Abstract
Age-related impaired T cell function is associated with increased mortality risk. The purpose of the present study was therefore to identify factors associated with the age-related decreased phytohaemagglutinin (PHA)-induced proliferative response of lymphocytes in a cohort of 174 81-year-old humans and in 91 young controls. Decreased proliferation was associated with a reduced number of true naive CD4+ cells (CD62L+CD45RO−). Furthermore, a low IL-2-stimulated proliferation was correlated with a decreased PHA response in the elderly cohort, whereas reciprocal interactions of IL-10- and IL-2-producing cells were of importance in both elderly and young subjects. Accordingly, a minimum of true naive CD4+ cells was required for a normal proliferative response to PHA, perhaps by providing sufficient IL-2 which is critical for growth of naive as well as memory cells.
Keywords: ageing, proliferation, cytokines, naive T lymphocytes
INTRODUCTION
T cell proliferation declines with ageing and this may be important for the ability of cells to undergo clonal expansion and to set up an effective response to antigenic stimulations in vivo [1]. Furthermore, age-related increases in the proportion of memory T cells to naive T cells have consistently been reported [1]. Associations between increased mortality and lack of response to mitogens have been demonstrated in healthy, elderly humans [2–4]. Furthermore, age-associated decreases in the proliferative response may be more pronounced when mitogens such as phytohaemagglutinin (PHA) are used compared with well-defined activators such as anti-CD3 [5]. It has been suggested that the age-associated increased pool of memory T cells may represent a potential for immune dysregulation through the overproduction of certain immunoregulatory cytokines [6].
Isoforms of the leucocyte common antigen CD45 have been promoted as potential markers of memory T cells [7]. CD45RA+ cells and CD45RO+ cells are considered as nearly reciprocal subsets in humans and have been used as markers of naive cells and memory cells, respectively [7,8]. However, within the CD45RA+ cell compartment there is also a subset which shows characteristics of activated cells with regard to cytokine production and effector functions [9–11]. Furthermore, CD45RO+ T cells are able to revert to the CD45RA phenotype [8]. Accordingly, ‘true’ naive T cells are not defined by CD45 isoforms alone. CD62L plays an important role in the generation of primary T cell responses [8]. Thus, ‘true’ naive T cells co-express CD62L and CD45RA [12,13] and show distinct functions from CD62L− CD45RA+ cells [9,10]. Cytokine-activated naive T cells express the CD62L−CD45RA+CD45RO− phenotype and show functional features intermediate between naive and memory cells in vitro when stimulated by the T cell receptor [10]. A T cell subset with the same phenotype has been described in the blood in vivo [10]. CD62L−CD45RA+CD45RO−CD8+ cells isolated from the blood of humans have a cytokine profile similar to antigen-experienced cells, including low IL-2 production, and this subset shows potent cytotoxicity without previous in vitro stimulation [9].
The purpose of the present study was to evaluate the importance of T cell subsets and cytokine production for the proliferative response to PHA in a cohort of 81-year-old humans. Associations were investigated between, on one hand, the proliferative response to PHA, and on the other the lymphocyte phenotype, cytokine profile, and proliferative response to different stimulators (IL-2, pokeweed mitogen (PWM) and candida). Naive and memory T cells were identified by their constellation of the CD62L (l-selectin) adhesion molecule and the CD45RO isoform of the CD45 glycoprotein. Naive T lymphocytes were defined as CD62L+CD45RO− cells; memory T lymphocytes were defined as CD45RO+ cells; CD62L−CD45RO− cells, which may correspond to CD62L−CD45RA+ cells, were defined as intermediate cells. Furthermore, the importance of CD28 glycoprotein expression, which has a critical role in the costimulatory events that occur along with engagement of the T cell antigen receptor [14], was investigated. The in vivo priming of cytokine production was investigated by cytokine levels in supernatants from short-lived PHA stimulations of whole blood. IL-2 and interferon-gamma (IFN-γ) represented T helper 1 (Th1) and/or T cytotoxic 1 (Tc1) cytokines and IL-10 represented a Th2/Tc2 cytokine [15]. Ratios of IL-2/IL-10 and IFN-γ/IL-10 were calculated as measures for the balance between Th1/Tc1 and Th2/Tc2 responses.
SUBJECTS AND METHODS
Elderly subjects
The elderly subjects were from the 1914 cohort in Glostrup, which is a longitudinal study of ageing [16,17]. In 1995, survivors were asked to participate in a survey of octogenarians [18]. One hundred and seventy-four individuals aged 80–81 years (88/86 women/men) accepted an extra visit to the laboratory in the Department of Infectious Diseases, Rigshospitalet, for the collection of blood samples used in the immunological surveys. Immunological data are not complete for all subjects due to technical laboratory problems.
