Abstract
Bacteroides fragilis toxin (BFT) has been shown to be capable of inducing intestinal mucosal inflammation in animals. Such inflammation may be responsible for diarrhoea, which occurs in some, but not all human carriers of enterotoxigenic strains of B. fragilis (ETBF). We have studied responses to BFT by different human intestinal epithelial cell lines and subsequently investigated the expression of IL-8 and TGF-β by T84 cells. The latter were selected because their responses to BFT, characterized by morphological changes and cell death by apoptosis, were similar to those we have recently observed in primary human colonocytes. We show that BFT dose-dependently increased the expression of transcripts and protein of the polymorphonuclear cell chemoattractant IL-8. BFT also dose-dependently induced the release of TGF-β, which has been shown to enhance the repair of the injured intestinal epithelium. However, the secreted TGF-β was almost exclusively in the biologically inactive form, as determined by Mv1Lu bioassay. Our studies therefore suggest that exposure of colonic epithelial cells in vivo to high concentrations of BFT can initiate an inflammatory response via secreted IL-8. BFT-induced release of latent TGF-β may facilitate the subsequent repair of the injured epithelium, following its activation by proteases from neighbouring cells. Variation in cytokine responses by colonic epithelial cells in vivo could be an important determinant in the development of mucosal disease and symptoms in response to ETBF.
Keywords: Bacteroides fragilis, epithelial cells, cytokines
INTRODUCTION
Enterotoxigenic strains of Bacteroides fragilis (ETBF) were first isolated from the faeces of young farm animals with diarrhoea [1–4]. The potential role of these strains in inducing diarrhoea in humans has been of recent interest. In three epidemiological studies carried out in three different geographical areas, faecal isolation of ETBF has been associated with acute watery diarrhoea in children [5–7]. The enterotoxin (BFT) produced by ETBF is a metalloprotease [8] that induces rounding of cells of the human carcinoma cell line HT29 [9–12]. Such BFT-specific enterotoxic activity has been demonstrated in faecal samples of children with diarrhoea in whom ETBF was also isolated [13,14]. We have recently demonstrated the capacity of purified BFT to induce cytotoxicity in primary adult human colonic epithelial cells in organ culture [15]. Morphologically, the responses by the epithelial cells were characterized by cell rounding, separation from adjacent cells, and detachment from the basement membrane. In many organ cultures, BFT-exposed detached epithelial cells demonstrated characteristic features of cells undergoing apoptosis. Such epithelial cell responses are similar to those seen following exposure of primary colonocytes to Clostridium difficile toxin A [16].
The mechanism by which BFT may induce diarrhoea in animals and humans is unknown, but it is likely to be related to mucosal inflammation. Exposure of intestinal loops to purified BFT has been shown to induce mucosal inflammation characterized by the presence of a large number of polymorphonuclear cells (PMN [17]). Such mucosal inflammation may be initiated following BFT-induced epithelial cell injury, leading to the release of IL-8 [16], which is a potent chemoattractant of PMN.
In addition to IL-8, epithelial cells are also capable of producing TGF-β, which has been shown to enhance repair of ‘wounded’ epithelium by a process designated restitution [18,19]. TGF-β is also capable of enhancing barrier function [20,21], which is largely mediated via epithelial tight junctions. Thus, biological activity of TGF-β on the injured colonic epithelium in vivo would limit exposure of underlying immune cells to luminal bacteria and their products. The amount of bioactive TGF-β released by epithelial cells may therefore determine the extent of mucosal inflammation and damage. TGF-β is secreted as an inactive complex in which the bioactive dimer remains associated with the cleaved N-terminal glycopeptide. The bioactive dimer can be released from the inactive complex by proteases and extremes of pH [22].
The aims of our study were to (i) investigate responses to BFT by different epithelial cell lines, and (ii) study BFT-induced changes in the expression of IL-8 and TGF-β by an epithelial cell line in which responses to BFT are similar to those seen in primary colonocytes. Of the cell lines studied, we show that BFT-induced responses by T84 cells most closely reflect those by primary colonic epithelial cells. In the T84 cells, BFT dose-dependently increased the expression of IL-8 transcripts and protein. There was also induction of TGF-β release, which was mainly in the biologically inactive form.
MATERIALS AND METHODS
Purification of B. fragilis enterotoxin
BFT was purified from a highly toxigenic strain of ETBF (NCTC 11295) as previously described [15,23]. Briefly, B. fragilis NCTC 11295 was grown anaerobically at 37°C in prereduced brain heart infusion broth for 16–18 h. BFT was purified from culture supernatant by sequential ammonium sulphate precipitation, ion-exchange chromatography on Q-Sepharose (Pharmacia Biotech, Brussels, Belgium), hydrophobic interaction chromatography on phenyl-agarose (Sigma Chemical Co., St Louis, MO) and high resolution ion-exchange chromatography on a Mono-Q column (Pharmacia Biotech).
