Abstract
Oil-induced arthritis is a genetically restricted polyarthritis that develops in the DA rat after injection of the mineral oil Freund's incomplete adjuvant. Here, we investigated the role of the potentially disease-limiting cell populations CD8+ T cells, γδ T cells, natural killer (NK) cells and NK T cells in inguinal lymph nodes for the development of this adjuvant-induced arthritis. Flow cytometry analysis before and at disease onset revealed a higher proportion of lymph node T cells expressing NKR-P1 in the disease-resistant LEW.1AV1 compared with the disease-susceptible DA strain, suggesting that NK T cells might be disease protective. However, prophylactic in vivo administration of an anti-NKR-P1 MoAb (clone 10/78) did not consistently affect the disease course. The proportion of CD8+ T cells and the ratio CD4+/CD8+ T cells in inguinal lymph nodes did not differ significantly between DA and LEW.1AV1 rats before or at disease onset. Nevertheless, prophylactic in vivo depletion of CD8+ cells by the OX8 MoAb in the DA strain resulted in an earlier disease onset compared with the control group, demonstrating that CD8+ cells regulate arthritis development. In vivo depletion of γδ T cells by the V65 MoAb did not alter the disease course, indicating that the disease-suppressive CD8+ cells are αβ T cells or NK cells.
Keywords: experimental arthritis, CD8, NK T cells, NK cells, γδ T cells
Introduction
Inflammatory joint disease with clinical and genetic similarities to rheumatoid arthritis (RA) can be induced in susceptible rat strains by hydrocarbons, such as squalene [1], pristane [2] and Freund's incomplete adjuvant (FIA) [3,4], which activate the immune system non-specifically. The mechanisms whereby these adjuvants trigger joint-restricted inflammation are unknown, but determining the role of different cell types for arthritis development can give clues to the pathogenesis. Oil-induced arthritis (OIA) develops in the susceptible DA rat after one intradermal injection of the mineral oil FIA, and has been demonstrated to be mediated by αβ T cells [4] expressing CD4 [5].
However, knowledge about cell populations that limit OIA is currently lacking. Such knowledge can be gained both from descriptive comparisons of cell populations in arthritis-resistant and -susceptible strains, and from in vivo depletion of distinct cell types before disease induction. Cell populations that have been reported to have disease down-regulating effects in various experimental autoimmune diseases are γδ T cells [6], natural killer (NK) cells [7–9], NK T cells [10] and CD8+ cells [11–13]. Moreover, recent genetic studies have highlighted the interest in the latter cell types in OIA, since the NK gene complex and the CD8 genes are located on chromosome 4 within a major quantitative trait locus determining susceptibility to OIA [14–17]. In addition, the arthritis-prone DA rat has a defect NK alloreactiviy mapped to the NK gene complex [15].
The aim of the present study was to determine the role of potentially disease-limiting cell populations in OIA. We focused our study on inguinal lymph nodes draining the injection site, since cell transfer studies have previously demonstrated these lymph nodes to be involved in disease development [5]. We determined the proportions of γδ T cells, NK cells, NK T cells and CD8+ T cells in inguinal lymph nodes from oil injection to disease onset, thus comparing the arthritis-susceptible DA rat with the resistant LEW.1AV1 to enable detection of changes associated with disease development. Finally, we performed a prophylactic in vivo depletion/modulation of TCRγδ+, NKR-P1+ and CD8+ cells to determine the importance of these cells for OIA development.
Materials and methods
Animals
The inbred MHC identical (RT1av1) rat strains DA and LEW.1AV1 were originally derived from Zentralinstitut für Versuchstierzucht (Hannover, Germany). They were bred and maintained at the animal departments at the Biomedical Centre (Uppsala, Sweden) and at the Centre of Molecular Medicine, Karolinska Institute (Stockholm, Sweden). They were free from rat pathogens as tested for in a health-monitoring programme at the National Veterinary Institute in Uppsala. They were kept in a 12-h light/12-h dark cycle and housed in polystyrene cages containing aspen wood shavings and autoclaved rodent chow (Lactamin R3; Vadstena, Sweden). All animal procedures were in accordance with national regulations on animal experiments. Female rats, 10–19 weeks of age, were used.