No one had dementia or suffered from acute illness prior to the collection of blood samples. Statistical analyses were done with and without subjects having disorders known or suspected to influence immune function: cancer at present or previously (n = 25), acute or chronic inflammatory disorders (n = 5); intakes of systemic corticosteroids (n = 7), > 100 mg acetyl salicylic acid (n = 8), or non-steroidal anti-inflammatory drugs (n = 20); and increased leucocyte counts in the blood (> 15 × 109/l, n = 1). In total, 55 elderly people were separated due to these criteria.
Young subjects
Forty-five healthy women volunteers and 46 healthy male volunteers aged median 25 years (range 19–31 years) were included as a young control group.
Blood samples were drawn in evacuated blood collection tubes supplemented with 25 IE heparin/ml blood between 7 a.m. and 10 a.m. after an overnight fast.
Isolation of BMNC
BMNC were isolated by density gradient centrifugation (Lymphoprep Nyegaard, Oslo, Norway) on LeucoSep tubes (Greiner, Frickenhausen, Germany) and washed three times in medium RPMI 1640 (Gibco, Grand Island, NY). Cells were frozen in freezing medium (50% RPMI 1640, 30% fetal calf serum (FCS; Gibco), and 20% dimethyl sulphoxide (DMSO; Bie Berntsen, Rødovre, Denmark)) and kept in liquid nitrogen until thawed for analysis.
Proliferative response
BMNC proliferation assay was performed as previously described [19]. Briefly, cell cultures were performed in triplicates in microtitre plates (Nunc, Roskilde, Denmark), 6 × 105BMNC were resuspended in 200 μl RPMI 1640/10% FCS and incubated with either medium alone, 20 μg/ml PHA, PWM diluted 1:2 × 103 to induce suboptimal lymphocyte proliferative responses (Life Technologies, Roskilde, Denmark), or 20 U/ml IL-2 (Boehringer, Mannheim, Germany). BMNC were also incubated for 7 days with medium or with ethanol-inactivated candida antigen titrated to the lowest concentration inducing an optimal lymphocyte proliferative response. During the last 24 h cells were exposed to 3H-thymidine. Cultures were collected on glassfibre filters with a harvesting machine (Micromate 196; Packard Instruments, Rockville, MD). 3H-thymidine incorporation was measured by a direct β-counter (Matrix 96 direct β-counter; Packard Instruments). For each triplicate the mean ct/min was calculated. BMNC from both young and elderly subjects were included in each experiment to ensure that the effect of day to day variation would affect the two groups similarly. Stimulation with PHA was chosen as a strong T cell mitogen [14]. In submaximal concentrations PWM stimulates mainly T cells through CD3 [20]. PWM has been shown to be a more sensitive predictor of disease progression than PHA in cohorts of HIV+ patients [21]. Exogenous IL-2 stimulates preferentially proliferation of memory T cells [22] and may compensate for an age-associated defect in the endogenous IL-2 production. Furthermore, IL-2 stimulates proliferation of natural killer (NK) cells. Candida is a recall antigen to which elderly as well as young subjects have been exposed.
Flow cytometry
BMNC were washed twice in PBS with 3% FCS. Cells (1.0 × 105) were resuspended in 100 μl PBS containing FCS and incubated for 30 min at 4°C with antibodies in volumes recommended by the manufacturer. Labelled cells were washed three times and analysed by a flow cytometer (Epics XL-MCL; Coulter, Hialeah, FL). The subsequent computer analyses were carried out using PC lysis software from Becton Dickinson (Oxnard, CA). The following antibodies were used: FITC-conjugated CD62L (clone FMC46; Dako, Glostrup, Denmark), PE-conjugated CD45R0 (clone UCHL1; Dako) and CD28 PE (clone L293; Becton Dickinson), and peridinin chlorophyll protein (PerCP)-conjugated CD3 (clone SK7; Becton Dickinson), CD4 PerCP (clone SK3; Becton Dickinson), and CD8 PerCP (clone SK1; Becton Dickinson). For determination of background staining, cells were incubated with the relevant mouse isotype antibodies as negative controls. Lymphocytes were distinguished from monocytes on the basis of their forward versus right angle light scatters, and controlled by a CD14 Fitch/CD45 PE double-stained sample. A lymphocyte gate was used for all the analyses. Figure 1 shows distinctions between high and low expression of CD62L and CD45RO.
Fig. 1.
