Abstract
Neuroendocrine peptides have a variety of physiological functions in the gastrointestinal tract. This study was carried out to investigate the impact of IL-2 deficiency on the neuroendocrine system in normal colon, and the neuroendocrine changes during colonic inflammation. Mice with homozygous disrupted IL-2 gene (IL-2−/–) spontaneously developed a bowel disease with similarities to human ulcerative colitis. Different types of colonic endocrine cells and myenteric nerves were analysed in the IL-2−/– mice using immunomorphometry. The neuropeptide contents in the colonic tissues were determined by radioimmunoassay. Age-matched healthy IL-2+/– and IL-2+/+ mice served as controls and the colonic IL-2 levels were compared between these two groups of mice by ELISA. Our data showed that less than half the amount of IL-2 was synthesized in the colon of IL-2+/– mice compared with the IL-2+/+ wild-type mice. Two major differences in the neuroendocrine colon were found between the mice with an intact and disrupted IL-2 gene. One was age-related. The frequencies of various endocrine cells and myenteric nerves increased with age in the IL-2+/+ mice. However, no such increases were seen in the mice with a disrupted IL-2 gene. Instead, the volume densities of enteroglucagon, serotonin cells and substance P (SP), vasoactive intestinal polypeptide (VIP) and total myenteric nerves were lower in the older IL-2+/– and IL-2−/– mice compared with the wild type. The other was disease-related. Polypeptide YY (PYY) cells and tissue levels of PYY, SP and VIP were significantly decreased in the IL-2−/– mice during the course of bowel inflammation compared with the healthy IL-2+/– and IL-2+/+ controls. These findings indicate that colonic neuroendocrine alterations did occur in the mice with a disrupted IL-2 gene and diminished local IL-2 level, suggesting a role of IL-2 in the regulation of the neuroendocrine system and a prevalent interaction between the immune and neuroendocrine systems in normal colon. On the other hand, there were some changes that seemed to correlate with the bowel inflammatory process. They might be associated with the impaired function in inflamed gut and contribute to the development and/or prolongation of disease.
Keywords: IL-2, gene knock-out, colonic inflammation, neuroendocrine system, mice
Introduction
Ulcerative colitis (UC) and Crohn's disease (CD) constitute the two main forms of chronic inflammatory bowel disease (IBD). The aetiology of both diseases still remains largely unknown. It is likely that neuroendocrine-immune mechanisms play an important role in the inflammatory process [1–5]. It has been suggested that UC is caused by a failure to maintain homeostasis with the normal gut flora, leading to an aberrant and uncontrolled immune response to one or a few normally occurring gut constituents [6,7]. The inflammation may be exacerbated and perpetuated by an autoimmune response against colonic antigens. Autoantibodies directed against colonic components [8] and immunological cross-reactivity of these autoantibodies with certain microbial antigens [9] have been demonstrated in UC. More recently, autoantibodies directed against intracellular components of epithelial cells [10], neutrophils [11], and antibodies against intestinal bacteria [12] have been reported to be produced in UC colon.
The gastrointestinal neuroendocrine peptides have potent modulatory activities on motility, absorption, secretion, epithelial cell proliferation, adaptation and probably also on the intestinal immune responses [13–17]. Abnormalities of the neuroendocrine system in the gut of IBD patients have been reported during the last decades, which have led to the hypothesis of a neural involvement. The observations included increased ganglion cells [18], neural necrosis, ganglion cell and axonal degeneration [19] as well as alterations in colonic endocrine cell populations [20]. Changes in neuropeptide levels [21–24] and distribution of peptidergic nerves [21,25–28] in IBD gut have also been demonstrated. The results however have mostly been conflicting.
Several genetically manipulated mice spontaneously develop bowel inflammation and may serve as animal models for IBD. An interesting model is the IL-2 gene knock-out (IL-2−/–) mouse [29,30]. IL-2 is a key regulatory cytokine in T cell-dependent immunity and thus its absence may cause disturbed immune response and inflammation. IL-2−/– mice are normal at birth but may die between the weeks 5 and 9 of an unidentified disease which is characterized by splenomegaly, lymphadenopathy and severe haemolytic anaemia. However, the surviving mice spontaneously develop a colonic inflammation which resembles human UC in many immunological aspects [31]. Large increases in the numbers of CD4+, CD8+, αβ TCR+ and γδ TCR+ T cells can been seen in the inflamed colon of these mice. Further characterization indicates a Th1-dominated cytokine response, with an increase in interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α) and IL-1 production in combination with a decrease in IL-4 and IL-10 [32]. In human UC, the local immune response has been suggested to be of Th2 type. However, the cytokine profile of T cells in UC colon is still unresolved [33]. Mice kept in germ-free conditions do not develop this disease [31,32].
The aim of the present study was to investigate the regulatory function of IL-2 on the neuroendocrine system in the colon and the alteration of the neuroendocrine colon during inflammation. The frequencies of various endocrine cells and peptidergic myenteric nerves as well as the tissue levels of neuropeptides in the colon were examined in IL-2−/– mice with bowel disease and age-matched healthy IL-2+/– and IL-2+/+ mice.
Materials and methods
Mice
IL-2+/– mice on a C57Bl/6 background were obtained from the Jackson Laboratories (Bar Harbor, ME). They were bred in our laboratory, at a constant temperature of 21°C, 50% humidity, and light and dark cycles of 12 h. They were fed a standard pellet diet and water ad libitum. The new generations of mice were genotyped for IL-2 gene disruption by polymerase chain reaction (PCR) analysis of DNA [29]. Mice were classified as IL-2−/– (homozygous), IL-2+/– (heterozygous) or IL-2+/+ (wild type).
To compare the colonic IL-2 levels between the healthy IL-2+/– and IL-2+/+ mice by ELISA, a total of 23 mice, 12 from wild-type and 11 from heterozygous groups, aged between 20 and 29 weeks, were examined.
For the morphometric study on colonic endocrine cells and peptidergic myenteric nerves, 19 IL-2−/– mice (five with acute, and 14 with chronic disease) were investigated. Seven heterozygous and eight wild-type age-matched mice were used as two different control groups for the IL-2−/– mice with acute bowel inflammation. For the chronically diseased IL-2−/– mice, eight heterozygous and nine wild-type age-matched mice served as controls. For radioimmunoassays of the neuropeptide levels in colonic tissues, eight IL-2−/– mice with chronic disease were used. Seven heterozygous and 10 wild-type age-matched mice served as controls.