Induction and evaluation of arthritis
Rats were anaesthetized and intradermally injected with FIA (Difco, Detroit, MI) at the base of the tail. The animals for the flow cytometry analysis received 150 μl FIA emulsified with 150 μl 0·1 m acetic acid, while the depletion animals received 200–300 μl FIA. The two different induction protocols resulted in a comparable arthritis. Paws were visually inspected, and arthritis in individual paws was evaluated in a blinded manner according to a scoring system where 0 = no signs of arthritis, 1 = one type of red and swollen joint, 2 = two types, 3 = three types, and 4 = the whole paw red and swollen. Thus, each rat was assigned a score between 0 and 16.
Flow cytometry analysis
For two-colour flow cytometry analysis, DA and LEW.1AV1 rats were killed on days 4, 7, 11 and 15 post-mineral oil injection. The draining inguinal lymph nodes were dissected out, passed through a steel mesh, washed three times in PBS and resuspended in PBS supplemented with 2% fetal calf serum (FCS). Cells (5 × 105/sample) were stained with MoAbs at saturating concentrations. First, cells were incubated with biotinylated anti-rat CD3 (clone G4.18; PharMingen, San Diego, CA) together with either direct-conjugated antibody FITC anti-rat TCRαβ (clone R73; Serotec, Oxford, UK), FITC anti-rat TCRγδ (clone V65; PharMingen), FITC anti-rat CD4 (clone OX35; PharMingen), FITC anti-rat CD8 (clone OX8; PharMingen) or FITC anti-rat NKR-P1 (clone 3.2.3; Serotec) for 30 min at 4°C. They were washed once with PBS/2% FCS, and thereafter incubated with streptavidin–PE (Becton Dickinson, Mountain View, CA) for 30 min at 4°C. After a final wash, cells were fixed in PBS/1% paraformaldehyde. Simultest Control γ1/γ2a (Becton Dickinson) were used as negative control. Cells were also incubated with streptavidin–PE alone to exclude unspecific staining from this reagent. Finally, 50 000 events per sample were acquired on a FACSsort flow cytometer (Becton Dickinson) and analysed using CellQuest software (Becton Dickinson). A wide lymphocyte gate was set to include also the NK cells.
Monoclonal antibody treatment
The hybridomas 10/78 and V65 were kind gifts from Dr T. Hünig (Würzburg, Germany). 10/78 produces IgG1 MoAb that recognizes NKR-P1 [18], an activating NK receptor expressed on NK cells, a subset of T cells and granulocytes [19]. The V65 hybridoma produces IgG1 MoAb against the γδ T cell receptor [20]. Anti-CD8 MoAbs (IgG1) from the OX8 hybridoma were also used [21], and an anti-TNP MoAb, clone C1406D10 (D10) [22], served as an isotype-matched control antibody. Antibodies were affinity-purified from cell culture supernatants on Hi-Trap rProtein A column (Amersham, Pharmacia Biotech, Uppsala, Sweden), dialysed against PBS, sterile filtered and stored at −70°C. The concentration was determined by absorbance measurements at 280 nm.
For prophylactic in vivo administration, 1 mg purified MoAb was injected intraperitoneally under anaesthesia 2 days before injection of FIA. Control animals received 1 mg D10 MoAb.
Statistical analysis
The Mann–Whitney U-test was used throughout the study to test the null hypothesis that no differences existed between two groups, except from the analysis of disease course where the survival analysis log rank test was used. P < 0·05 was regarded as significant.
Results
Comparison between susceptible and resistant strains in lymph node cell populations
Flow cytometry analysis before and at arthritis onset revealed a difference from day 4 and thereafter in the proportion of lymph node T cells expressing NKR-P1, the arthritis-resistant LEW.1AV1 having a larger NK T cell population than the susceptible DA strain (Fig. 1c).
Fig. 1.