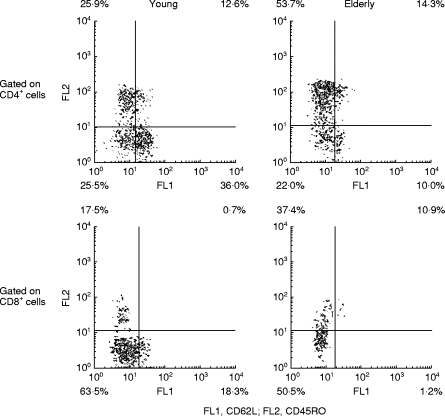
Distinctions between high and low expression of CD62L and CD45RO on CD4+ cells. BMNC were stained by CD62L–Fitch, CD45RO–PE, and CD4–peridinin chlorophyll protein (PerCP) or CD8–PerCP and analysed by flow cytometry. CD4+ or CD8+ lymphocytes were gated based on their forward versus right angle light scatters together with a high signal within the emission spectrum of PerCP. Distinctions between high and low expression of CD62L and CD45RO on T lymphocytes were based on mouse isotype antibodies as negative controls. Distributions of the different CD4+ and CD8+ subsets within lymphocytes were subsequently calculated.
Whole blood production of IL-2, IL-10 and IFN-γ
Whole blood was diluted 1:4 in RPMI 1640 (Kibbutz Beit Haemen, Biological Industries, Israel) and stimulated for 24 h with 5 μg/ml PHA (Difco, Detroit, MI) at 37°C. After centrifugation, supernatants were harvested and kept at −80°C until analysis in duplicates by commercially available ELISA kits (Quantikine; R&D Systems, Minneapolis, WI); the mean was used in the statistical analysis. Levels in unstimulated blood were below the detection limit of the assays.
Statistical analysis
Statistical calculations were performed using systat statistical software 7.0 (systat, Evanston, IL). Data are presented as medians and quartiles. Non-normally distributed data were square root (sqr) or log10 transformed in the statistical analyses. Differences between independent groups were tested by a t-test. Relationships between the proliferative response to PHA on one hand and the lymphocyte phenotype, cytokine production per lymphocyte, and proliferative response to different stimulations on the other were investigated by linear regression analysis with independent variables on a sqr or log10 scale. Each independent variable was tested for an interaction with the age group. If an interaction was found (P < 0.05), separate slopes were calculated for the elderly and the young controls. Analyses were performed univariately and in a multivariate model including naive and intermediate CD4+ cells (%). P < 0.05 was considered significant.
RESULTS
Elderly versus young humans
Proliferative responses to PHA, PWM, IL-2 and candida were reduced in the elderly cohort compared with young controls (Fig. 2). Furthermore, the elderly had reduced percentages of naive CD4+ and CD8+ cells (CD62L+CD45RO−), percent CD8+ cells (total), percent CD62L−CD45RO−CD8+ intermediate cells, and percent CD28+CD8+ cells compared with young controls, whereas percent CD62L−CD45RO−CD4+ intermediate cells were only borderline to be significantly decreased (P = 0.08). However, elderly people showed increased percentages of memory CD4+ and CD8+ cells (CD45RO+), percent CD28−CD8+ cells, and percent CD28−CD4+ cells. Percent CD4+ cells (total) and percent CD28+CD4+ cells were unaltered (Fig. 2). The unadjusted production of IL-10 was reduced in the elderly cohort, whereas there was no difference in the production of IFN-γ and IL-2 or in ratios of cytokines (Table 1).
Fig. 2.
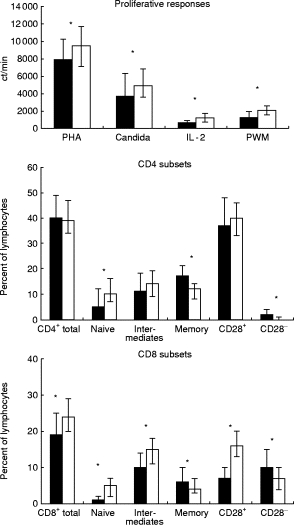
Proliferative responses, CD4 and CD8 subsets in elderly humans (▪) and in young controls (□). Medians and quartiles are shown. *P < 0.05. Proliferative responses to phytohaemagglutinin (PHA): elderly, n = 161 versus young, n = 87; candida: elderly, n = 118 versus young, n = 68; IL-2: elderly, n = 154 versus young, n = 87; pokeweed mitogen (PWM): elderly, n = 159 versus young, n = 87. CD4 subsets, CD4 total: elderly, n = 169 versus young, n = 90; naive, intermediates and memory subsets: elderly, n = 159 versus young, n = 86; CD28 subsets: elderly, n = 154 versus young, n = 79. CD8 subsets, CD8 total: elderly, n = 168 versus young, n = 91; naive, intermediates and memory subsets: elderly, n = 160 versus young, n = 86; CD28 subsets: elderly, n = 150 versus young, n = 79. Naive, CD62L+CD45RO− cells; intermediates, CD62L−CD45RO− cells; memory cells, CD45RO+ cells.
Table 1.