The investigation was approved by the local ethics committee on animal experiments, Northern Sweden.
Tissue specimens
Mice were killed in a CO2 chamber and the distal colon was immediately excised. The specimens were divided into two pieces: one was directly frozen in liquid nitrogen and kept at −70°C for radioimmunoassay, the other was fixed in 4% phosphate-buffered formaldehyde overnight and embedded in paraffin for histological and immunomorphometric studies. In some cases, an extra piece was taken for ELISA determinations of tissue IL-2 level.
Histological and immunohistochemical techniques
Sections (5 μm thick) were cut from the fixed tissue specimens and stained with haematoxylin–eosin for histopathological examination. Immunohistochemical demonstration of the myenteric nerves and endocrine cells was performed on 10- or 5-μm tissue sections, respectively, using the avidin-biotin complex (ABC) method as described earlier [34,35]. Immunostaining of nerves was performed after microwave antigen retrieval [36]. The various types of colonic endocrine cells were identified by polyclonal rabbit antisera against polypeptide YY (PYY), glucagon, serotonin, pancreatic polypeptide (PP) and somatostatin. Antisera against protein gene product 9.5 (PGP 9.5), substance P (SP) and vasoactive intestinal polypeptide (VIP) were used for the detection of the myenteric nerves. Details of the antisera used are given in Table 1.
Table 1.
Details of rabbit antisera used
| Antisera raised against | Working dilution | Code no. | Source |
|---|---|---|---|
| Polypeptide YY (PYY) | 1:1000 | R841303-B4 | Euro-Diagnostica, Malmö, Sweden |
| Glucagon (porcine)* | 1:1000 | R781101-B3 | Euro-Diagnostica |
| Serotonin | 1:400 | R871204-B4 | Euro-Diagnostica |
| Somatostatin | 1:2000 | A566 | Dakopatts, Glostrup, Denmark |
| Pancreatic polypeptide (PP) | 1:500 | A619 | Dakopatts |
| Protein gene product 9.5 (PGP 9.5) | 1:600 | RA95101 | Ultra Clone, Isle of Wight, UK |
| Substance P (SP) | 1:1000 | SP2–840517-B6 | Euro-Diagnostica |
| Vasoactive intestinal polypeptide (VIP) | 1:2000 | 7854/01-B5 | Euro-Diagnostica |
Cross-reacts with murine enteroglucagon.
Specificity controls were: (i) replacement of the primary antisera with non-immune rabbit serum; (ii) preincubation of the primary antisera with an excess of the corresponding or structurally related peptides (75–100 μg peptide/ml diluted antibody solution) for 24 h at 4°C; and (iii) substitution of the secondary antibody with non-immune swine serum. Sections from normal human colon processed in parallel served as positive controls, since the antisera used are known to be cross-reactive between species.
Computerized image analysis
Immunomorphometric analysis was performed using the Quantimet 500 MC image processing and analysis system (Leica, Cambridge, UK) connected to an Olympus BX50 microscope. The computer programs used were QWIN and QUIPS (an interactive system). Endocrine cells with visible nuclei were counted manually and the area of epithelial cells was measured by use of threshold setting. Twenty fields randomly chosen from three sections (at least 80 μm apart from each other) were examined for each specimen and antigen. Measurements were performed using a × 20 objective and in a frame representing an area of 0·034 mm2 of tissue. Knowing the thickness of the section (5 μm), the number of endocrine cells per mm3 of epithelium was calculated.
The relative volume densities of myenteric nerve fibres were determined using a classical stereological point-counting method adapted for computerized image analysis [36]. Briefly, a regular 400-point lattice was superimposed on a frame containing an area of 0·01 mm2 of tissue. Points covering the immunoreactive nerve fibres and the muscle tissues were counted, and the ratio was summed up automatically. Forty randomly chosen fields from each sample were analysed for each marker using a × 40 objective. All measurements were carried out by the same investigator (B.-F.Q.).
Radioimmunoassay
Forty to 140 mg thawed wet colonic tissues were boiled in 3 ml 0·5 m acetic acid, followed by homogenization and centrifugation for 20 min at 4000 rev/min. The supernatant was then aspirated and used for immunoassay [36]. The neuropeptide levels were determined using commercial competitive radioimmunoassay (RIA) kits with the antisera raised in rabbit: PYY (Peninsula, Merseyside, UK), SP and VIP (Euro-Diagnostica). The assays were performed according to the manufacturers' instructions using duplicates of undiluted, 1:2 and 1:4 diluted extracts. In brief, the standards, samples and controls were incubated with the corresponding antisera and 125I-tracer sequentially. The antibody-bound 125I-tracers were separated from the unbound fraction by the double antibody solid-phase technique in combination with centrifugation. The supernatant (free tracers) was removed and the radioactivity of the precipitates (bound tracers) was quantified with a gamma-counter.
ELISA
The tissue extracts were prepared by homogenization and sonication, and treated with mild detergent in order to get high recovery of IL-2 and still preserve the epitopes of the cytokine [37,38]. In brief, 30–120 mg wet colonic tissue specimens were placed in 3 ml of chilled PBS containing 0·25% NP-40 (Sigma Chemical Co., St Louis, MO), aprotinin (Sigma; 0·06 Ti U/ml) and ε-amino caproic acid (Sigma; 10 g/l), and homogenized. They were then sonicated for 3 min using a Branson Sonifer (Model 250/450; Danbury, CT) and kept at 4°C overnight. The supernatant was collected after centrifugation (14 000 rev/min; 15 min) and concentrated six times in a speed vacuum concentrator. The amounts of IL-2 were measured in duplicates using a Quantitative Colormetric Sandwich ELISA kit (Quantikine M mouse IL-2; R&D Systems Europe, Abingdon, UK), according to the protocol provided by the manufacturer. The encountered concentrations of the controls supplied with the ELISA kit were within the limits given.
Statistical analysis
The significance of observed differences between the groups was evaluated by two-tailed non-parametric Mann–Whitney test. Analyses of correlation between various endocrine cells or myenteric nerves and age were performed using the Spearman rank correlation test. P < 0·05 was considered statistically significant.
