Abstract
Cachexia is a prominent feature of advanced tuberculosis, in association with increased expression of the monokine tumour necrosis factor (TNF)-α. Monocytes, have high affinity receptors (mannose, complement and Fc γ1 and γ111) which mediate antigen uptake and subsequent cytokine activation. Several mycobacterial proteins, including PPD, can stimulate TNF-α secretion from monocytes. However, the role of various receptors in stimulating or regulating TNF-α secretion is still unclear. We have previously shown selective augmentation of opsonic antibodies (IgG1 and IgG3) in tuberculosis patients with advanced pulmonary disease. We now analyse the role of opsonizing antibodies in modulating TNF-α expression in antigen stimulated monocytes. PPD was used as the prototypic mycobacterial antigen to stimulate monocytes from PPD skin test negative donors (n = 7) in the presence of plasma from tuberculosis patients (n = 8), containing known amounts of IgG1 and IgG3 anti-PPD antibodies. TNF-α secretion was enhanced in the presence of TB plasma (4/8) but not in the presence of control plasma. Using Spearman Rank analysis (two-tailed Fisher exact test), a significant correlation (rho = 0.762; P = 0.04) was observed between IgG1 antibodies and enhancement of TNF-α secretion. No significant association was observed with IgG2 (rho = 0.310; P = 0.41), IgG3 (rho = 0.089; P = 0.81) or IgG4 (rho = − 0.357; P = 0.347) subclass antibodies. Absorption of IgG1 with protein ‘A’ removed the enhancement of TNF-α secretion activity from the plasma samples. Our results therefore indicate that IgG1 antibodies may enhance the chronic release of TNF-α in TB patients with progressive disease and, for the first time, show a direct link between disease pathogenesis and raised antibody levels.
Keywords: IgG antibody subclasses, TNF-α, tuberculosis, monocyte, cytokines
INTRODUCTION
The rational design of a more effective vaccine and chemotherapeutic agents against tuberculosis requires a better understanding of the pathogenesis of infection and the early steps in uptake by phagocytes and immune activation. Virulent organisms enter the macrophages via the mannose receptor and can inhibit the phago-lysosome fusion [1]. The presence of serum components, such as complement factors which mediate uptake via the CR1/CR3 complement receptors [2], can also bind to mycobacteria and influence this event. It has been shown that while the mannose receptor selectively mediates uptake of virulent organisms, complement receptors can mediate uptake of both virulent and nonvirulent organisms [1]. Another serum factor that may influence macrophage mediated uptake is opsonising antibody for which macrophages have high affinity Fc receptors [3]. Among the four subclasses of IgG, we have recently shown a selective shift in opsonising antibodies (IgG1 and IgG3 subclass) in patients with disseminated leprosy [4] as well as in advanced pulmonary tuberculosis [5]. However, to date, these antibodies are considered surrogate markers of disease progression and no direct link between IgG1 and IgG3 antibodies to disease pathogenesis has been established. We addressed this issue in the current study.
The four subclasses of human IgG differ in terms of their serum concentrations and biological activity, as well as in their ability to activate other cells of the immune system [6]. IgG1 constitutes the highest proportion (70%) of serum IgG and activates the complement cascade, thus being part of the pro-inflammatory arm of the immune system. Mononuclear phagocytes, in which, mycobacteria reside and multiply, have high affinity receptors (Fc γ1 and γ3) for IgG1 and IgG3 antibody subclasses [6]. Mycobacterial specific IgG1/IgG3 antibodies could therefore facilitate entry of mycobacteria into monocytes via the Fc receptors, as opposed to uptake via mannose or the complement receptors, which may result in differential activation of monocytes and modulation of the effector function such as cytokine secretion. Clinical and pathological features of tuberculosis include fever, anorexia and prolonged acute phase response and recruitment of T lymphocytes to granuloma. Such clinical manifestations and tissue events depend in part upon the orchestrated secretion of a number of pro-inflammatory cytokines including tumor necrosis factor (TNF), IL-1 and IL-6. TNF and IL-1 are endogenous pyrogens while IL-6 induces the hepatic acute phase response. TNF was originally described as chacectin and was implicated in anorexia and fever [7, 8]. TNF has also been shown to be the key modulator of pathogenesis in endotoxin shock, bacterial sepsis and severe complications in diseases such as malaria, leishmania and reactional complications of leprosy [9,10,11–12]. Several mycobacterial proteins, including PPD, can directly stimulate TNF-α secretion from monocytes [13, 14]. This observation was considered a rational explanation for chronic release of TNF-α in advanced or progressive tuberculosis. In the current study, we analysed the effect of opsonizing (IgG1/IgG3) antibodies present in the plasma of tuberculosis patients, on the secretion of TNF-α from PPD-stimulated monocytes obtained from healthy donors.