Purification of the toxin was monitored by its cytotoxic effect on HT29 cells (obtained from the European Collection of Animal Cell Cultures (ECACC), Porton Down, UK) characterized by cell rounding [9,10,12]. The cytotoxic titre of BFT was expressed as the reciprocal of the highest dilution of the toxin that causes rounding of >50% of HT29 cells after 4 h [10,24]. A cytotoxic unit (CU) was defined as the lowest amount of the toxin that elicited a positive response in HT29 cells (50% cell rounding at 4 h). The cytotoxic titre of our purified BFT ranged from 10 240 to 40 960 CU (protein concentration 1–5 μg/ml). BFT was frozen (−20°C) in aliquots immediately after purification.
Epithelial cell lines
The intestinal epithelial cell lines HT29, T84, Caco-2 and IEC-6 were obtained from the ECACC. They were grown to confluence in 96-well plates (Nunc, Gibco BRL, Gaithersburg, MD) for MTT assay or in 35-mm2 culture dishes (Costar, High Wycombe, UK) for transmission electron microscopy (TEM; see below).
Caco-2 cells (passages 40–43) were grown to confluence in Dulbecco’s minimal essential medium (DMEM) containing 10% fetal calf serum (FCS; Gibco BRL), 10 μg/ml transferrin (Sigma) and 2 mmol/l glutamine (Sigma). T84 cells (passages 80–85) were cultured in 50% DMEM −50% Ham’s F-12 medium (Gibco BRL) containing 10% FCS and 2 mmol/l glutamine. HT29 cells (passages 185–190) were grown to confluence in DMEM containing 10% FCS and 2 mmol/l glutamine. IEC-6 cells (passages 27–30) were grown in DMEM containing 5% FCS, insulin 5 μg/ml (Sigma) and 2 mmol/l glutamine.
The following antibiotics were present in all the culture media: 100 U/ml penicillin G (Britannia Pharmaceuticals, Surrey, UK), 0·1 mg/ml streptomycin (Evans Medical, Surrey, UK) and 0·1 mg/ml gentamycin (Roussel Labs Ltd, Uxbridge, UK).
Upon reaching confluence, the epithelial cell monolayers were washed and cultured in 1% FCS/DMEM, in the absence or presence of different concentrations of BFT.
For studies of IL-8 and TGF-β expression, T84 cells were grown in 12-well plates (see below). For IL-8 and TGF-β protein and TGF-β bioactivity, confluent monolayers of T84 cells were exposed to varying concentrations of BFT for 24 h before collection of supernatants. The supernatants were centrifuged (13 000 g for 5 min) and stored at −80°C before use in ELISA and bioassay (see below).
Assay for mitochondrial dehydrogenase activity
This assay was performed to assess the viability of different epithelial cell lines exposed to BFT. Metabolism by mitochondrial dehydrogenase of the yellow tetrazolium salt, 3-(4,5-dimetylthiozol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to the purple formazan reaction product can be quantified spectrophotometrically [16].
Confluent monolayers of epithelial cells (HT29, T84, Caco-2, IEC-6) were cultured in 96-well tissue culture plates (Nunc, Gibco BRL) and the MTT assays were performed in triplicate. After culture with BFT for varying time periods (24, 48, 72 and 96 h) MTT (Sigma) was added to the final concentration of 0·5 mg/ml to each well and incubation continued for 4 h before addition of 100 μl of solubilization solution (5% SDS in 0·1 mm HCl). Following overnight incubation, spectrophotometric absorbance of the samples was performed using an ELISA plate reader (Labsystem iEMS Reader MF) equipped with a 570-nm filter.
Transmission electron microscopy
After culture in the presence or absence of BFT for 96 h, T84 monolayers were fixed in 2·5% glutaraldehyde (in 0·5 m cacodylate buffer) for 4 h. Subsequently they were washed in PBS and postfixed in 1% osmium tetroxide for 1 h before dehydrating in ethanol and embedding in Epon resin, according to standard procedures [25]. Suitable areas for TEM were selected from 0·5 μm toluidine-stained sections. They were stained with uranyl acetate and lead citrate, before being observed in a Jeol 1200 EX transmission electron microscope.
Reverse transcriptase-polymerase chain reaction
Confluent monolayers of T84 cells were incubated in the presence of different concentrations of BFT for 24 h (at 37°C). The cells were then harvested for RNA extraction, as described below. The culture supernatants were filtered by a 0·22 μm pore size filter and stored at −20°C until use in ELISA or bioassay.
Reverse transcriptase-polymerase chain reaction (RT-PCR) was carried out as described previously [26]. Briefly, total RNA was obtained by cell lysis in RNAzol B (Biogenesis Ltd, Poole, UK), extraction with chloroform, and precipitation with isopropanol. Reverse transcription was performed using random primers and Moloney murine leukaemia virus reverse transcriptase (Gibco).