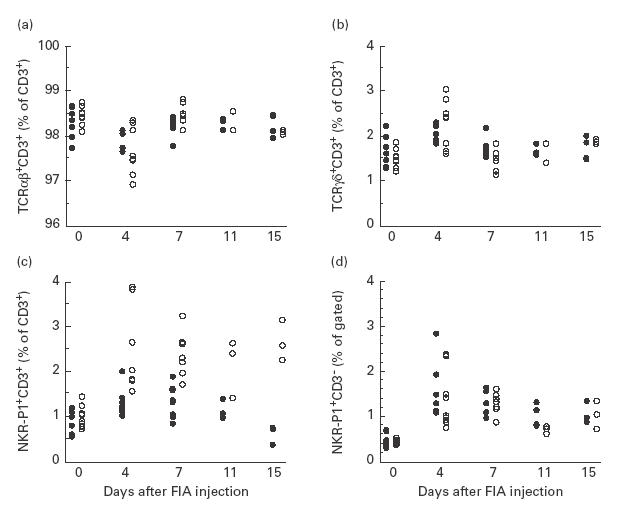
Proportions of (a) αβ T cells, (b) γδ T cells, (c) natural killer (NK) T cells and (d) NK cells in inguinal lymph nodes of arthritis-susceptible DA rats (•) and resistant LEW.1AV1 (○) from induction of oil-induced arthritis (OIA) (day 0 untreated animals) to disease onset (day 15), determined by flow cytometry analysis. Significant differences between the two strains were observed in (a) at day 7 (P = 0·02), in (b) at day 7 (P = 0·02) and in (c) at day 4 (P = 0·004), day 7 (P = 0·002), day 11 (P = 0·03) and day 15 (P = 0·03). The figure depicts pooled data from two similar experiments (n = 7–9/group on days 0, 4 and 7, n = 3–4/group on days 11 and 15).
For αβ and γδ T cell populations no long-lasting differences between strains were detected, although a difference on day 7 was observed (Fig. 1a,b).
The two strains had similar frequencies of NK cells in the lymph nodes throughout the study, but both strains had an increased proportion of NK cells on day 4 compared with day 0 (Fig. 1d).
In a separate experiment, the proportions of CD4+ T cells and CD8+ T cells in inguinal lymph nodes during arthritis development were analysed (Fig. 2). The percentage of CD4+ T cells was significantly higher in LEW.1AV1 compared with DA at all time points (Fig. 2a). Neither the proportion of CD8+ T cells nor the ratio CD4+ T cells/CD8+ T cells differed significantly between the two strains at any time point (Fig. 2b,c).
Fig. 2.
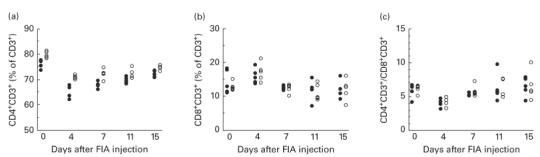
Proportions of (a) CD4+ T cells and (b) CD8+ T cells in inguinal lymph nodes of arthritis-susceptible DA rats (•) and resistant LEW.1AV1 (○) from induction of oil-induced arthritis (OIA) (day 0 untreated animals) to disease onset (day 15) as determined by flow cytometry analysis. (c) Ratio CD4+ T cells/CD8+ T cells calculated from (a) and (b). There are significant differences between the groups at all time points in (a): P = 0·009 on day 0, P = 0·009 on day 4, P = 0·03 on day 11 and P = 0·02 on day 15 (n = 5).
In vivo depletion/modulation of distinct cell populations before disease induction
In vivo administration of 0·5 mg OX8 MoAb, recognizing CD8, has been reported to eliminate completely CD8-expressing cells in spleen and lymph nodes for at least 2 weeks, as determined by immunohistochemistry [11]. This was confirmed by injection of 1 mg OX8, which eliminated CD8+ T cells from inguinal lymph nodes and spleen for at least 10 days as determined by flow cytometry analysis (data not shown). Injection of 1 mg of OX8 into DA rats 2 days before disease induction resulted in an earlier onset of disease in OX8-treated animals compared with animals receiving the isotype-matched control antibody D10 (Fig. 3, Table 1). The two groups had significantly different disease courses as determined by the survival analysis log rank test (P = 0·005; Fig. 3). Day of disease onset differed significantly between the two groups as determined by the Mann–Whitney U-test (P = 0·0005), the OX8-treated group having a median of 11 days and the control group 14 days (Table 1). A difference in the day of maximal arthritis score was also observed (P = 0·037), the median being 14 days for the OX8 group and 20 days for the control group (Table 1). No difference in maximal arthritis score could be detected (Table 1).
Fig. 3.
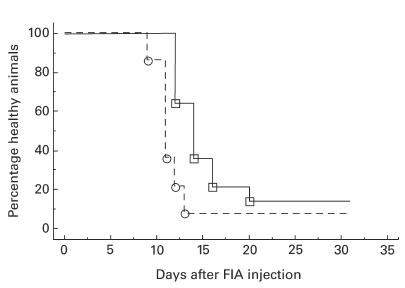
Percentage healthy animals over time, after prophylactically having received a depleting dose of anti-CD8 MoAb (– – – –, clone OX8, n = 14) or isotype-matched control MoAb (––––––, clone D10, n = 14) before induction of oil-induced arthritis (OIA). There is a significant difference between the two groups (log rank test, P = 0·005). The figure depicts pooled data from two similar experiments.