Results
Distribution of endocrine cells and myenteric nerve fibres in the colonic tissue
Endocrine cells were identified as cells stained by antisera against PYY, glucagon, serotonin, PP or somatostatin. A significant number of PYY-, glucagon- and serotonin-positive cells were seen in samples both from diseased and control mice, while PP- and somatostatin-containing cells were few or absent in all groups. The positively stained cells occurred mostly in the lower-middle part of the crypts and varied in shape from flask- to basket-shaped. Immunoreactive granules were observed around the nucleus, or at the basal portion of the cells opposite to the luminal pole (Fig. 1A,B).
Fig. 1.
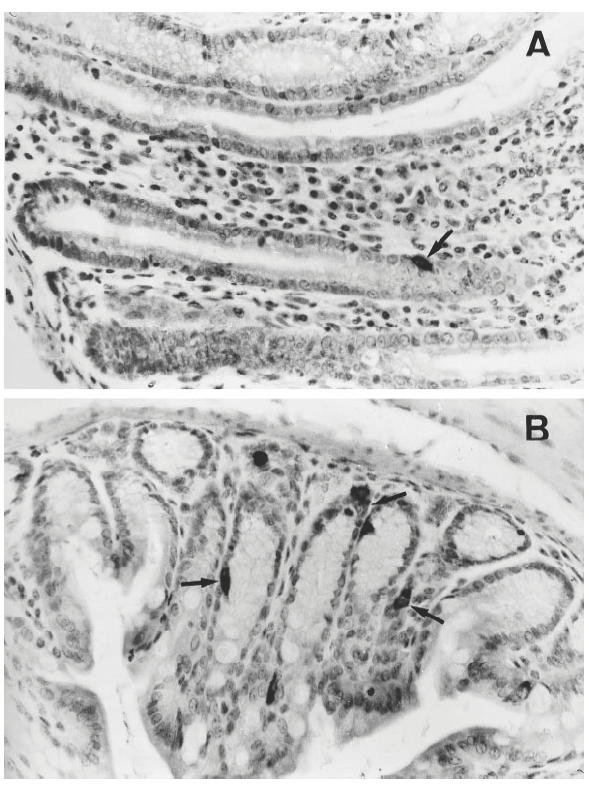
Anti-polypeptide YY (PYY) immunohistochemical staining of colonic tissues from an IL-2−/– mouse with chronic colitis (A) and a healthy IL-2+/+ control (B). The number of PYY-containing cells (arrows) was fewer in the IL-2−/– mouse compared with the IL-2+/+ mouse. Note the elongation of crypts, goblet cell depletion, and the infiltration of inflammatory cells in the lamina propria of the IL-2−/– mouse (A). (Original mag. × 400.)
Antiserum against PGP 9.5 was used to stain all types of efferent and afferent enteric nerves. The nerves immunoreactive to antisera against PGP 9.5 as well as SP and VIP were detected in all three groups of mice (IL-2−/–, IL-2+/– and IL-2+/+). These nerve fibres were abundant within the circular muscular layer but relatively sparse in the other parts of the bowel wall (Fig. 2A,B). In addition, positively stained neuronal cell bodies in the ganglions could be observed in the myenteric and submucosal plexuses.
Fig. 2.
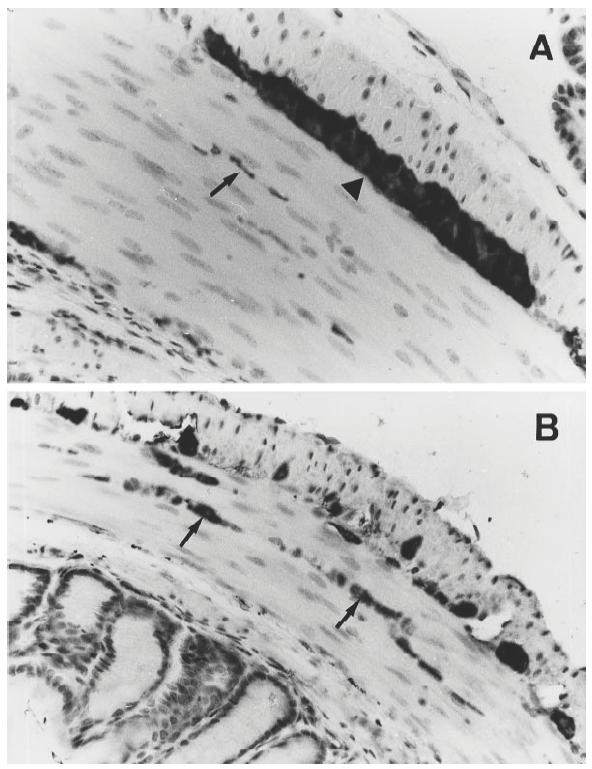
Protein gene product (PGP) 9.5-immunoreactive nerve fibres in the muscular layer of colon of an IL-2−/– mouse with chronic colitis (A) and a healthy IL-2+/+ control (B). The arrows point to the positively stained nerve fibres, and the arrowhead indicates a myenteric ganglia. Note that the frequency of the immunoreactive nerves was decreased in the IL-2−/– mouse compared with the IL-2+/+ control. (Original mag. × 400.)
Replacement of the primary or secondary antisera by non-immune rabbit or swine serum, or preincubation of the primary antisera with the corresponding peptides did not result in immunostaining, while preincubation of the primary antisera with irrelevant, structurally related peptides had no adsorptive effect. All antisera specifically stained sections from normal human colon.
Healthy IL-2+/– mice have decreased levels of IL-2 in colonic tissue
IL-2 was detected in the colonic tissue extracts of all mice analysed. The mean concentrations (± s.e.m.) of IL-2 in the IL-2+/– and IL-2+/+ mice were 78·2 ± 17·0 and 232·8 ± 48·2 pg/g colon tissue, respectively. Thus, there was a two-thirds decrease in IL-2 levels in the IL-2+/– mice compared with the IL-2+/+ mice (P = 0·009).
Colonic endocrine cells and peptidergic myenteric nerves increase in frequencies with age in IL-2+/+ but not IL-2+/– mice
The frequencies of various endocrine cells and peptidergic myenteric nerves were analysed in colonic tissues of healthy IL-2+/+ and IL-2+/– mouse littermates between 10 and 18 weeks of age. The numbers of PYY-, glucagon-, and serotonin-containing cells were all increased with increasing age in the IL-2+/+ mice. The relative volume densities of VIP-immunoreactive nerves were also increased with age in these mice, while no such changes were noted for PGP 9·5- and SP-immunoreactive nerves. In contrast, no age-related increases in the frequencies of either endocrine cells or myenteric nerves were seen in the IL-2+/– mice (Fig. 3 and Table 2).