The following primer pairs were used in PCR to amplify cDNA: 5′ TTG GCA GCC TTC CTG ATT TCT 3′ and 5′ TTT CCT TGG GGT CCA GAC AGA 3′ to amplify a 220-bp IL-8 product; 5′ GGT GAA GTT CGG AGT CAA CGG A 3′ and 5′ GAG GGA TCT CGC TCC TGG AAG A 3′ to amplify a 240-bp glyceraldehyde-3-phosphate dehydrogenase (GAPDH) product. PCR cycles consisted of denaturation for 45 s at 95°C, annealing at 54°C for 90 s and extension at 72°C for 90 s. A total of 28 cycles were used to amplify IL-8 transcripts and 23 cycles for GAPDH. These cycles of amplification were optimized for the linear range of the PCR reaction [26,27]. PCR was completed by heating to 54°C for 2 min and 72°C for 3 min.
IL-8 and GAPDH PCR products were analysed by gel electrophoresis to confirm their size. They were subsequently applied to a nylon membrane and quantified using digoxigenin (DIG)-labelled (R&D Systems, Minneapolis, MN) oligonucleotide probes specific for each PCR product (5′ TCA TTG AGA GTG GAC C 3′ for IL-8 product and 5′ GCC TTG ACG GTG CCA 3′ for GAPDH product) [26,27].
Following hybridization and incubation with alkaline phosphatase-conjugated rabbit anti-DIG antibody (Boehringer Mannheim, Lewes, UK), the membranes were incubated with chemiluminescent substrate (CPS-Star; Boehringer Mannheim) and the photons emitted were measured by microplate scintillation spectrophotometer. The signal for the IL-8 product was recorded as counts per second and expressed as a ratio of the counts per second for the GAPDH product.
Control experiments to investigate for genomic DNA contamination of the RNA samples were always incorporated during the reverse transcription stage. An aliquot of each extracted RNA sample was set up without any reverse transcriptase enzyme and subsequently taken through the PCR amplification steps. No PCR product was detected in any of these control samples, confirming the lack of contamination by genomic DNA.
Sandwich ELISA for IL-8 and TGF-β1
Levels of IL-8 and TGF-β1 protein in culture supernatants of BFT-treated T84 cells were measured by ELISA. For IL-8, plates were coated with MoAb 208 (capture antibody; R&D Systems) and bound IL-8 was detected by biotinylated antibody (BAF 208; R&D Systems). Recombinant human IL-8 (208-IL-010; R&D Systems) was used as standard cytokine and sensitivity of the assay was 32 pg/ml, with inter- and intra-assay coefficient of variation <5%.
The amount of TGF-β1 secreted into the culture supernatant was determined using a human TGF-β1 quantikine kit from R&D Systems. This assay employs the quantitative sandwich enzyme immunoassay technique, using plates precoated with TGF-β soluble receptor type II. Samples were activated by acidification with 1 m HCl (to pH 2·0) for 10 min followed by neutralization (to pH 7·2–7·6) to transform latent TGF-β1 to the immunoreactive form (therefore total TGF-β1 in sample assayed). The sensitivity of the ELISA was 7 pg/ml, with inter- and intra-assay coefficient of variation <7·5%.
TGF-β bioassay
TGF-β bioactivity in culture supernatants of T84 cells exposed to BFT or control medium was assessed using the mink lung cell line Mv1Lu (ECACC) [28,29]. Cells (105/well) were seeded in 0·2% FCS/DMEM using 24-well plates (Costar). After 3 h, recombinant TGF-β1 (R&D Systems) or culture supernatants were added and culture continued for 24 h. Incorporation of 3H-thymidine over the last 3 h of culture was determined. Total content of TGF-β in culture supernatants was determined following acidification to pH 2·0 (by addition of HCl) for 1 h followed by neutralization using NaOH and HEPES (to a final concentration of 16 mmol/l).
Statistical analysis
The results were analysed by one-way analysis of variance and Student’s t-test.
RESULTS
Assessment of viability of intestinal epithelial cell lines exposed to BFT
Confluent monolayers of different epithelial cell lines (HT29, T84, Caco-2 and IEC-6) were exposed to different concentrations of BFT (4000–4 CU/ml) for 24, 48, 72 and 96 h. After each 24-h interval, viability was assessed by measurement of cellular mitochondrial dehydrogenase activity (MTT assay).
BFT exposure induced a dose- and time-dependent decrease in mitochondrial dehydrogenase activity in T84 cells (Fig. 1). After 96 h incubation at 4000 CU/ml and 400 CU/ml of the toxin, the mitochondrial dehydrogenase activity was virtually abolished.
Fig. 1.