Levels of cytokines (ng/ml) in whole blood supernatants after phytohaemagglutinin stimulation for 24 h in young and elderly humans
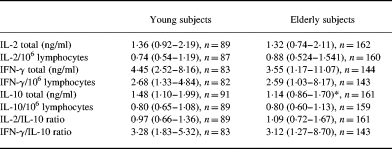
Medians (quartiles) are shown.
*P = 0.004.
Blood levels of CD4+ and CD8+ cells were decreased in the elderly cohort due to decreased concentrations of naive CD4+ and CD8+ cells (CD62L+CD45RO−), intermediate CD4+ and CD8+ cells (CD62L−CD45RO−), CD28+ cells, and CD28−CD4+ cells (Table 2). There was no difference with regard to concentrations of CD45RO+CD8+ memory cells and CD28−CD8+ cells, whereas the number of CD45RO+CD4+ memory cells was increased in the elderly subjects (Table 2).
Table 2.
Blood concentrations of CD4+ and CD8+ subsets in elderly and young humans
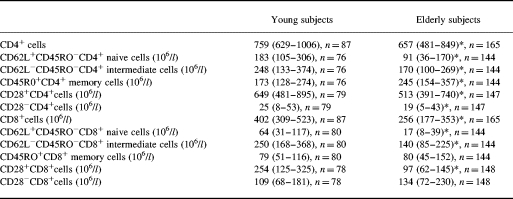
Medians (quartiles are shown).
*Significant difference (P < 0.05) from young controls.
Linear regression analyses (Table 3)
Table 3.
Linear regression analysis of the lymphocyte phenotype, cytokine profile, and proliferation after different stimulations on the proliferative response to phytohaemagglutinin (PHA) in elderly and young subjects
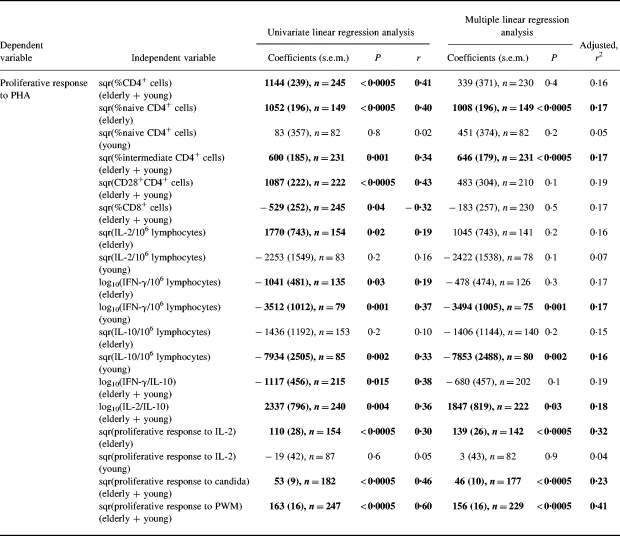
Each independent variable was tested for an interaction with age group—if an interaction was found (P < 0.05) separate slopes were calculated for elderly and young subjects. Multiple linear regression analysis refers to the model including percent CD62L+CD45RO−CD4+ naive cells and percent CD62L−CD45RO−CD4+ intermediate cells. Independent variables are square root (sqr) transformed or log10 transformed.
Naive: CD62L+CD45RO−; intermediates: CD62L−CD45RO−; memory: CD45RO+.
In both old and young people the proliferative response to PHA was positively associated with percent CD4+ cells (total), percent CD62L−CD45RO−CD4+ intermediate cells and percent CD28+ CD4+ cells. However, in the elderly cohort the proliferative response to PHA showed the strongest correlation to percent CD62L+CD45RO−CD4+ naive cells which, on the other hand, did not play a significant part in the response in the young group. Furthermore, multiple linear regression analyses showed that the effects of total percent CD4+ cells and percent CD28+CD4+ cells were not significant when adjusting for percent CD62L+ CD45RO−CD4+ naive cells and percent CD62L−CD45RO− CD4+ intermediate cells. In the following, associations between PHA-induced proliferation and other parameters were analysed with and without adjusting for the effect of naive and intermediate CD4+ cells (multiple linear regression analysis) because these two subsets together explained 17% of the variability in the proliferative response in both age groups.
The proliferative response to PHA was negatively correlated with percent CD8+ cells in a univariate analysis, but not when adjusting for the effect of naive and intermediate CD4+ cells. No associations were found to any particular subsets within the CD8+ cell compartment or to CD28−CD4+ cells and CD45RO+CD4+ memory cells (data not shown).