MATERIALS AND METHODS
Antigens
Mycobacterium tuberculosis PPD and culture filtrates were prepared in Dr Wallis's laboratory using the standard methodology [15]. Briefly M. tuberculosis strain H37 Rv was grown in roller bottles in Proskauer Beck medium (Difco, Middlebrook, MI). Bacilli were removed by sedimentation and filtration. Proteins were precipitated in 60% saturated ammonium sulphate, redissolved in H2O and dialysed against ultra pure water. Protein concentration was determined using the Bradford method [14]. Escherichia coli endotoxin (LPS) and Polymyxin B were obtained from Sigma Chemicals (St Louis, MO, USA).
Plasma samples from tuberculosis patients and controls
Plasma samples were obtained from pulmonary tuberculosis patients (n = 8) with microscopically and culture proven tuberculosis in Karachi, Pakistan. Patients had moderate (PMD) to advanced disease (PAD) which was ranked according to the tissue involvement as described previously [16]. Venous blood was collected in heparinized syringes. Plasma was separated from heparinized blood separated on a ficoll layer and the top layer was carefully removed to avoid mixing with ficoll. In addition, plasma from three healthy PPD skin test negative control donors was also obtained as described above. Sterile endotoxin free conditions were used for handling and separation of blood samples. The plasma samples were distributed in small aliquots and stored at − 70°C until further use. Samples were only thawed once before use in the assays.
Reagents monoclonal antibodies and conjugates
Monoclonal antibodies specific for human IgG subclasses were: HP 6001(anti IgG1), HP 6002 (anti IgG2), HP 6047 (anti IgG3), HP 6023 (anti IgG4) and HP 6029 (anti IgE), all prepared at the Center for Disease Control (Atlanta, GA, USA), and were a gift from Dr Reimer. The specificity evaluation and performance characteristics of these antibodies are described in detail elsewhere [17, 18]. Goat antihuman IgG (Fc specific) and goat antimouse IgG (H + L chain specific), conjugated to alkaline phosphatase were commercially obtained (Jackson Immuno Research Laboratories, Westgrove, PA, USA) and diluted according to the manufacturer's recommendations.
Determination of IgG subclass activity in TB plasma
IgG subclass antibodies to PPD in TB plasma were determined using an ELISA based assay described in detail previously [5]. Briefly, Immulon four plates were coated with 100 μl culture filtrate antigen at 1 μg/ml in carbonate buffer pH 9.6 for 2 h at 37°C and then overnight at 4°C. PBS containing 5% BSA was added for 2 h at 37°C to block free sites. Some 100 μl of sera diluted in PBS containing 0.05% Tween 20 and 1.0% BSA were added and incubated for 2 h at 37°C and subsequently overnight at 4°C. For IgG subclasses, monoclonal antibodies specific for each of the IgG subclasses and IgE were added at saturation concentrations of 1:1000 for HP 6001, HP6002 and HP 6047 and further incubated overnight at 4°C. Alkaline phosphatase-labelled goat antimouse IgG was added and incubated for 2 h at 37°C. The plates were finally developed with alkaline phosphatase substrate. Each incubation was followed by three washes with PBS containing 0.05% Tween 20 to remove unbound protein. All test sera were run at a minimum of three dilution and the activity was expressed as optical density in the linear range × dilution of the sera. In each assay, a reference pool containing high titre of IgG subclass antibodies was used as a calibrator for interassay variability. Pooled sera from normal healthy donors were used as the negative control for each assay. The samples were transported to Case Western Reserve University, Cleveland, OH, USA, on dry ice for further testing in monocyte stimulation assays. In addition, antibodies were also determined in three healthy donors who were skin test negative. The distribution of antibody activity in all the plasma samples is shown in Table 1.
Table 1.
Distribution of IgG subclass antibodies to PPD in plasma of tuberculosis patients with pulmonary disease
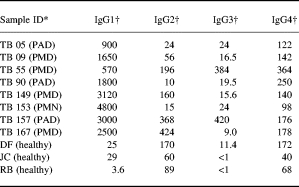
* PMD, moderate disease; PAD, advanced disease.
† Expressed as U/ml (1U = 0.1OD).
Absorption of plasma with protein ‘A’ to remove antibody activity
Insolubilized protein ‘A’ (Sigma chemicals, St Louis, MO, USA) was used to absorb IgG antibodies. Protein A was reconstituted in PBS and washed several times by repeated suspension and centrifugation. Protein A was then equilibrated with RPMI to a final concentration of 50% volume by volume suspension. Aliquots (0.5 ml) of the suspension were prepared and an equal volume of plasma was added to the suspension. The tubes were rotated for 30 min at 37°C and the suspension was filtered through a 5.0 ml of autoclavable syringe containing a small layer of glass wool. The clear supernate was collected and distributed in small aliquots and stored at − 70°C until further use.