Fig. 3.
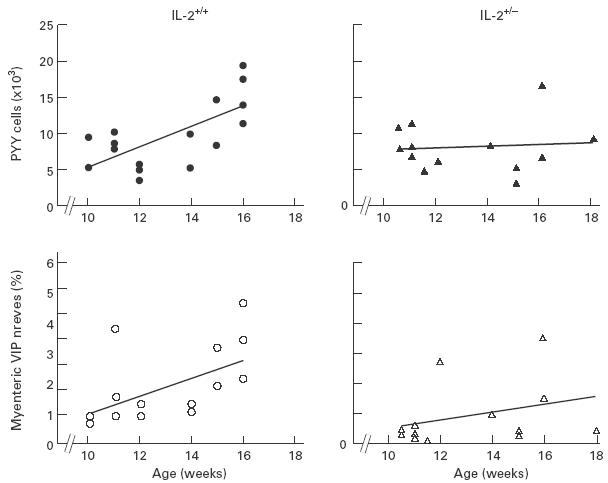
Correlation between colonic polypeptide YY (PYY)-containing endocrine cells (closed symbols), vasoactive intestinal polypeptide (VIP)-immunoreactive myenteric nerves (open symbols) and age in the IL-2+/+ (circles) and IL-2+/– mice (triangles). Values on the ordinate are the number of PYY cells per mm3 epithelium and percentage of VIP nerve fibres in the muscle tissues, respectively. Because of overlapping, some of the variables are not discernible.
Table 2.
Influence of age on the frequencies of endocrine cells and myenteric nerves in the colon of healthy IL-2+/+ and IL-2+/– mice
| Changes caused by increasing age | ||||||
|---|---|---|---|---|---|---|
| Numbers of endocrine cells expressing | Densities of myenteric nerves expressing | |||||
| PYY | Glucagon | Serotonin | PGP 9.5 | SP | VIP | |
| IL-2+/+ | Increased | Increased | Increased | Unchanged | Unchanged | Increased |
| n = 16 | rs = 0·59 | rs = 0·51 | rs = 0·57 | rs = 0·47 | rs = 0·23 | rs = 0·65 |
| (3 F, 13 M) | P = 0·02 | P = 0·04 | P = 0·02 | P = 0·006 | ||
| IL-2+/– | Unchanged | Unchanged | Unchanged | Unchanged | Unchanged | Unchanged |
| n = 13 | rs = − 0·11 | rs = 0·02 | rs = 0·02 | rs = 0·27 | rs = 0·23 | rs = 0·34 |
| (1 F, 12 M) | ||||||
PYY, Polypeptide YY; PGP 9.5, protein gene product 9.5; SP, substance P; VIP, vasoactive intestinal polypeptide; n, number of mice/group; F, female; M, male; rs, correlation coefficient as determined by Spearman rank correlation test; P, probability that the observed correlation is due to chance alone; unchanged, no significant correlation with age and P > 0·05.
The numbers of PYY-, glucagon- and serotonin-containing cells were the same in the younger mice (10–13 weeks old) of both IL-2+/– and IL-2+/+ groups (Fig. 4a,b,c). In the older mice (> 13 weeks) however, the numbers of these endocrine cells were lower in IL-2+/– than in IL-2+/+ mice (Fig. 4d,e,f). In the case of glucagon-containing cells, this difference was statistically significant (P = 0·02). With regard to the myenteric nerves, the differences were even more pronounced and the relative volume densities of PGP 9.5-, SP- and VIP-immunoreactive nerves were all significantly lower in the older IL-2+/– than in IL-2+/+ mice (Fig. 5). Interestingly, no significant differences in the concentrations of neuropeptides PYY, SP and VIP were observed in the colonic tissues between the older IL-2+/– and IL-2+/+ mice (Fig. 6).
Fig. 4.
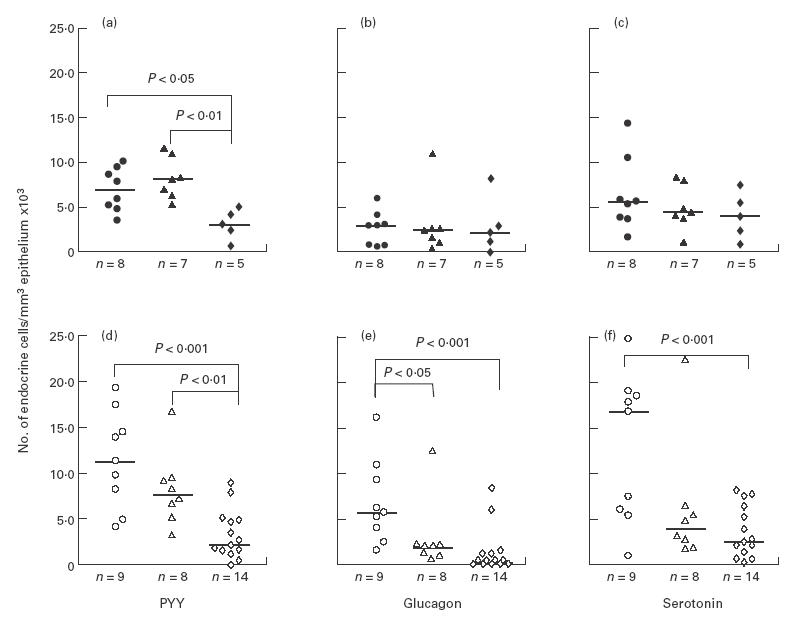
Frequencies of polypeptide YY (PYY; a,d), glucagon (b,e) and serotonin (c,f)-containing endocrine cells in the colon of IL-2−/– mice (diamonds) at acute (a,b,c; closed symbols) or chronic (d,e,f; open symbols) stage of disease, compared with the age-matched healthy IL-2+/+ (circles) and IL-2+/– mice (triangles). Medians are indicated by the horizontal bars. n, Number of mice/group.
Fig. 5.