Table 1.
Modulation of oil-induced arthritis (OIA) by in vivo administration of an anti-CD8 monoclonal antibody
| Treatment† | Incidence | Day of onset‡ | Day of maximal arthritis score‡ | Maximal arthritis score‡ | Sum score‡ |
|---|---|---|---|---|---|
| D10 | 12/14 | 14* | 20** | 6 | 46 |
| OX8 | 13/14 | 11* | 14** | 6 | 67 |
1 mg anti-CD8 MoAb (clone OX8, n = 14) or isotype-matched control MoAb (clone D10, n = 14) was administered to DA rats 2 days before induction of OIA. Each group represents pooled data from two experiments.
Median value of affected animals.
Significant difference between the two groups, P = 0·0005.
Significant difference between the two groups, P = 0·037.
In vivo administration of 1 mg V65 MoAb against the γδ TCR deleted the CD3+αβ− cell population from lymph nodes for at least 10 days as determined by flow cytometry (data not shown). Injection of this dose 2 days before disease induction did not affect disease development compared with the isotype-matched control MoAb D10 (data not shown).
Flow cytometry analysis and a standard 4-h 51Cr-release assay on spleen cells after in vivo administration of anti-NKR-P1 MoAb clone 10/78 suggest that this antibody has a limited capacity to deplete NK cells (data not shown). Prophylactic in vivo administration of 10/78 MoAb did not reproducibly modulate arthritis development, but a significantly different disease course was observed in the group receiving 10/78 compared with the control group in one experiment out of three, as determined by survival analysis (log-rank test, P = 0·048; Table 2). Incidence, day of onset, day of maximal arthritis score, maximal arthritis score and sum score did not differ significantly between the two groups in any of the three experiments (Table 2).
Table 2.
Modulation of oil-induced arthritis (OIA) by in vivo administration of an anti-NKR-P1 monoclonal antibody, clone 10/78
| Treatment† | Experiment | Incidence | Day of onset‡ | Day of maximal arthritis score‡ | Maximal arthritis score‡ | Sum score‡ | Log rank test§ |
|---|---|---|---|---|---|---|---|
| D10 | 1 | 5/6 | 12 | 17 | 5 | 52 | NS* |
| NKR-P1 | 1 | 6/7 | 14 | 18 | 6 | 41 | |
| D10 | 2 | 7/7 | 12 | 20 | 7 | 57 | 0·048 |
| NKR-P1 | 2 | 5/7 | 14 | 18 | 6 | 61 | |
| D10 | 3 | 5/7 | 14 | 20 | 4 | 19 | NS |
| NKR-P1 | 3 | 6/7 | 13 | 17 | 5 | 30 |
1 mg anti-NKR-P1 MoAb (clone 10/78) or isotype-matched control MoAb (clone D10) was administered to DA rats 2 days before induction of OIA.
Median value of affected animals.
P value when testing the null hypothesis that there is no difference between the groups when taking the whole disease progress into account, using the survival analysis log-rank test.
Not significant.
Discussion
The aim of this study was to identify cell populations that can suppress the development of arthritis induced by non-specific activation of the immune system. We here demonstrate that CD8+ cells can be down-regulatory, since in vivo depletion of these cells results in an earlier onset of OIA. This is in contrast to the classical Mycobacteria-induced adjuvant arthritis, which has been reported not to be affected by OX8 treatment [23,24]. Reports of clinical effects on other adjuvant-induced arthritides are lacking, but the antigen-induced streptococcal cell wall-induced arthritis demonstrates a more chronic and rapid disease course after CD8 depletion [13].
Analysis of CD4+ and CD8+ T cells in inguinal lymph nodes revealed no difference between the arthritis-prone DA rat and the disease-resistant MHC congenic LEW.1AV1 rat in CD8+ T cell numbers. This suggests that the susceptibility of the DA rat is not associated with a deficiency in influx and expansion of CD8+ cells in draining lymph nodes. Instead, the results might be explained by a difference in CD8 cell function between the susceptible and the resistant strain.