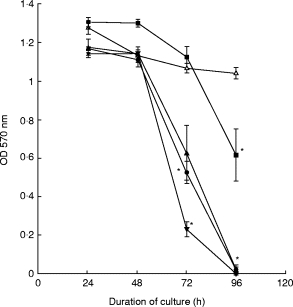
MTT assays on T84 monolayers exposed to Bacteroides fragilis toxin (BFT). Confluent monolayers of T84 cells were grown in 96-well plates in medium only or in medium containing 4 (▪), 40 (▴), 400 (•) or 4000 (▾) cytotoxic units (CU)/ml of BFT. After culture for varying time periods, MTT assays (which reflect cellular mitochondrial dehydrogenase activity) were performed. Assays were performed in triplicate and data points represent means (± s.e.m.). The figure shows one of three representative experiments. Δ, Control cells. *Statistically significant compared with control cells (P < 0·05).
In T84, Caco-2 and HT29 monolayers cell rounding was seen within 24 h of application of BFT. However, unlike T84 cells, there was no significant change in mitochondrial dehydrogenase activity in Caco-2 and HT29 monolayers despite exposure to 4000 CU/ml of BFT for 96 h (data not shown). In IEC-6 (non-transformed rat intestinal epithelial cell line) no morphological changes were observed by light microscopy despite exposure to high concentrations (4000 CU/ml) of BFT for 96 h (data not shown). In these cultures there was also no significant change in mitochondrial dehydrogenase activity (data not shown).
Electron microscopy
TEM of T84 cells exposed to 4000 CU/ml of BFT for 96 h showed an increase in space between intercellular tight junctions and cell rounding. Cells also became detached from the culture dish and from each other, and some of them showed characteristic morphological features of apoptosis (Fig. 2a,b).
Fig. 2.
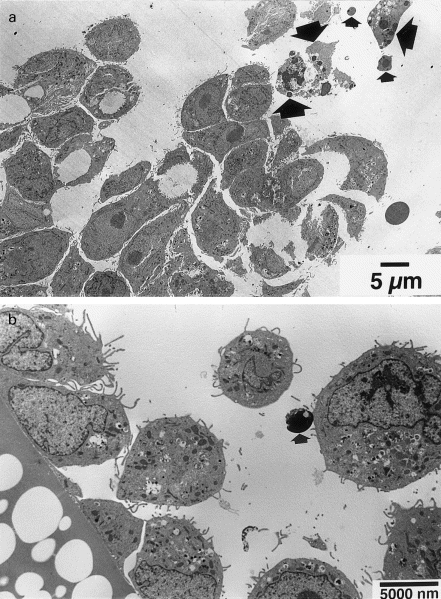
Transmission electron micrographs (TEM) of T84 cells exposed to Bacteroides fragilis toxin (BFT). Confluent monolayers of T84 cells were exposed to 4000 cytotoxic units (CU)/ml of BFT for 96 h before examination by TEM. In both (a) and (b), detached rounded epithelial cells are seen and some of these show characteristic morphological features of cells undergoing apoptosis (large arrows) and also apoptotic bodies (small arrows).
Expression of IL-8 and TGF-β
Of the epithelial cell lines studied, responses to BFT by T84 cells most closely resembled those by primary colonocytes exposed to the toxin [30], as shown by morphological changes and cell death by apoptosis. The T84 cell line was therefore chosen to investigate BFT-induced changes in expression of IL-8 and TGF-β. Semiquantitative RT-PCR showed that exposure to BFT, at a concentration of 4000 CU/ml, significantly enhanced the expression of IL-8 transcripts (Fig. 3).
Fig. 3.
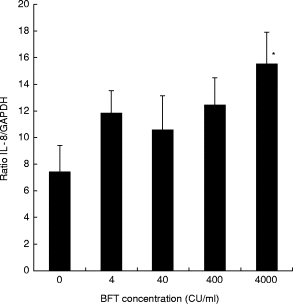
Expression of IL-8 transcripts by T84 cells exposed to Bacteroides fragilis toxin (BFT). Monolayers of T84 cells were exposed for 24 h to medium only or medium containing different concentrations of the toxin (4, 40, 400 or 4000 cytotoxic units (CU)/ml). Total RNA extracted from each sample was used for semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and enzyme-linked oligonucleotide chemiluminescent assay, as explained in Materials and Methods. The expression of IL-8 transcripts in each sample, relative to that of the constitutive marker glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was calculated by dividing the quantified IL-8 PCR products by the corresponding value for GAPDH products. The data represent mean (± s.e.m.) of four separate experiments. *Statistically significant compared with cells grown in control medium (P < 0·05).
Studies by ELISA showed that BFT induced the release of IL-8 by T84 cells in a dose-dependent fashion (Fig. 4).
Fig. 4.
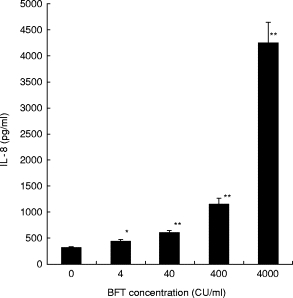
IL-8 production by T84 monolayers exposed to Bacteroides fragilis toxin (BFT). Monolayers of T84 cells were grown to confluence in 12-well plates and exposed to different concentrations of BFT (4, 40, 400 or 4000 cytotoxic units (CU)/ml) or control medium. After 24 h, culture supernatants were collected and assayed for IL-8 levels by ELISA. The data represent mean (± s.e.m.) of six separate experiments. *P < 0·05; **P < 0·001, versus cells grown in control medium.