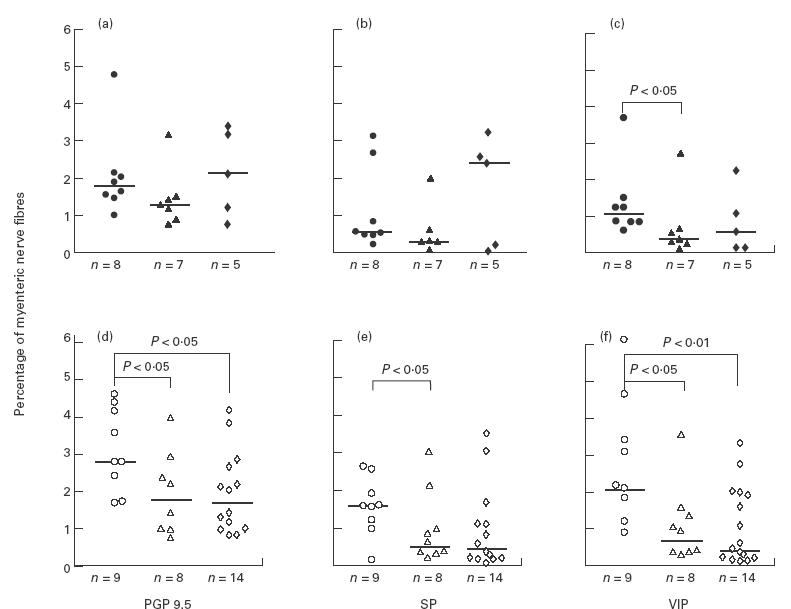
Relative volume densities of protein gene product 9.5 (PGP 9.5; a,d); substance P (SP; b,e) and vasoactive intestinal polypeptide (VIP; c,f)-immunoreactive myenteric nerve fibres in the colon of IL-2−/– mice (diamonds) at acute (a,b,c; closed symbols) or chronic (d,e,f; open symbols) stage of disease, compared with the age-matched healthy IL-2+/+ (circles) and IL-2+/– mice (triangles). Values are expressed as percentage of the nerve fibres in muscle tissues. Medians are indicated by the horizontal bars. n, Number of mice/group.
Fig. 6.
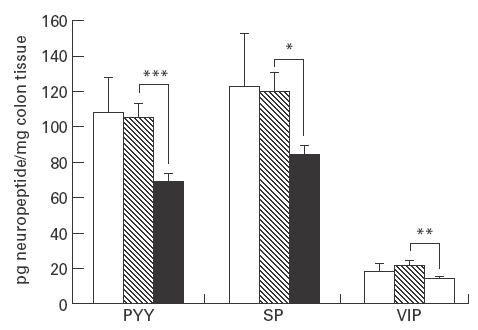
Colonic contents of various neuropeptides in the IL-2−/– mice with chronic bowel inflammation (▪, n = 8), and the age-matched healthy IL-2+/– (hatched, n = 7) or IL-2+/+ controls (□, n = 10). Results are presented as mean + s.e.m. *0·01 < P < 0·05; **0·001 < P < 0·01; ***P < 0·001. n, Number of mice/group. PYY, Polypeptide YY; SP, substance P; VIP, vasoactive intestinal polypeptide.
Clinical and histopathological characterizations of IBD in IL-2−/– mice
The IL-2−/– mice suffering from colitis were first detected between 10 and 13 weeks of age. This group of mice developed a fulminant form of colitis with marked weight loss, diarrhoea and fatigue, and had to be killed early after onset of disease. The other group of IL-2−/– mice displayed a chronic IBD at an age older than 13 weeks. They manifested weight loss and a hunched stance. The gastrointestinal symptoms included diarrhoea, intermittent intestinal bleeding and rectal prolapse. Gross examination showed that the entire colon was enlarged and thickened. Moreover, the mesenteric lymph nodes and spleen were markedly increased in size. Histopathological changes in colon were usually restricted to the mucosa layer, with general inflammation of the lamina propria, epithelial hyperplasia and ulcerations. Crypt elongation and branching, presence of microabscesses and decreased number of goblet cells were other characteristic features of the colonic mucosa in diseased mice (Fig. 1A). None of the IL-2+/– and IL-2+/+ mice which were housed in the same or adjacent cages as their IL-2−/– littermates exhibited clinical or histopathological signs of colonic inflammation (Fig. 1B).
Colonic inflammation causes a significant decrease in the frequency of endocrine cells containing PYY
In the younger, acutely ill IL-2−/– mice there was a disease-related decrease in the endocrine cells containing PYY compared with the IL-2+/– mice (P = 0·002, Fig. 4a), while no significant changes in glucagon- and serotonin-containing cells were noted (Fig. 4b,c). Similarly, the number of PYY-containing cells was significantly lower in the older IL-2−/– mice with chronic disease compared with the healthy IL-2+/– mice (P = 0·003; Fig. 1 and Fig. 4d). In the case of glucagon-containing cells, there was no significant difference in the frequencies between IL-2+/– and IL-2−/– mice. Instead, both IL-2+/– and IL-2−/– mice had lower numbers of these cells than IL-2+/+ mice (Fig. 4e), suggesting an influence of reduced or absent IL-2 levels rather than of colitis in this case. Serotonin-producing cells showed the same tendency as glucagon cells (Fig. 4f).
Inflammatory process has no significant influence on myenteric innervation in colon
Estimation of the relative volume densities of PGP 9·5-, SP- and VIP-immunoreactive nerve fibres in the muscular layer of colon in the diseased and control mice is shown in Fig. 5. There were no significant disease-related changes in the IL-2−/– mice with regard to the volume densities of myenteric nerves studied, either in younger, acutely ill mice (Fig. 5a,b,c) or in older mice with chronic colitis (Fig. 5d,e,f), compared with the IL-2+/– mice.
However, in the groups of older mice, PGP 9.5-, SP- and VIP-immunoreactive nerves were fewer both in the IL-2−/– and IL-2+/– mice compared with the IL-2+/+ controls (Fig. 2 and Fig. 5d,e,f). In contrast, in the younger mouse groups no statistically significant differences were found between IL-2−/–, IL-2+/– and IL-2+/+ mice except that VIP-immunoreactive nerves were decreased in the IL-2+/– mice compared with the IL-2+/+ mice (Fig. 5a,b,c). These findings suggest that IL-2 levels influenced also the densities of colonic myenteric innervation, mainly in older mice.