We here describe an expansion of the NK T cell population in inguinal lymph nodes in the OIA-resistant but not in the susceptible strain, suggesting a protective role for NK T cells. In vivo administration of anti-NKR-P1 MoAb did not consistently change the disease course. However, our preliminary data indicate that the 10/78 MoAb has a limited capacity to deplete NK cells in vivo, and the action of the MoAb in terms of NK T cell activity is unknown. NKR-P1 is expressed at a lower level on NK T cells than on NK cells [25], favouring the hypothesis that the MoAb might not deplete the NK T cells. Moreover, it has been reported that signalling through NK1.1 (an activating NK receptor on mice NK cells and NK T cells), induced by in vivo administration of a MoAb against this molecule, triggers NK cell death but induces IL-4 production by NK T cells [26]. Such activation of NK T cells could promote a type 2 immune response, which has been reported to be disease down-regulatory in other experimental arthritides [27,28]. Further studies are necessary to address a role for NK cells and NK T cells in the regulation of OIA.
Our data indicate that γδ T cells are not involved in the development of OIA, in line with a report on γδ T cell depletion in Mycobacteria-induced classical adjuvant arthritis, where no clinical effect was observed, although microscopic aggravation of joint destruction was reported [6].
In conclusion, we describe a disease-counteracting role for CD8+ cells in OIA. Since depletion of γδ T cells did not result in a significant aggravation of the disease, we suggest that the protective CD8+ cells are of the TCR αβ subtype or NK cells.
Acknowledgments
We thank Professor Lars Klareskog for critically reading the manuscript, M. D. Ronald van Vollenhoven for linguistic advice and Anna Sjöström for performing NK cytotoxic assays. This study was supported by grants from Alex and Eva Wallström Foundation, Anna-Greta and Holger Crafoord's Foundation, Börje Dahlin's Foundation, King Gustaf V's 80th Jubilee Foundation, Nanna Svartz' Foundation, the Swedish Medical Research Council, the Swedish Rheumatism Association, Ulla and Gustaf af Ugglas Foundation for Medical Research, and Åke Wiberg Foundation.
REFERENCES
- 1.Lorentzen Jc. Identification of arthritogenic adjuvants of self and foreign origin. Scand J Immunol. 1999;49:45–50. doi: 10.1046/j.1365-3083.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 2.Vingsbo C, Sahlstrand P, Brun JG, Jonsson R, Saxne T, Holmdahl R. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149:1675–83. [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinau S, Erlandsson H, Holmdahl R, Klareskog L. Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence for an immunological involvement. J Autoimmun. 1991;4:871–80. doi: 10.1016/0896-8411(91)90050-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmdahl R, Goldschmidt TJ, Kleinau S, Kvick C, Jonsson R. Arthritis induced in rats with adjuvant oil is a genetically restricted, alpha beta T-cell dependent autoimmune disease. Immunology. 1992;76:197–202. [PMC free article] [PubMed] [Google Scholar]
- 5.Svelander L, Mussener A, Erlandsson-Harris H, Kleinau S. Polyclonal Th1 cells transfer oil-induced arthritis. Immunology. 1997;91:260–5. doi: 10.1046/j.1365-2567.1997.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelegri C, Kuhnlein P, Buchner E, et al. Depletion of gamma/delta T cells does not prevent or ameliorate, but rather aggravates, rat adjuvant arthritis. Arthritis Rheum. 1996;39:204–15. doi: 10.1002/art.1780390206. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–87. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–8. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson N, Bremell T, Tarkowski A, Carlsten H. Protective role of NK1.1+ cells in experimental Staphylococcus aureus arthritis. Clin Exp Immunol. 1999;117:63–69. doi: 10.1046/j.1365-2249.1999.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter Ag. alpha/beta-T cell receptor (TCR)+CD4–CD8−(NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–56. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmdahl R, Olsson T, Moran T, Klareskog L. In vivo treatment of rats with monoclonal anti-T-cell antibodies. Immunohistochemical and functional analysis in normal rats and in experimental allergic neuritis. Scand J Immunol. 1985;22:157–69. doi: 10.1111/j.1365-3083.1985.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SB, Diamantstein T, Weetman Ap. The effect of T cell subset depletion on autoimmune thyroiditis in the Buffalo strain rat. Immunol Letters. 1990;23:263–8. doi: 10.1016/0165-2478(90)90070-7. [DOI] [PubMed] [Google Scholar]