BFT also dose-dependently induced the release of TGF-β1 by T84 cells, when assessed by ELISA (Fig. 5). The ELISA is able to detect only the bioactive form of TGF-β, and the assay was therefore performed following acid treatment (and subsequent neutralization) of supernatants of control and BFT-exposed T84 cells. In order to determine whether the untreated supernatants contained TGF-β in the biologically active and/or inactive forms, studies were performed using a specific bioassay. No TGF-β bioactivity was detected in untreated supernatants of BFT-exposed T84 cells (Table 1). However, following acidification (and subsequent neutralization) specific TGF-β bioactivity was detected in these supernatants. These studies suggest that the TGF-β released by BFT-exposed T84 cells is present almost exclusively in the biologically inactive (latent) form.
Fig. 5.
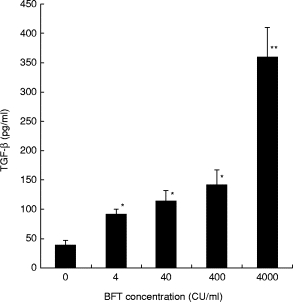
TGF-β1 production by T84 cells exposed to Bacteroides fragilis toxin (BFT). Monolayers of T84 cells were grown to confluence in 12-well plates and exposed to different concentrations of BFT (4, 40, 400 or 4000 cytotoxic units (CU)/ml) or control medium. After 24 h, culture supernatants were collected and acid-treated to activate latent TGF-β. Following neutralization, the supernatant samples were assayed for TGF-β1 levels by ELISA. The data represent mean (± s.e.m.) of six separate experiments. *P < 0·05; **P < 0·001, versus cells grown in control medium.
Table 1.
TGF-β bioactivity in culture supernatants of T84 cells exposed to Bacteroides fragilis toxin (BFT)†
| TGF-β (pg/ml)‡ | ||
|---|---|---|
| BFT concentration (CU/ml) | UTS | ATS |
| None | UD | 198·0 (±72·7) |
| 400 | UD | 284·0 (±14·0) |
| 4000 | UD | 478·0 (±51·4)* |
Confluent monolayers of T84 cells were exposed to BFT or control medium for 24 h. The culture supernatants were collected and TGF-β bioactivity was determined using Mv1Lu cells before (UTS) and after acid treatment (ATS).
The data represent mean (±s.e.m.) of three separate experiments. In each experiment, the supernatant samples were studied in triplicate. ATS, Acid-treated supernatants; UTS, untreated supernatants; UD, undetectable.
P < 0·05 versus cells grown in control medium.
DISCUSSION
Injection of rabbit intestinal loops with BFT induces epithelial cell injury and inflammation characterized by infiltration of the mucosa by polymorphonuclear cells [17]. The mechanisms by which BFT induces mucosal inflammation are unknown. Although BFT has been implicated in diarrhoeal disease in children [5–7], its capacity to induce mucosal inflammation in humans remains to be demonstrated. We have recently shown that purified BFT induces injury to primary human colonic epithelial cells [15]. This injury was characterized by cell rounding, separation from adjacent cells and detachment from the basement membrane. Many of the detached cells demonstrated characteristic features of cells undergoing apoptosis. It is of interest that the rate of BFT-induced colonic epithelial cell injury varied between individuals and in some no morphological changes to epithelial cells were seen despite exposure to high concentrations of the toxin [15]. ETBF and BFT have also been detected in stool samples of healthy individuals [14,31].
The aim of this study was to investigate cytokine responses to epithelial cells exposed to BFT. Of the two cytokines studied, IL-8 is a potent chemoattractant of polymorphonuclear cells [32], whereas TGF-β has been shown to enhance repair of the ‘wounded’ epithelium [18,19] and also to enhance epithelial barrier function [20,21]. Thus, the biological actions of these two cytokines could be important in not only the induction of mucosal inflammation but also the subsequent recovery of the epithelium. Because of difficulties in maintaining cultures of isolated primary colonic epithelial cells, initial studies were performed on four intestinal cell lines with the aim of identifying one that showed BFT-induced responses similar to those seen in our recent studies of primary colonocytes in organ culture [15]. Of the cell lines studied, T84 cells showed similarities (in response to BFT) most closely resembling those of primary colonocytes. These include morphological changes and evidence of cell death by apoptosis. Although Caco-2 and HT29 cells showed morphological changes characterized by cell rounding, cell death did not occur. The likely explanation for this is the presence of chromosomal abnormalities that regulate the capacity of the carcinoma-derived cells to undergo apoptosis. Similar, but less severe abnormalities may explain the delay in cell death in T84 cells, compared with primary colonocytes [15]. Lack of any BFT-induced morphological and cell viability changes in IEC-6 cells was surprising, as this is a non-transformed cell line [33]. The cause of this lack of an effect of BFT on IEC-6 is currently unknown.