Reductions of tissue neuropeptide levels in inflamed colon of IL-2−/– mice
The morphometrical studies revealed that the neuroendocrine alterations were more pronounced in the gene knock-out mice older than 13 weeks. Therefore, the determination of neuropeptide levels in colonic tissue was focused on the chronically diseased IL-2−/– mice and age-matched IL-2+/– as well as IL-2+/+ mice. The results are presented in Fig. 6. The levels of PYY, SP and VIP in colonic tissues were lower in the IL-2−/– mice compared with the two control groups of non-diseased mice. These changes were significant when comparing the IL-2−/– with IL-2+/– mice, suggesting that they were all related to the colitis. No significant differences were observed between the IL-2+/– and IL-2+/+ mice in the levels of the neuropeptides studied.
Discussion
IL-2−/– mice which survived the first 10 weeks of life spontaneously developed a severe wasting disorder with striking clinical and histopathological similarities to human UC. In this genetically predisposed animal model with either acute or chronic bowel inflammation, we found that the colonic neuroendocrine system was altered. In general, two types of changes could be noted: (i) alterations apparently caused by the disruption of the IL-2 gene per se; and (ii) alterations related to the development of colonic inflammation in the IL-2−/– mice.
Ten-week-old mice with wild-type (IL-2+/+), heterozygous (IL-2+/–) and homozygous (IL-2−/–) phenotypes generally had the same amounts of endocrine cells and myenteric nerves in their colonic tissues. In the wild-type mice, a gradual increase in various endocrine cells and myenteric nerves expressing VIP occurred from 10 to 18 weeks of age. No such increase was seen in the healthy IL-2+/– or diseased IL-2−/– mice. In contrast, the low numbers of glucagon- and serotonin-containing endocrine cells and relative volume densities of PGP 9.5-, SP-, and VIP-immunoreactive myenteric nerves remained unchanged in the older mouse groups. The lack of age-related changes may be caused by lower levels and absence of IL-2 in IL-2+/– and IL-2−/– mice, respectively. This hypothesis was supported by the finding from the present study that the IL-2+/– mice contained only one-third the amount of IL-2 in colonic tissues compared with the IL-2+/+ mice. It suggests a cross-talk between the neuroendocrine and immune systems in normal colon. Indeed, previous results indicate that the immune system not only receives modulatory signals from the neuroendocrine system, but also provides information mainly through its secretory products, e.g. cytokines, to influence the neuroendocrine components [1,39–41]. However, the role of IL-2 in this interaction remains obscure, but is most probably indirect. One possibility is that intraepithelial lymphocytes regulate the differentiation of endocrine cells. Different kinds of intestinal epithelial cells, including endocrine cells, are all generated from the epithelial progenitor cells in the lower part of the crypts [42]. Intraepithelial lymphocytes in normal human intestine express IL-2 mRNA [43] and studies on TCR-γδ−/– mice have suggested a role for intraepithelial γδ T cells in epithelial cell differentiation [44]. Thus, one component in the regulation of the neuroendocrine system in colon could involve IL-2-dependent activation of intraepithelial lymphocytes.
A decrease in PYY-producing endocrine cells correlated with the presence of colonic inflammation. The frequency of PYY-containing cells was significantly lower in both the acute and chronic diseased IL-2−/– mice compared with the age-matched healthy IL-2+/+ mice and notably also healthy IL-2+/– mice. Furthermore, the PYY concentration in the colonic tissue was markedly decreased in the IL-2−/– mice with chronic colitis. PYY is a neuropeptide localized exclusively to the ileocolonic region. Its biological actions include vasoconstriction, inhibition of gut secretion and motility [45]. The peptide-containing cells are scattered amongst the epithelial cells lining the mucosa and have cytoplasmic processes directed towards the neighbouring goblet cells, which may suggest a paracrine effect. In UC patients, a reduction in colonic PYY level has been reported [46]. A decrease in PYY-containing cells in colon of patients with severe UC compared with patients with mild inflammation was also demonstrated in a previous study from our group [20]. The location of PYY cells and the known actions of the peptide raise the interesting possibility that the decrease in PYY in the inflamed colon may result in malabsorption, hypersecretion and disturbed gut motility observed in IBD. We did not find any disease-related changes in serotonin- or glucagon-containing cells in the same samples of inflamed colon. This suggests that the alteration of PYY cells in the IL-2−/– mice is indeed related to the inflammation rather than only a function of disordered mucosal architecture.
It has been reported that the majority of the intrinsic nerves that supply the circular muscles of intestine are immunoreactive for SP and VIP [47]. In the gastrointestinal tract, SP provides the excitatory control, whereas VIP plays the inhibitory role, ensuring a physiological balance in the regulation of a variety of gut functions. Previous studies on these peptidergic nerves in UC patients have given inconsistent results, with either increased, decreased or unchanged values [21,25–28]. In the present study no differences were found between the IL-2+/– and IL-2−/– mice concerning the relative volume densities of total as well as SP- and VIP-immunoreactive nerves in the muscular layer of colon.
In contrast to the morphometric findings, the local tissue levels of both SP and VIP were decreased in the IL-2−/– mice with established chronic bowel inflammation, compared with the healthy IL-2+/– mice. Recently it was shown that human intestinal lymphocytes express receptors for SP [17], suggesting a role of SP in the neuro-immune modulation in the gut. Decrease in SP levels could influence lymphocyte functions, thereby perpetuating the inflammation. The decrease in SP and VIP concentration may mainly be caused by the altered physiological activities of neuropeptide-producing anatomic units, since the peptidergic innervation in the IL-2−/– mice did not differ from that in their IL-2+/– littermates. These observations are in accordance with the early investigations on SP and VIP contents in intestinal tissue samples from UC patients [24], but in disagreement with other studies where an increased or unchanged amount of SP or VIP was detected in the inflamed mucosa [21,23]. These discrepancies could partially be explained by a varying degree of oedema and accumulation of inflammatory cells in gut mucosa. Different distribution of the neuropeptides throughout the layers of intestine in man and mice may also influence the results. In man, about 40% of total SP or VIP content in the distal ileum and colon originates from the external muscle layer, 20% from the submucosa, and 40% from the lamina propria [48]. Conversely, we found that the great majority of SP- and VIP-immunoreactive nerves is localized in the muscle layer of mouse colon. Therefore, it is reasonable to believe that the variation of neuropeptide contents during inflammation may differ in the various layers of intestine in different species.