IL-2 production per lymphocyte in response to PHA was positively correlated with the proliferative response in the elderly but not in the young group. However, IL-2 production per lymphocyte showed no significant effect when adjusting for the effect of naive and intermediate CD4+ cells. IL-10 production per lymphocyte in response to PHA was not correlated to the proliferative response in the elderly, whereas it was negatively correlated to the proliferative response in the young group in both univariate and multiple linear regression analyses. IFN-γ production was negatively correlated to the proliferative response in both young and elderly subjects but the slope was significantly steeper in the young group. The effect was only significant in univariate regression analysis in the old cohort, whereas it was also significant in multiple linear regression analysis in young controls. The IL-2/IL-10 ratio was positively correlated to the proliferative response in both age groups in univariate as well as in multiple linear regression analyses. The IFN-γ/IL-10 ratio was only negatively correlated with PHA-induced proliferation in univariate linear regression analysis.
The proliferative response to PHA was correlated to proliferative responses following stimulation by candida or PWM. The effect was still significant when adjusting for the effect of naive and intermediate CD4+ cells (no age-associated differences in the slopes). The proliferative response to IL-2 was only correlated to the PHA response in the old group.
Conclusions were the same when elderly individuals with medical intakes or medical conditions known or suspected to influence the immune system were excluded (data not shown).
DISCUSSION
The major findings in the present study were that in 81-year-old people the PHA-induced proliferative response of BMNC was associated with: (i) percent CD62L+CD45RO−CD4+ naive cells; (ii) PHA-induced IL-2 production; (iii) the proliferative response to exogenous IL-2. However, these associations were not found in young controls. In both elderly and young people PHA-induced BMNC proliferation was correlated with the IL-2/IL-10 ratio and the proliferative response to PWM and candida.
With regard to linear interrelations between proliferative responses and phenotypes of lymphocytes, PHA-induced proliferation in the elderly cohort showed the strongest correlation to true naive CD4+ cells, whereas the association to the intermediate subtype was weak. In contrast, PHA-induced proliferation was only correlated with the intermediate CD4+ subset in the young group. Thus, the decreased presence of true naive CD4+ cells appeared to be a limiting factor in the elderly group, whereas the number might be far above the threshold that limits BMNC proliferation in the young group. Consistent with the present findings, PHA has been described mainly to stimulate proliferation of CD45RA+CD4+ cells [7], and CD62L has been shown to be crucial in primary T cell responses in CD62L-deficient mice [23]. Negative associations between percent CD8+ cells and PHA-induced BMNC proliferation seemed to be secondary to low percentages of CD4+ subsets (multiple linear regression analysis). Thus, a low percentage of CD4+ cells will often be accompanied by a high percentage of CD8+ cells.
In the elderly cohort IL-2 production showed a significant correlation with proliferation to PHA in univariate regression analysis, but not when the analysis was adjusted for the effect of naive and intermediate CD4+ cells. IL-2 production was also correlated with the number of true naive CD4+ cells (data not shown). This finding indicated that one major purpose of naive CD4+ cells might be to provide sufficient IL-2, which again was a limiting factor in the elderly but not in young controls. Whereas production of cytokines such as IFN-γ and IL-10 contributed negatively to BMNC proliferation in the young group, it was only of secondary importance to the reduced number of naive CD4+ cells in the elderly cohort. In contrast, a low IL-2/IL-10 ratio was still associated with a decreased proliferative response in multiple linear regression analysis. However, this effect did not seem to be age-dependent, as the slopes were the same for the two age groups. Thus, no evidence was found for an age-related decline in the proliferative response due to dysregulation of cytokines produced by memory cells or due to a shift from Th1/Tc1to Th2/Tc2 cytokine patterns. The last conclusion is limited by the fact that synthesis of IL-2 and IL-10 in humans is not as tightly restricted to a single subset as in mouse T cells [15], e.g. IL-10 is produced by human Th1 as well as Th2 clones, but Th2 clones show higher IL-10 mRNA levels [24]. Despite these considerations, we chose to let IL-10 represent an approximation to the Th2/Tc2 arm of the immune system because this cytokine, but not the classical Th2/Tc2 cytokine IL-4, can be detected following PHA stimulation. Furthermore, IL-10 is important to consider in the present context, due to the strong anti-proliferative effects. Consistent with the findings in the present study, the age-associated decrease in the proliferative response of BMNC has often been connected to decreased IL-2 production, but data are controversial and there is no consensus [25]. In accordance with the present study, IL-2 production by naive T cells were crucial for the proliferative response of naive as well as memory cells from mice, suggesting that low IL-2 production by memory cells limited their growth in cultures [26]. In addition to the finding that the IL-2/IL-10 ratio was important for the proliferative response in the present study, IL-10 blocked proliferation of isolated naive cells in mice [26]. Naive CD4+ cells from aged mice have been reported to show decreased [27–29] as well as unaltered [30,31] IL-2 production.
