 μg/ml versus 5·9 ± 0·5 μg/ml; P = 0·001). This transient increase followed by a slow decline in mean serum sCD14 concentrations was seen in both TB+HIV− (n = 10) (Fig. 2) and TB+HIV+ (n = 10) subgroups of patients. The changes in serum sCD14 concentrations from pretreatment values in these subgroups were not statistically significant, probably as a result of the small numbers of patients (n = 10) in each group. However, after 3 months of treatment mean serum concentrations of sCD14 were still significantly elevated in both the TB+HIV+ group (7·0 ± 0·9 μg/ml) and the TB+HIV− group (4·7 ± 0·9 μg/ml) compared with the TB−HIV− group (3·1 ± 0·6 μg/ml) (P < 0·001 for each).
Fig. 2.
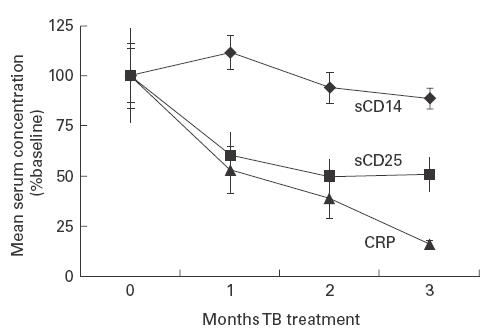
Mean ± s.e.m. serum concentrations of sCD14, sCD25 and C-reactive protein (CRP) in patients receiving treatment for pulmonary TB (TB+HIV−, n = 10). Mean serum concentrations are expressed as a percentage of the mean pretreatment concentration.
Serum sCD14, CRP and sCD25 concentrations during anti-TB treatment
In TB+HIV− patients (n = 10) receiving treatment for TB, mean serum concentrations of sCD14 were compared with changes in serum concentrations of the acute-phase protein, CRP, and sCD25 (Fig. 2). There was no significant decline in mean serum sCD14 concentration from pretreatment levels during the first 3 months of anti-TB treatment. However, mean serum CRP concentrations declined by 47·1% during the first month of treatment (84·6 ± 16·3 mg/l versus 44·8 ± 12·0 mg/l; P < 0·01) (Fig. 2) and continued to decline to near normal levels by the third month (13·5 ± 1·5 mg/l), in keeping with the microbiological and clinical response to therapy. During the first month of treatment there was also a steep decline in mean serum sCD25 concentrations from pretreatment levels (2856 ± 626 pg/ml versus 1714 ± 311 pg/ml, respectively; P < 0·01) (Fig. 2). After 2 months of treatment, mean sCD25 levels reached a plateau in the normal range (mean ± 2 s.d. serum concentration in 35 healthy West African subjects; unpublished data). Thus, serum concentrations of sCD14 were dissociated from those of CRP and sCD25 during resolution of TB.
Discussion
In this study we have confirmed and expanded findings of previous studies by demonstrating that serum concentrations of total sCD14, sCD14α and sCD14β are all elevated in patients with active TB compared with healthy controls. In addition, while serum concentrations of sCD14 were also significantly increased in patients with HIV infection, serum levels were most markedly elevated in those with TB and HIV co-infection. Furthermore, we demonstrated that rapid reductions in serum concentrations of the acute-phase protein, CRP, and the lymphocyte activation marker, sCD25, occurred during the first 3 months of anti-TB treatment, whereas levels of sCD14 were slow to decline. These data show that serum sCD14 concentrations do not provide a sensitive index of response to anti-TB treatment, and also suggest that cellular activation resolves more slowly in the macrophage pool compared with the lymphocyte pool during treatment.
Increased concentrations of sCD14 are detectable in BALF obtained from patients with active pulmonary TB [11], and our finding that sCD14 concentrations are also increased in serum of such patients confirms the findings of Juffermans et al. [12]. Since CD14 serves as a receptor for mycobacterial LAM [4,8], the increased levels of sCD14 in peripheral venous blood distant from the principal site of disease may be a response to LAM antigenaemia, which is detectable in most patients with active TB [22]. This systemic increase may represent an important component of the host response to M. tuberculosis, helping to limit systemic spread of the organism. In normal individuals, plasma concentrations of sCD14α are either similar to or, as in this study, higher than concentrations of sCD14β[7]. Our finding that α and β forms were elevated proportionately in patients with newly diagnosed TB suggests that both forms may play a role in the host response to mycobacterial disease. However, since increased shedding of sCD14 is not specific to patients with TB [13–18], the serum concentration of this soluble receptor is very unlikely to serve in a diagnostic role for this disease.