Our studies do however suggest that in addition to heterogeneity in responses by primary colonocytes, there is also heterogeneity in responses by intestinal epithelial cell lines. Such heterogeneity in responses by different epithelial cell lines has also been reported previously [12,34]. Our recent studies have shown that BFT does not have any morphological effects on primary intestinal subepithelial myofibroblasts (unpublished observations), which are normally resident below the basement membrane [35]. MTT assays did not show loss of viability in response to BFT in these cells, nor in lymphocyte preparations (unpublished observations). This is in contrast to C. difficile toxin A, which induces cell death by apoptosis in T cells [30] as well as epithelial cells [16]. Such differences are likely to be related to the sites and mechanisms of action of the two toxins. Toxin A is internalized [36], whereas BFT appears to mediate its activity by proteolytic action on proteins involved in cell–cell [23,34,37] and matrix–cell interactions [8]. Indeed, recent studies have shown that BFT cleaves the zonula adherens protein, E-cadherin, in epithelial cells [38].
In view of the similarity of their response to primary colonocytes, T84 cells were chosen for studies to investigate BFT-induced changes in expression of IL-8 and TGF-β. Following exposure to the toxin, there was a dose-dependent increase in the expression of IL-8 mRNA and protein in T84 cells. This expression was most prominent in response to the highest concentration of BFT. Since IL-8 is a potent chemoattractant for polymorphonuclear cells, these studies suggest that BFT is capable of initiating an inflammatory response by interaction with epithelial cells. Previous in vitro studies on human intestinal epithelial cell lines have demonstrated the capacity of pathogenic microorganisms [39–42] or their toxic products [16] to induce the release of IL-8.
The mechanism by which BFT induces IL-8 production by epithelial cells remains to be determined. Since BFT is not internalized but appears to affect predominantly the cell–cell [34,37] and probably cell–matrix interactions, we postulate that the BFT-induced IL-8 production by epithelial cells occurs as a consequence of this specific type of cell injury. Our previous studies have shown that similar injury to primary colonic epithelial cells, by EDTA, which leads to detachment of cells from the basement membrane and neighbouring cells, induces the synthesis of IL-8 and subsequent cell death by apoptosis [16].
Exposure to BFT also induced the expression of TGF-β by T84 cells. Studies by ELISA and bioassay, following acidification to activate latent TGF-β, showed that the total amount of the peptide factor secreted by the epithelial cells increased in a dose-dependent fashion in response to BFT. TGF-β is usually secreted by cells in a biologically inactive latent form in which the bioactive dimer remains associated with the cleaved N-terminal glycopeptide [22]. Proteases and extremes of pH can release the bioactive TGF-β dimer from the inactive complex [22]. In our studies, no TGF-β bioactivity (by Mv1Lu assay) was detected in acid untreated supernatants of T84 cells exposed to BFT. This suggests that BFT induces the release of the latent form of TGF-β by T84 cells. BFT is a metalloprotease and was present in the supernatants studied for TGF-β bioactivity. Our study therefore suggests that BFT itself is not capable of activating the latent form of TGF-β and also that it does not induce T84 cells to release proteases capable of such activation. In vivo, activation of the released latent form of TGF-β may occur via proteases secreted by neighbouring cells such as myofibroblasts [43] and/or recruited inflammatory cells such as polymorphonuclear cells and macrophages. Such activation of latent TGF-β by a cell type other than the one secreting it has been demonstrated before [44].
Our studies therefore suggest that exposure of colonic epithelial cells in vivo to high concentrations of BFT can initiate an inflammatory response via secreted IL-8. BFT-induced release of latent TGF-β would be expected to facilitate the subsequent repair of the denuded epithelium, following its activation by proteases from neighbouring cells. Continual exposure of colonic epithelial cells to high concentrations of BFT (and consequent persistence of IL-8 release) would be postulated to lead to diarrhoea secondary to mucosal inflammation. Exposure of epithelial cells to low concentrations of BFT may not be enough to induce and/or sustain an inflammatory response. Thus luminal concentrations of BFT could explain the occurrence of mucosal inflammation (and diarrhoea) in some carriers of ETBF but not others. Variation in IL-8- and TGF-β-producing capacity of epithelial cells of different individuals could also be important determinants in the development of mucosal disease and symptoms.
Acknowledgments
This study was supported by the Medical Research Council. The electron micrograph studies used equipment funded by the Wellcome Trust. We thank Mr Trevor Gray for assistance. L.S. was on leave of absence from the Istituto di Microbiologia, Parma, Italy and was supported by the MURST ex 60% 1998 (02/16/01-04/23/48) and CNR (contract no. 97.04196.CT04).