No age-associated decline in IL-2 production was found in the present study. This is in accordance with a study by Sindermann et al. [32], in which a similar whole blood culture system was used, although the incubation time was 48 h. IFN-γ and IL-10 are preferentially produced by memory cells. There is no consensus about IFN-γ production in human ageing [25]. Production of IL-10 by T cells has been reported increased in old humans [33,34] in contrast to the lack of age-associated changes in IL-10 and IFN-γ production in the present study. Discrepancies may be due to different incubation times. In this study, short-term stimulations were chosen, attempting to measure in vivo priming of cytokine production instead of stimulus-driven T cell differentiation in vitro. The former may be important for the proliferative response whereas the latter may have an impact on more differentiated effector functions. Whole blood culture stimulations were chosen because they mimic the in vivo environment and are the most reproducible condition of culture [35].
The correlation between proliferative responses to PHA and exogenous IL-2 in the elderly cohort but not in young controls suggested that a part of the age-related decrease in proliferative responses was associated with IL-2 insensitivity, and this could be isolated from the effect of decreased percentages of naive CD4+ cells. Thus, the multiple linear regression model including IL-2-induced BMNC proliferation and the percentage of naive and intermediate CD4+ cells explained 32% (R2) of the variability in PHA-induced proliferation in the elderly cohort. It is consistent with reports of an age-related deficit in the proliferative response even when endogenous IL-2 is high, lack of restoration by exogenous IL-2, and decreased IL-2 receptor expression [25]. However, it should be taken into account that proliferation of NK cells may also contribute to the IL-2 response in the present study. The strong correlations between proliferative responses to PHA on one hand and responses to PWM and candida on the other in both young and elderly people do not support the idea of a preserved T cell proliferation to recall antigens or stimuli mainly involving the CD3 receptor in ageing [36].
The expression of CD28 on CD8+ and CD4+ cells was decreased, as reported by others [37–39]. However, lack of CD28 expression was not associated with declined proliferative responses to PHA.
The elderly cohort in the present study represents an approximation to a normal population. Relatively few immunogerontological studies have based their investigations on well-described cohorts. Accordingly, it is poorly described to what extent normal elderly humans with and without chronic and/or age-associated diseases suffer from cellular immunosenescence. Furthermore, we attempted to investigate associations between various immune parameters, which are known to change with ageing, across and within two different age groups. Accordingly, it was important to include a large number of elderly subjects representing a wide spectrum of ‘physiological’ ageing but with the same chronological age. Exclusions of subjects with severe medical diseases did not influence the conclusions of the study. This is in accordance with the findings by others using even more strict exclusion criteria [40]. However, we chose not to use ‘The SENIEUR protocol’ which attempts to separate the influence of disease from physiological ageing by strict selection criteria [41]. This was based on the following considerations. With increasing age a growing proportion of individuals do not fulfil the SENIEUR protocol. Thus, only about 10% of the residents of a home for aged could be admitted in accordance with this protocol [42]. This leads to the question if the SENIEUR Protocol selects exceptional individuals instead of normal aged individuals. Furthermore, the protocol may exclude the subjects who have suffered most from the process of ageing, although these individuals may constitute the very population in which it is important to assess dysregulated immune function for its clinical relevance [43]. Additionally, changes in immune parameters may reflect ongoing pathological processes (e.g. atherosclerosis and dementia) which develop over decades. However, clinical symptoms may first show up late in the process, and in the meantime these immunological changes will be recognized as representing ‘physiological’ and/or ‘successful’ ageing, although they may be pathological. We thought it was questionable if physiological ageing could be separated from age-associated diseases. Furthermore, we found it would be bizarre to exclude 70–90% of the population we wanted to study. We chose to solve these problems by selecting our elderly individuals on epidemiological instead of biological criteria.
In conclusion, the present study demonstrated that in a cohort of elderly humans the decreased proliferative response to PHA was associated with a reduced number of true naive CD4+ cells. This indicates that a minimum of true naive CD4+ cells is required for a normal proliferative response to PHA, perhaps by providing sufficient IL-2 which is critical for growth of naive as well as memory cells. Furthermore, low IL-2 responsiveness was associated with decreased PHA response in the elderly cohort but not in young controls. The age-associated decreased proliferative response to PHA and lack of naive CD4+ cells may have clinical implications by inducing an immunodeficiency similar to what is seen in HIV+ subjects [44].
Acknowledgments
The excellent assistance of Hanne Willumsen, Ruth Rousing, Leila Jacobsen, Birgit Jensen and Gitte Petersen is acknowledged. The research was supported by the Velux Foundation and The Danish Foundation for the Advancement of Medical Science. H.B. was supported by a grant from The Danish Research Council 9503346.