This study confirms the finding that serum concentrations of sCD14 are elevated in patients with HIV infection and that the increase is dependent upon stage of disease [18]. This increase has been attributed to progressive activation of the macrophage cellular pool associated with advancement of HIV infection [18]. However, in contrast to the findings of Juffermans et al., who studied a limited number of TB/HIV co-infected patients [12], we demonstrated that serum sCD14 concentrations are markedly increased in such patients. It is likely that this increase reflects costimulation of macrophages by M. tuberculosis and HIV; the high incidence of mycobacteraemia in HIV+ patients with TB [23] may enhance the activation of macrophages systemically. Such activation of the macrophage cell pool in HIV+ patients with mycobacterial disease leads to increased replication of HIV-1 in these cells [20,24], and macrophages may thus contribute to the increase in plasma HIV-1 load that accompanies the development of active TB in such patients [19,25]. Broad systemic activation of the immune system also occurs in patients with TB and HIV co-infection [26,27], and sCD14 may serve as an important receptor mechanism facilitating this immunological activation.
A previous cross-sectional study found that serum concentrations of sCD14 were lower in patients with TB receiving treatment compared with those with newly diagnosed disease, and that levels were normal in those that had completed curative treatment [12]. In this study we prospectively monitored changes in serum sCD14 concentration during the first 3 months of anti-TB treatment and correlated these with other parameters of disease resolution. The significant rise in serum sCD14 in these patients (TB +HIV− and TB+HIV+) after 1 month of treatment corresponds to transient increases in serum tumour necrosis factor-alpha (TNF-α) concentration previously observed at the same time point of treatment in both TB+HIV− patients [28] and TB+HIV+ patients [19]. This initial rise in TNF-α is thought to be due to release of mycobacterial antigens [28], and the coincident rise in sCD14 may represent a component of the host response to the increased antigen load, which results in up-regulation of TNF-α secretion. The slow decline in serum sCD14 concentrations that occurs during anti-TB treatment parallels similar changes in serum TNF-α in TB+HIV+ patients [19].
The rapid decline in mean serum CRP concentrations reflected the exponential decline in mycobacterial burden that occurs during the first months of anti-TB treatment [29]. In contrast, persistently elevated serum concentrations of sCD14 after 3 months of treatment indicate that, unlike CRP, sCD14 is not a sensitive correlate of the response to treatment. Although the half-life of sCD14 in the circulation has not been defined, the changes in serum concentrations of sCD14 in patients responding to anti-TB treatment suggest that macrophage activation, after initially increasing, declined slowly thereafter. In contrast, the mean concentration of sCD25, a well-characterized marker of lymphocyte activation [30], reduced sharply during treatment. This suggests that cellular activation in the lymphocyte cell pool declines more rapidly than in the macrophage pool during recovery from pulmonary TB. Slow resolution of macrophage activation may, in part, explain the persistent elevation of plasma TNF-α and HIV-1 load observed in African patients treated for pulmonary TB [19,21].
Acknowledgments
S.D.L. is funded by the Wellcome Trust, London, UK. We thank Dr Salvatore T. Butera for his helpful review of this manuscript.
REFERENCES
- 1.Ziegler-Heitbrock HWL, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–5. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- 2.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14 serves as the cellular receptor for complexes of lipopolysaccharide with lipopolysaccharide binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 3.Dentener MA, Bazil V, Von Asmuth EJU, Ceska M, Buurman WA. Involvement of CD14 in lipopolysaccharide-induced tumour necrosis factor-α, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–91. [PubMed] [Google Scholar]
- 4.Pugin J, Heumann ID, Tomasz A, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–16. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 5.Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, Wright SD. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–71. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labeta MO, Durieux JJ, Fernandez N, Herrman R, Ferrara P. Release from a human monocyte-like cell line of two different soluble forms of the lipopolysaccharide receptor, CD14. Eur J Immunol. 1993;23:2144–51. doi: 10.1002/eji.1830230915. [DOI] [PubMed] [Google Scholar]
- 7.Durieux JJ, Vita N, Popescu O, et al. The two soluble forms of the lipopolysaccharide receptor, CD14: characterization and release by normal human monocytes. Eur J Immunol. 1994;24:2006–12. doi: 10.1002/eji.1830240911. [DOI] [PubMed] [Google Scholar]