In conclusion, an altered neuroendocrine system was demonstrated in the colon of mice with a disrupted IL-2 gene and diminished local IL-2 tissue level. These changes included a decrease in glucagon- and serotonin-producing endocrine cells and myenteric nerve fibres, and an abrogation/retardation of a normally occurring age-related increase in various endocrine cells and peptidergic nerves. IL-2−/– mice also exhibited some neuroendocrine changes that appeared to be a direct reflection of colonic inflammation. Decreases in PYY-containing endocrine cells and concentrations of neuropeptides PYY, SP and VIP did correlate with the colitis. Whether these changes are primary abnormalities or secondary to the disease process could not be addressed here. However, in any case, they could have relevance for the physiological dysfunction in IBD gut and exert an impact on the development of disease. Taken together, these findings suggest an ongoing interaction between the neuroendocrine and immune systems in normal colon as well as a role for neuroendocrine peptides in the inflammatory process. The colonic inflammation in IL-2−/– mice shows resemblance to human UC with respect to neuroendocrine changes, suggesting that IL-2−/– mice indeed constitute an attractive model for UC study.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (19X-11240), the Swedish Natural Science Council (B-AA/BV 11234-302), the Medical Faculty Research Fund, Umeå University, the County of Västerbotten, Bengt Ihre's Foundation, and Stiftelsen J C Kempes Minnes Stipendiefond.
REFERENCES
- 1.Shanahan F, Anton P. Neuroendocrine modulation of the immune system. Possible implications for inflammatory bowel disease. Dig Dis Sci. 1988;33:41S–49S. doi: 10.1007/BF01538130. [DOI] [PubMed] [Google Scholar]
- 2.Debas HT, Mulvihill Sj. Neuroendocrine design of the gut. Am J Surg. 1991;161:243–9. doi: 10.1016/0002-9610(91)91139-a. [DOI] [PubMed] [Google Scholar]
- 3.Braegger CP, MacDonald Tt. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy. 1994;72:135–41. [PubMed] [Google Scholar]
- 4.Nielsen OH, Rask-Madsen J. Mediators of inflammation in chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31(Suppl. 216):149–59. doi: 10.3109/00365529609094569. [DOI] [PubMed] [Google Scholar]
- 5.Ottaway Ca. Role of the neuroendocrine system in cytokine pathways in inflammatory bowel disease. Aliment Pharmacol Ther. 1996;10(Suppl. 2):10–15. doi: 10.1046/j.1365-2036.1996.22164015.x. [DOI] [PubMed] [Google Scholar]
- 6.Beagley KW, Elson Co. Cells and cytokines in mucosal immunity and inflammation. Gastroenterol Clinics N Am. 1992;21:347–66. [PubMed] [Google Scholar]
- 7.Schreiber S, Raedler A, Stenson WF, MacDermott Rp. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clinics N Am. 1992;21:451–502. [PubMed] [Google Scholar]
- 8.Broberger O, Perlmann P. Autoantibodies in ulcerative colitis. J Exp Med. 1959;110:657–74. doi: 10.1084/jem.110.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagercrantz R, Hammarström S, Perlmann P, Gustafsson Be. Immunological studies in ulcerative colitis. IV. Origin of autoantibodies. J Exp Med. 1968;128:1339–52. doi: 10.1084/jem.128.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das KM, Dasgupta A, Mandal A, Geng X. Autoimmunity to cytoskeletal protein tropomyosin. A clue to the pathogenic mechanism for ulcerative colitis. J Immunol. 1993;150:2487–93. [PubMed] [Google Scholar]
- 11.Targan SR, Landers CJ, Cobb L, MacDermott RP, Vidrich A. Perinuclear antineutrophil cytoplasmic antibodies are spontaneously produced by mucosal B cells of ulcerative colitis. J Immunol. 1995;155:3262–7. [PubMed] [Google Scholar]
- 12.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–75. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanisz Am. Neuronal factors modulating immunity. Neuroimmunomodulation. 1994;1:217–30. doi: 10.1159/000097169. [DOI] [PubMed] [Google Scholar]
- 14.Taylor RG, Fuller Pj. Humoral regulation of intestinal adaptation. Bailliere's Clin Endocr Metab. 1994;8:165–83. doi: 10.1016/s0950-351x(05)80230-7. [DOI] [PubMed] [Google Scholar]
- 15.Mulvihill SJ, Debas Ht. Regulatory peptides of the gut. In: Greenspan FS, Baxter JD, editors. Basic and clinical endocrinology. 4. Norwalk: Appleton & Lange; 1994. pp. 551–70. [Google Scholar]
- 16.Adrian TE, Thompson JS, Quigley Emm. Time course of adaptive regulatory peptide changes following massive small bowel resection in the dog. Dig Dis Sci. 1996;41:1194–203. doi: 10.1007/BF02088237. [DOI] [PubMed] [Google Scholar]
- 17.Goode T, O'connell J, Sternini C, Anton P, Wong H, O'sullivian GC, Collins JK, Shanahan F. Substance P (neurokinin-1) receptor is a marker of human mucosal but not peripheral mononuclear cells: molecular quantitation and localization. J Immunol. 1998;161:2232–40. [PubMed] [Google Scholar]
- 18.Storsteen KA, Kernohan JW, Bargen Ja. The myenteric plexus in chronic ulcerative colitis. Surg Gynecol Obstet. 1953;97:335–43. [PubMed] [Google Scholar]
- 19.Koch TR, Sonnenberg A, Carney Ja. Gut neuropeptides and the pathophysiology of inflammatory bowel disease. In: Snape WJ, Collins SM, editors. Effects of immune cells and inflammation on smooth muscle and enteric nerves. Boca Raton: CRC Press Inc; 1991. pp. 169–80. [Google Scholar]
- 20.El-Salhy M, Danielsson Å, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–9. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 21.O'morain C, Bishop AE, McGregor GP, Levi AJ, Bloom SR, Polak JM, Peters Tj. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut. 1984;25:57–61. doi: 10.1136/gut.25.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch TR, Carney JA, Go Vlw. Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel disease. Dig Dis Sci. 1987;32:369–76. doi: 10.1007/BF01296290. [DOI] [PubMed] [Google Scholar]