References
- 1.Myers LL, Firehammer BD, Shoop DS, Border MM. Bacteroides fragilis: a possible cause of acute diarrheal disease in newborn lambs. Infect Immun. 1984;44:241–4. doi: 10.1128/iai.44.2.241-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers LL, Shoop DS, Firehammer BD, Border MM. Association of enterotoxigenic Bacteroides fragilis with diarrhea disease in calves. J Infect Dis. 1985;152:344–7. doi: 10.1093/infdis/152.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers LL, Shoop DS, Byars TD. Diarrhea associated with enterotoxigenic Bacteroides fragilis in foals. Am J Vet Res. 1987;48:1565–7. [PubMed] [Google Scholar]
- 4.Myers LL, Shoop DS. Association of enterotoxigenic Bacteroides fragilis with diarrhea disease in young pigs. Am J Vet Res. 1987;48:774–5. [PubMed] [Google Scholar]
- 5.Sack RB, Myers LL, Almeido-Hill J, Shoop DS, Bradbury WC, Reid R, Santosham M. Enterotoxigenic Bacteroides fragilis: epidemiologic studies of its role as a human diarrhoeal pathogen. J Diarrhoeal Dis Res. 1992;10:4–9. [PubMed] [Google Scholar]
- 6.Sack RB, Albert MJ, Alam K, Neogi PKB, Akbar MS. Isolation of enterotoxigenic Bacteroides fragilis from Bangladeshi children with diarrhea: a controlled study. J Clin Microbiol. 1994;32:960–3. doi: 10.1128/jcm.32.4.960-963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.San Joakin VH, Griffis JC, Lee C, Sears CL. Association of Bacteroides fragilis with childhood diarrhea. Scand J Infect Dis. 1995;27:211–5. doi: 10.3109/00365549509019011. [DOI] [PubMed] [Google Scholar]
- 8.Moncrief JF, Obiso R, Barroso LA, Kling JJ, Wright RL, Van Tassel RL, Lyerly DM, Wilkins TD. The enterotoxin of Bacteroides fragilis is a metalloprotease. Infect Immun. 1995;63:175–81. doi: 10.1128/iai.63.1.175-181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mundy ML, Sears CL. Detection of toxin production by Bacteroides fragilis: assay development and screening of extra-intestinal clinical isolates. Clin Infect Dis. 1996;23:269–76. doi: 10.1093/clinids/23.2.269. [DOI] [PubMed] [Google Scholar]
- 10.Pantosti A, Cerquetti M, Colangeli R, D’Ambrosio F. Detection of intestinal and extra-intestinal strains of enterotoxigenic Bacteroides fragilis by the HT-29 cytotoxicity assay. J Med Microb. 1994;41:191–6. doi: 10.1099/00222615-41-3-191. [DOI] [PubMed] [Google Scholar]
- 11.Van Tassel RL, Lyerly DM, Wilkins TD. Production of antisera against the enterotoxin of Bacteroides fragilis and their use in a cytotoxicity neutralization assay of HT-29 cells. Clin Diagn Lab Immun. 1994;1:473–6. doi: 10.1128/cdli.1.4.473-476.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weikel CS, Grieco FD, Reuben J, Myers LL, Sack RB. Human colonic epithelial cells, HT-29, treated with crude Bacteroides fragilis enterotoxin dramatically alter their morphology. Infect Immun. 1992;60:321–7. doi: 10.1128/iai.60.2.321-327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantosti A, Piersimoni C, Perissi G. Detection of Bacteroides fragilis enterotoxin in the feces of a child with diarrhea. Clin Infec Dis. 1994;19:809–10. doi: 10.1093/clinids/19.4.809. [DOI] [PubMed] [Google Scholar]
- 14.Pantosti A, Menozzi MG, Frate A, Sanfilippo L, D’Ambrosio F, Malpeli M. Detection of enterotoxigenic Bacteroides fragilis and its toxin in stool sample from adults and children in Italy. Clin Infect Dis. 1997;24:12–16. doi: 10.1093/clinids/24.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Sanfilippo L, Baldwin T, Borriello SP, Menozzi MG, Mahida YR. Heterogeneity in responses by primary adult human colonic epithelial cells to purified enterotoxin of Bacteroides fragilis. Gut. 1998;43:651–5. doi: 10.1136/gut.43.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahida YR, Makh S, Gray T, Borriello SP. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–47. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obiso RJ, Lyerly DM, Van Tassel RL, Wilkins TD. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid accumulation and intestinal damage in vivo. Infect Immun. 1995;63:3820–6. doi: 10.1128/iai.63.10.3820-3826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciacci C, Lind SE, Podolsky DK. Transforming growth factor β regulation of migration in wounded intestinal epithelial monolayers. Gastroenterology. 1993;105:93–101. doi: 10.1016/0016-5085(93)90014-4. [DOI] [PubMed] [Google Scholar]
- 19.Dignass AU, Podolsky D. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor β. Gastroenterology. 1993;105:1323–32. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 20.McKay DM, Singh PK. Superantigen activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via IFNγ and TNFα. J Immunol. 1997;159:2382–90. [PubMed] [Google Scholar]
- 21.Planchon SM, Martins C, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function by TGFβ1—evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–9. [PubMed] [Google Scholar]