References
- 1.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 2.Murasko DM, Weiner P, Kaye D. Decline in mitogen induced proliferation of lymphocytes with increasing age. Clin Exp Immunol. 1987;70:440–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts TI, Whittingham S, Youngchaiyud U, Mackay IR. Ageing, immune response, and mortality. Lancet. 1974;2:368–70. doi: 10.1016/s0140-6736(74)91755-3. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol Biol Sci. 1995;50:B378–B382. doi: 10.1093/gerona/50a.6.b378. [DOI] [PubMed] [Google Scholar]
- 5.Sansoni P, Brianti V, Fagnoni F, et al. NK cell activity and T-lymphocyte proliferation in healthy centenarians. Ann NY Acad Sci. 1992;663:505–7. doi: 10.1111/j.1749-6632.1992.tb38717.x. [DOI] [PubMed] [Google Scholar]
- 6.Ernst DN, Weigle WO, Hobbs MV. Aging and lymphokine production by T cell subsets. Stem Cells. 1993;11:487–98. doi: 10.1002/stem.5530110618. [DOI] [PubMed] [Google Scholar]
- 7.Akbar AN, Salmon M, Janossy G. The synergy between naive and memory T cells during activation. Immunol Today. 1991;12:184–98. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- 8.Bell EB, Spartshott S, Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen—a unifying concept. Immunol Today. 1998;19:60–64. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unutmaz D, Baldoni F, Abrignani S. Human naive T cells activated by cytokines differentiate into a split phenotype with functional features intermediate between naive and memory T cells. Int Immunol. 1995;7:1417–24. doi: 10.1093/intimm/7.9.1417. [DOI] [PubMed] [Google Scholar]
- 11.Rothstein DM, Sohen S, Daley JF, Schlossman SF, Morimoto C. CD4+CD45RA+ and CD4+CD45RA− T cell subsets in man maintain distinct function and CD45RA expression persists on a subpopulation of CD45RA+ cells after activation with Con A. Cell Immunol. 1990;129:449–67. doi: 10.1016/0008-8749(90)90220-l. [DOI] [PubMed] [Google Scholar]
- 12.Hengel RL, Jones BM, Kennedy MS, Hubbard MR, McDougal JS. Lymphocyte kinetics and precursor frequency-dependent recovery of CD4 (+) CD45RA (+) CD62L (+) naive T cells following triple-drug therapy for HIV type 1 infection. AIDS Res Hum Retrovir. 1999;15:435–43. doi: 10.1089/088922299311187. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman J, Trimble LA, Friedman RS, et al. Expansion of CD57 and CD62L−CD45RA+ CD8 T lymphocytes correlates with reduced viral plasma RNA after primary HIV infection. AIDS. 1999;13:891–9. doi: 10.1097/00002030-199905280-00004. [DOI] [PubMed] [Google Scholar]
- 14.Weis A. T lymphocyte activation. In: Paul WE, editor. Fundamental immunology. New York: Raven Press; 1993. pp. 467–504. [Google Scholar]
- 15.Mosmann TR, Sad S. The expanding universe of T cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 16.Schroll M, Jorgensen T, Ingerslev J. The Glostrup Population Studies, 1964–92. Dan Med Bull. 1992;39:204–7. [PubMed] [Google Scholar]
- 17.Schroll M. A ten-year prospective study, 1964–74, of cardiovascular risk factors in men and women from the Glostrup population born in 1914. Dan Med Bull. 1982;29:213–52. [PubMed] [Google Scholar]
- 18.Schroll M, Avlund K, Davidsen M. Predictors of five-year functional ability in a longitudinal survey of men and women aged 75–80. The 1914-population in Glostrup, Denmark. Aging Milano. 1997;9:143–52. doi: 10.1007/BF03340140. [DOI] [PubMed] [Google Scholar]
- 19.Tvede N, Pedersen BK, Hansen FR, et al. Effect of physical exercise on blood mononuclear cell subpopulations and in vitro proliferative responses. Scand J Immunol. 1989;29:383–9. doi: 10.1111/j.1365-3083.1989.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann B, Ødum N, Platz P, Ryder LP, Svejgaard A. Immunological studies in acquired immunodeficiency syndrome. Scand J Immunol. 1985;21:235–43. doi: 10.1111/j.1365-3083.1985.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann B, Lindhardt BO, Gerstoft J, et al. Lymphocyte transformation response to pokeweed mitogen as a predictive marker for development of AIDS and AIDS related symptoms in homosexual men with HIV antibodies. Br Med J Clin Res Ed. 1987;295:293–6. doi: 10.1136/bmj.295.6593.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taga K, Kasahara Y, Yachie A, Miyawaki T, Taniguchi N. Preferential expression of IL-2 receptor subunits on memory populations within CD4+ and CD8+ T cells. Immunology. 1991;72:15–19. [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183:589–98. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del PG, De CM, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 25.Hobbs MV, Ernst DN. The role of cytokines in aging. In: Aggarwal BB, Puri RK, editors. Human cytokines: their role in disease and therapy. Cambridge, MA: Blackwell Science; 1996. pp. 353–63. [Google Scholar]