- 23.Goldin E, Karmeli F, Selinger Z, Rachmilewitz D. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci. 1989;34:754–7. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- 24.Renzi D, Mantellini P, Calabro A, et al. Substance P and vasoactive intestinal polypeptide but not calcitonin gene-related peptide concentrations are reduced in patients with moderate and severe ulcerative colitis. Ital J Gastroenterol Hepatol. 1998;30:62–70. [PubMed] [Google Scholar]
- 25.Björck S, Dahlström A, Ahlman H. Topical treatment of ulcerative proctitis with lidocaine. Scand J Gastroenterol. 1989;24:1061–72. doi: 10.3109/00365528909089256. [DOI] [PubMed] [Google Scholar]
- 26.Mazumdar S, Das Km. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am J Gastroenterol. 1992;87:176–81. [PubMed] [Google Scholar]
- 27.Kimura M, Masuda T, Hiwatashi N, Toyota T, Nagura H. Changes in neuropeptide-containing nerves in human colonic mucosa with inflammatory bowel disease. Pathol Int. 1994;44:624–34. doi: 10.1111/j.1440-1827.1994.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 28.Keränen U, Kiviluoto T, Järvinen H, Bäck N, Kivilaakso E, Soinila S. Changes in substance P-immunoreactive innervation of human colon associated with ulcerative colitis. Dig Dis Sci. 1995;40:2250–8. doi: 10.1007/BF02209015. [DOI] [PubMed] [Google Scholar]
- 29.Schorle H, Holtschke T, Hunig T, Schimple A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–4. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 30.Horak I, Löhler J, Ma A, Smith K. Interleukin-2 deficient mice: a new model to study autoimmunity and self-tolerance. Immunol Rev. 1995;148:35–44. doi: 10.1111/j.1600-065x.1995.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 31.Sadlack B, Merz H, Schorle H, Schimple A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 32.McDonald SAC, Palmen MJHJ, Vanrees EP, MacDonald Tt. Characterization of the mucosal cell-mediated immune response in IL-2 knockout mice before and after the onset of colitis. Immunology. 1997;91:73–80. doi: 10.1046/j.1365-2567.1997.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald TT, Monteleone G, Pender Sl. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 34.El-Salhy M, Stenling R, Grimelius L. Peptidergic innervation and endocrine cells in the human liver. Scand J Gastroenterol. 1993;28:809–15. doi: 10.3109/00365529309104014. [DOI] [PubMed] [Google Scholar]
- 35.Qian B-F, El-Salhy M, Danielsson Å, Shalaby A, Axelsson H. Effects of unilateral cervical vagotomy on antral endocrine cells in mouse. Histol Histopathol. 1999;14:705–9. doi: 10.14670/HH-14.705. [DOI] [PubMed] [Google Scholar]
- 36.El-Salhy M, Spångéus A. Substance P in the gastrointestinal tract of non-obese diabetic mice. Scand J Gastroenterol. 1998;33:394–400. doi: 10.1080/00365529850171026. [DOI] [PubMed] [Google Scholar]
- 37.Dillner-Centerlind M-L, Axelsson B, Hammarström S, Hellström U, Perlmann P. Interaction of lectins with human T lymphocytes mitogenic properties, inhibitory effects, binding to the cell membrane and to isolated surface glycopeptides. Eur J Immunol. 1980;10:434–42. [Google Scholar]
- 38.Brynskov J, Tvede N, Andersen CB, Vilien M. Increased concentrations of interleukin 1β, interleukin-2, and soluble interleukin-2 receptors in endoscopical mucosal biopsy specimens with active inflammatory bowel disease. Gut. 1992;33:55–58. doi: 10.1136/gut.33.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbany G, Friedman WJ, Persson H. Lymphocyte-mediated regulation of neurotransmitter gene expression in rat sympathetic ganglia. J Neuroimmunol. 1991;32:97–104. doi: 10.1016/0165-5728(91)90001-n. [DOI] [PubMed] [Google Scholar]
- 40.Hart RP, Shadiack AM, Jonakait Gm. Substance P gene expression is regulated by interleukin-1 in cultured sympathetic ganglia. J Neurosci Res. 1991;29:282–91. doi: 10.1002/jnr.490290303. [DOI] [PubMed] [Google Scholar]
- 41.Straub RH, Westermann J, Schölmerich J, Falk W. Dialogue between the CNS and the immune system in lymphoid organs. Immunol Today. 1998;19:409–13. doi: 10.1016/s0167-5699(98)01297-3. [DOI] [PubMed] [Google Scholar]
- 42.Inokuchi H, Fujimoto S, Kawai K. Cellular kinetics of gastrointestinal mucosa, with special reference to gut endocrine cells. Arch Histol Jap. 1983;46:137–57. doi: 10.1679/aohc.46.137. [DOI] [PubMed] [Google Scholar]
- 43.Lundqvist C, Melgar S, Yeung MM, Hammarström S, Hammarström M-L. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J Immunol. 1996;157:1926–34. [PubMed] [Google Scholar]
- 44.Komano H, Fujiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 1995;92:6147–51. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adrian TE, Savage AP, Bacarese-Hamilton AJ, Wolfe K, Besterman HS, Bloom Sr. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90:379–84. doi: 10.1016/0016-5085(86)90936-4. [DOI] [PubMed] [Google Scholar]
- 46.Koch TR, Roddy DR, Carney JA, Go Vlw. Peptide YY concentration in normal ileum and colon and in idiopathic inflammatory bowel disease. Dig Dis Sci. 1988;33:1322–8. doi: 10.1007/BF01536686. [DOI] [PubMed] [Google Scholar]
- 47.Llewellyn-Smith IJ, Furness JB, Gibbins IL, Costa M. Quantitative ultrastructural analysis of enkephalin-, substance P-, and VIP-immunoreactive nerve fibers in the circular muscle of the guinea pig small intestine. J Comp Neurol. 1988;272:139–48. doi: 10.1002/cne.902720110. [DOI] [PubMed] [Google Scholar]
- 48.Ferri G-L, Adrian TE, Ghatei MA, et al. Tissue localization and relative distribution of regulatory peptides in separated layers from the human bowel. Gastroenterology. 1983;84:777–86. [PubMed] [Google Scholar]