- 22.Lyons RM, Gentry LE, Purchio AP, Moses HL. Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J Cell Biol. 1990;110:1361–7. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Tassel RL, Lyerly DM, Wilkins TD. Purification and characterization of an enterotoxin from Bacteroides fragilis. Infect Immun. 1992;60:1343–50. doi: 10.1128/iai.60.4.1343-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells CL, Van De Westerlo EMA, Jechorek RP, Feltis BA, Wilkins TD, Erlandsen SL. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology. 1996;110:1429–37. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- 25.Robinson G, Gray T. Electron microscopy 2. Tissue preparation, sectioning and staining. In: Bancroft JD, Stevens A, editors. Theory and practice of histological techniques. 3. London: Churchill Livingstone; 1990. pp. 525–62. [Google Scholar]
- 26.Li CKF, Seth R, Gray T, Bayston R, Mahida YR, Wakelin D. Production of proinflammatory cytokines and inflammatory mediators in human intestinal epithelial cells after invasion by Trichinella spiralis. Infect Immun. 1998;66:2200–6. doi: 10.1128/iai.66.5.2200-2206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlan JM, Seth R, Vautier G, Robins A, Scott B, Hawkey CJ, Jenkins D. Interleukin-8 and inducible nitric oxide synthetase mRNA levels in inflammatory bowel disease at first presentation. J Pathol. 1997;81:87–92. doi: 10.1002/(SICI)1096-9896(199701)181:1<87::AID-PATH736>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Danielpour D, Dart LL, Flanders KC, Roberts AB, Sporn MB. Immunodetection and quantitation of the two forms of transforming growth factor β (TGFβ1 and TGFβ2) secreted by cells in culture. J Cell Physiol. 1989;138:79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- 29.Mahida YR, Djelloul S, Ciacci C, deBeaumont M, Chevalier S, Gespach C, Podolsky DK. Resistance to TGFβ in SV40 large T-immortalized rat intestinal epithelial cells is associated with down-regulation of TGFβ type 1 receptor. Int J Oncol. 1996;9:365–74. [PubMed] [Google Scholar]
- 30.Mahida YR, Galvin A, Makh S, Hyde S, Sanfilippo L, Borriello SP, Sewell HF. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T cell apoptosis. Infect Immun. 1998;66:5462–9. doi: 10.1128/iai.66.11.5462-5469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers LL, Shoop DS, Stackhouse LL, Newman FS, Flaherty RJ, Letson GW, Sack RB. Isolation of enterotoxigenic Bacteroides fragilis from human with diarrhea. J Clin Microbiol. 1987;25:2330–3. doi: 10.1128/jcm.25.12.2330-2333.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oppenheim JJ, Zachariae COC, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene ‘intercrine’ cytokine family. Annu Rev Immunol. 1991;9:617–48. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 33.Quaroni A, Wands J, Trelstad RL, Isselbacher K. Epithelioid cell cultures from rat small intestine. J Cell Biol. 1979;80:245–65. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obiso RJ, Azghani AO, Wilkins TD. The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells. Infect Immun. 1997;65:1431–9. doi: 10.1128/iai.65.4.1431-1439.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273(Gastrointest Liver Physiol 36):G1341–8. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 36.Henriques B, Florin I, Thelestam M. Cellular internalisation of Clostridium difficile toxin A. Microb Pathol. 1987;2:455–63. doi: 10.1016/0882-4010(87)90052-0. [DOI] [PubMed] [Google Scholar]
- 37.Chambers FG, Koshy SS, Saidi RF, Clark DP, Moore RD, Sears CL. Bacteroides fragilis toxin exhibits polar activity on monolayers of human intestinal epithelial cells (T84 cells) in vitro. Infect Immun. 1997;65:3561–70. doi: 10.1128/iai.65.9.3561-3570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaoguang W, Lim K, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA. 1998;95:14979–84. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agace W, Hedges S, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–9. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckman L, Jung HC, Schurer-Maly C, Panja A, Morzycka-Wrobleska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of IL-8. Gastroenterology. 1993;105:1689–97. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 41.Schulte R, Wattiau P, Hartland EL, Robins-Browne RM, Cornelis GR. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent and nonvirulent Yersinia enterocolitica. Infect Immun. 1996;64:2106–13. doi: 10.1128/iai.64.6.2106-2113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–66. [PubMed] [Google Scholar]
- 43.McKaig BC, Makh SS, Hawkey CJ, Podolsky DK, Mahida YR. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGFβ3. Am J Physiol (Gastrointest Liver Physiol. 1999;276:G1087–93. doi: 10.1152/ajpgi.1999.276.5.G1087. [DOI] [PubMed] [Google Scholar]
- 44.Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor β1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–15. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]