- 26.Dozmorov IM, Miller RA. Regulatory interactions between virgin and memory CD4 T lymphocytes. Cell Immunol. 1996;172:141–8. doi: 10.1006/cimm.1996.0226. [DOI] [PubMed] [Google Scholar]
- 27.Haynes L, Linton PJ, Swain SL. Age-related changes in CD4 T cells of T cell receptor transgenic mice. Mech Ageing Dev. 1997;93:95–105. doi: 10.1016/s0047-6374(96)01826-x. [DOI] [PubMed] [Google Scholar]
- 28.Nagelkerken L, Hertogh HA, Dobber R, Drager A. Age-related changes in lymphokine production related to a decreased number of CD45RBhi CD4+ T cells. Eur J Immunol. 1991;21:273–81. doi: 10.1002/eji.1830210206. [DOI] [PubMed] [Google Scholar]
- 29.Kurashima C, Utsuyama M. Age-related changes of cytokine production by murine helper T cell subpopulations. Pathobiology. 1997;65:155–62. doi: 10.1159/000164117. [DOI] [PubMed] [Google Scholar]
- 30.Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur J Immunol. 1989;19:977–82. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 31.Flurkey K, Stadecker M, Miller RA. Memory T lymphocyte hyporesponsiveness to non-cognate stimuli: a key factor in age-related immunodeficiency. Eur J Immunol. 1992;22:931–5. doi: 10.1002/eji.1830220408. [DOI] [PubMed] [Google Scholar]
- 32.Sindermann J, Kruse A, Frercks HJ, Schutz RM, Kirchner H. Investigations of the lymphokine system in elderly individuals. Mech Ageing Dev. 1993;70:149–59. doi: 10.1016/0047-6374(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 33.Castle S, Uyemura K, Wong W, Modlin R, Effros R. Evidence of enhanced type 2 immune response and impaired upregulation of a type 1 response in frail elderly nursing home residents. Mech Ageing Dev. 1997;94:7–16. doi: 10.1016/s0047-6374(96)01821-0. [DOI] [PubMed] [Google Scholar]
- 34.Cakman I, Rohwer J, Schutz RM, Kirchner H, Rink L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech Ageing Dev. 1996;87:197–209. doi: 10.1016/0047-6374(96)01708-3. [DOI] [PubMed] [Google Scholar]
- 35.De GD, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–48. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 36.Sansoni P, Fagnoni F, Vescovini R, et al. T lymphocyte proliferative capability to defined stimuli and costimulatory CD28 pathway is not impaired in healthy centenarians. Mech Ageing Dev. 1997;96:127–36. doi: 10.1016/s0047-6374(97)01887-3. [DOI] [PubMed] [Google Scholar]
- 37.Fagnoni F, Vescovini R, Mazzola M, et al. Expansion of cytotoxic CD8 (+) CD28 (−) T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–7. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Effros RB, Walford RL, Porter V, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 39.Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4 (+) CD28 (−) T cells in the aging immune system. Mech Ageing Dev. 1998;102:131–47. doi: 10.1016/s0047-6374(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 40.Traill KN, Schonitzer D, Jurgens G, et al. Age-related changes in lymphocyte subset proportions, surface differentiation antigen density and plasma membrane fluidity: application of the eurage senieur protocol admission criteria. Mech Ageing Dev. 1985;33:39–66. doi: 10.1016/0047-6374(85)90108-3. [DOI] [PubMed] [Google Scholar]
- 41.Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 42.Ligthart GJ, Corberand JX, Geertzen HG, Meinders AE, Knook DL, Hijmans W. Necessity of the assessment of health status in human immunogerontological studies: evaluation of the SENIEUR protocol. Mech Ageing Dev. 1990;55:89–105. doi: 10.1016/0047-6374(90)90108-r. [DOI] [PubMed] [Google Scholar]
- 43.Pawelec G, Adibzadeh M, Pohla H, Schaudt K. Immunosenescence: ageing of the immune system. Immunol Today. 1995;16:420–2. doi: 10.1016/0167-5699(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 44.Ullum H, Lepri AC, Victor J, Skinhoj P, Phillips AN, Pedersen BK. Increased losses of CD4+CD45RA+ cells in late stages of HIV infection is related to increased risk of death: evidence from a cohort of 347 HIV-infected individuals. AIDS. 1997;11:1479–85. doi: 10.1097/00002030-199712000-00012. [DOI] [PubMed] [Google Scholar]