- 13.van den Broek MF, de Heer E, van Bruggen MC, de Roo G, Kleiverda K, Eulderink F, van den Berg Wb. Immunomodulation of streptococcal cell wall-induced arthritis. Identification of inflammatory cells and regulatory T cell subsets by mercuric chloride and in vivo CD8 depletion. Eur J Immunol. 1992;22:3091–5. doi: 10.1002/eji.1830221210. [DOI] [PubMed] [Google Scholar]
- 14.Dissen E, Fossum S. Chromosomal localization of the genes encoding rat CD4, CD8alpha, and CD8beta. Immunogenetics. 1996;44:312–4. doi: 10.1007/BF02602563. [DOI] [PubMed] [Google Scholar]
- 15.Dissen E, Ryan JC, Seaman WE, Fossum S. An autosomal dominant locus, Nka, mapping to the Ly-49 region of a rat natural killer (NK) gene complex, controls NK cell lysis of allogeneic lymphocytes. J Exp Med. 1996;183:2197–207. doi: 10.1084/jem.183.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorentzen JC, Glaser A, Jacobsson L, Galli J, Fakhrairad H, Klareskog L, Luthman H. Identification of rat susceptibility loci for adjuvant-oil-induced arthritis. Proc Natl Acad Sci USA. 1998;95:6383–7. doi: 10.1073/pnas.95.11.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansson AM, Jacobsson L, Luthman H, Lorentzen Jc. Susceptibility to oil-induced arthritis is linked to Oia2 on in a DA (DA x PVG.1AV1) backcross. Transplant Proc. 1999;31:1597–9. doi: 10.1016/s0041-1345(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 18.Kraus E, Lambracht D, Wonigeit K, Hunig T. Negative regulation of rat natural killer cell activity by major histocompatibility complex class I recognition. Eur J Immunol. 1996;26:2582–6. doi: 10.1002/eji.1830261107. [DOI] [PubMed] [Google Scholar]
- 19.Chambers WH, Vujanovic NL, DeLeo AB, Olszowy MW, Herberman RB, Hiserodt Jc. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–89. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhnlein P, Park JH, Herrmann T, Elbe A, Hunig T. Identification and characterization of rat gamma/delta T lymphocytes in peripheral lymphoid organs, small intestine, and skin with a monoclonal antibody to a constant determinant of the gamma/delta T cell receptor. J Immunol. 1994;153:979–86. [PubMed] [Google Scholar]
- 21.Brideau RJ, Carter PB, McMaster WR, Mason DW, Williams Af. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980;10:609–15. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- 22.Coulie PG, Van Snick J. Enhancement of IgG anti-carrier responses by IgG2 anti-hapten antibodies in mice. Eur J Immunol. 1985;15:793–8. doi: 10.1002/eji.1830150810. [DOI] [PubMed] [Google Scholar]
- 23.Larsson P, Holmdahl R, Dencker L, Klareskog L. In vivo treatment with W3/13 (anti-pan T) but not with OX8 (anti-suppressor/cytotoxic T) monoclonal antibodies impedes the development of adjuvant arthritis in rats. Immunology. 1985;56:383–91. [PMC free article] [PubMed] [Google Scholar]
- 24.Billingham ME, Hicks C, Carney S. Monoclonal antibodies and arthritis. Agents Actions. 1990;29:77–87. doi: 10.1007/BF01964727. [DOI] [PubMed] [Google Scholar]
- 25.Brissette-Storkus C, Kaufman CL, Pasewicz L, Worsey HM, Lakomy R, Ildstad ST, Chambers Wh. Characterization and function of the NKR-P1dim/T cell receptor-alpha beta+ subset of rat T cells. J Immunol. 1994;152:388–96. [PubMed] [Google Scholar]
- 26.Asea A, Stein-Streilein J. Signalling through NK1.1 triggers NK cells to die but induces NK T cells to produce interleukin-4. Immunology. 1998;93:296–305. doi: 10.1046/j.1365-2567.1998.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcelletti JF, Ohara J, Katz Dh. Collagen-induced arthritis in mice. Relationship of collagen-specific and total IgE synthesis to disease. J Immunol. 1991;147:4185–91. [PubMed] [Google Scholar]
- 28.Allen JB, Wong HL, Costa GL, Bienkowski MJ, Wahl Sm. Suppression of monocyte function and differential regulation of IL-1 and IL-1ra by IL-4 contribute to resolution of experimental arthritis. J Immunol. 1993;151:4344–51. [PubMed] [Google Scholar]