- 8.Vita N, Lefort S, Sozzani P, Reeb R, Richards S, Borysiewicz LK, Ferrara P, Labeta M. Detection and biochemical characteristics of the receptor for soluble complexes of soluble CD14 and bacterial polysaccharide. J Immunol. 1997;158:3457–62. [PubMed] [Google Scholar]
- 9.Yu W, Soprana E, Cosetino G, Volta M, Lichenstein HS, Viale G, Vercelli D. Soluble CD14 confers responsiveness to both lipoarabinomannan and lipopolysaccharide in a novel HL-60 cell bioassay. J Immunol. 1998;161:4244–51. [PubMed] [Google Scholar]
- 10.Zhang Y, Doerfler M, Lee TC, Guillemin B, Rom WN. Mechanisms of stimulation of IL-1 beta and TNF-alpha by M. tuberculosis components. J Clin Invest. 1993;91:2076–83. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoheisel G, Zheng L, Teschler H, Striz I, Costabel U. Increased soluble CD14 levels in BAL fluid in pulmonary tuberculosis. Chest. 1995;108:1614–6. doi: 10.1378/chest.108.6.1614. [DOI] [PubMed] [Google Scholar]
- 12.Juffermans NP, Verbon A, van Deventer SJH, Buurman WA, van Deutkom H, Speelman P, van der Poll T. Serum concentrations of lipopolysaccharide activity-modulating proteins during tuberculosis. J Infect Dis. 1998;178:1839–42. doi: 10.1086/314492. [DOI] [PubMed] [Google Scholar]
- 13.Wiersma SR, Ortega J, Sobel E, Weinberg KI. Clinical importance of myeloid-antigen expression in acute lymphoblastic leukemia of childhood. N Engl J Med. 1991;324:800–8. doi: 10.1056/NEJM199103213241204. [DOI] [PubMed] [Google Scholar]
- 14.Pforte A, Schiessler A, Gais P, Beer B, Strobel M, Schutt C, Ziegler-Heitbrock HWL. Increased expression of the monocyte differentiation antigen CD14 in extrinsic allergic alveolitis. Monaldi Arch Chest Dis. 1993;48:607–12. [PubMed] [Google Scholar]
- 15.Pforte A, Schiessler A, Gais P, Beer B, Ehlers M, Schutt C, Ziegler-Heitbrock HWL. Expression of CD14 correlates with lung function impairment in pulmonary sarcoidosis. Chest. 1994;105:349–54. doi: 10.1378/chest.105.2.349. [DOI] [PubMed] [Google Scholar]
- 16.Kruger C, Schutt C, Obertacke U, et al. Serum CD14 levels in polytraumatised and severely burned patients. Clin Exp Immunol. 1991;85:297–301. doi: 10.1111/j.1365-2249.1991.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landmann R, Reber M, Sansano S, Zimmerli W. Function of soluble CD14 in serum of patients with septic shock. J Infect Dis. 1996;173:661–8. doi: 10.1093/infdis/173.3.661. [DOI] [PubMed] [Google Scholar]
- 18.Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–92. [PubMed] [Google Scholar]
- 19.Lawn SD, Shattock RJ, Acheampong JW, Lal RB, Folks TM, Griffin GE, Butera ST. Sustained plasma TNF-α and HIV-1 load despite resolution of other immune activation parameters during treatment for tuberculosis in Africans. AIDS. 1999;13:2231–7. doi: 10.1097/00002030-199911120-00005. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Roberts BD, Griffin GE, Folks TM, Butera ST. Cellular compartments of HIV-1 replication: determination by virion-associated host proteins and the impact of opportunistic infection in vivo. J Virol. 2000;74:139–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Wallis RS, Nsubuga P, Whalen C, et al. Pentoxifylline therapy in human immunodeficiency virus-seropositive persons with tuberculosis: a randomized, controlled trial. J Infect Dis. 1996;174:727–33. doi: 10.1093/infdis/174.4.727. [DOI] [PubMed] [Google Scholar]
- 22.Sada E, Aguilar D, Torres M, Herrera T. Detection of lipoarabinomannan as a diagnostic test for tuberculosis. J Clin Microbiol. 1992;30:2415–8. doi: 10.1128/jcm.30.9.2415-2418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilks CF, Brindle RJ, Otieno LS, et al. Extrapulmonary and disseminated tuberculosis in HIV-1 seropositive patients presenting to the acute medical services in Nairobi. AIDS. 1990;4:981–5. doi: 10.1097/00002030-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–61. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 25.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 26.Vanham G, Edmonds K, Qing L, et al. Generalized immune activation in pulmonary tuberculosis: coactivation with HIV infection. Clin Exp Immunol. 1996;103:30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallis RS, Vjecha M, Amir-Tahmassch M, et al. Influence of tuberculosis on human immunodeficiency virus (HIV-1): enhanced cytokine expression and elevated β2-microglobulin in HIV-1-associated tuberculosis. J Infect Dis. 1993;167:43–48. doi: 10.1093/infdis/167.1.43. [DOI] [PubMed] [Google Scholar]
- 28.Bekker LG, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178:580–4. doi: 10.1086/517479. [DOI] [PubMed] [Google Scholar]
- 29.Toman K. Tuberculosis: case-finding and chemotherapy. Geneva: WHO; 1979. p. 91. [Google Scholar]
- 30.Rubin LA, Kurman CC, Fritz ME, Biddison WE, Boutin B, Yarchoan R, Nelson DL. Soluble interleukin-2-receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135:3172–7. [PubMed] [Google Scholar]