Isolation of peripheral blood mononuclear cells and monocytes
Healthy volunteers (n = 7) who were PPD skin test negative were used as donors. Five males and two females were included of whom six donors were caucasions of European origin and one was an oriental. Venous blood was collected in heparinized syringes diluted 1:2 with RPMI 1640 and separated on Ficoll gradient (Pharmacia, Piscataway. NJ, USA) at a centrifugal speed of 1200 r.p.m. for 30 min at room temperature. Peripheral blood mononuclear cells (PBMC) were isolated from the interface and washed three times in RPMI 1640. Approximately 50 × 106 cells were plated in 100 mm polystyrene tissue culture plates (no. 25020; Corning, NY, USA) which had been precoated with 1.5 ml of heat-inactivated pooled human serum (PHS) for 30 min at 37°C. After 1 h of incubation at 37°C, plates were washed twice with 10% foetal calf serum in RPMI 1640. Five ml of cold HBSS was added and plates were placed in the refrigerator for 10–20 min. Adherent cells were scraped off and suspended in Iecove's modified Dulbecca's medium (IMDM) medium containing 1% heat inactivated autologous serum. IMDM medium allows culture of monocytes with lower serum concentrations. Both pooled human serum and autologous serum was heat inactivated at 56°C for 30 min to minimize any complement-mediated effects in the subsequent monocyte stimulation assay.
Stimulation of monocytes
PPD (1 μg/ml) was used to stimulate purified adherent cells. E. coli LPS (0.1 μg/ml) was used as a positive control to assess the functionality of the culture conditions. Polymyxin B was added at a final concentration of 10 μg/ml to detect LPS contamination in PPD and plasma samples. For monocyte stimulation in the presence of IgG antibodies, antigens were preincubated with plasma (1:500) at 37°C for 1 h in 48-well tissue culture plates (Costar, Cambridge, MA, USA) in a volume of 250 μl per well. Purified adherent cells were later added in a volume of 250 μl per well and at a density of 1 × 106 cells per ml. Monocytes were incubated at 37°C for 24 h and supernatants collected and frozen in 100 μl aliquots − 70°C for cytokine assessment.
Assessment of TNF-α secretion
Monoclonal antibody pairs (capture and probe) for TNF-α detection was purchased from Pharmingen (San Diego, CA, USA). Assessment of TNF-α in supernatants was carried out using the standard methodology recommended by the manufacturer.
Statistical analysis
Spearman rank analysis was carried out using the two-tailed Fisher exact test. Graphs were generated using Macintosh Cricket Graph 111 software package (Computer Associates, Inc., USA).
RESULTS
Assessment of the functionality of the monocyte stimulation assay
Optimal amounts of PPD and LPS were used to stimulate purified monocytes. LPS is a highly sensitive indicator of monocyte activation. Polymyxin B, which is a potent inhibitor of LPS mediated activation, was also included as a control to detect LPS contamination of PPD and plasma samples being tested in the study. TNF-α secretion was assessed in supernatants after 24 h of stimulation. Figure 1 shows the results in one PPD skin test negative donor. As expected, LPS induced high concentrations of TNF-α secretion and was completely inhibitable by polymyxin B indicating that our monocyte culture system was functioning optimally. As previously reported, direct stimulation of TNF-α secretion was also observed with PPD but this release was not inhibitable by polymyxin B indicating that the TNF-α secretion in PPD stimulated monocytes was not due to LPS contamination. This assay system was then used to test the effect of opsonising antibodies in PPD stimulated cultures.
Fig. 1.
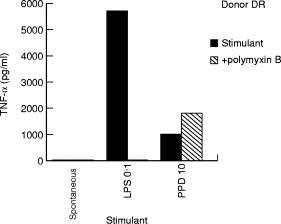
Functional assessment of adherent cell assay for TNF release from a single PPD skin test negative donor (DR). Purified adherent cells (1 × 106 cells in 500 μl) were stimulated with either LPS (0.1 μg) or PPD (10 μg) in the presence and absence of polymyxin B (10 μg). Spontaneous release of TNF-α by adherent cells was also assessed without any added stimulant (spontaneous), and was <10 pg/ml for all plasma samples tested.
Enhancement of TNF-α secretion by mycobacterial antigens in the presence of plasma from tuberculosis patients
The distribution of IgG subclass antibody to PPD in the TB plasma selected for testing is shown in Table 1. Plasma samples were chosen to represent varying concentrations of IgG1 and IgG3 antibodies. PPD negative donors showed low to undetectable levels of antibodies for IgG1 and IgG3 subclasses. These plasma samples were then tested for their ability to modulate TNF-α secretion in PPD stimulated monocytes. Figure 2 shows the TNF-α secretion from PPD unstimulated and PPD stimulated monocytes in the presence of plasma samples. Very little secretion was induced in the presence of plasma without concurrent PPD stimulation, indicating absence of LPS contamination in the plasma samples. Fifty percent of TB plasma samples (4/8) in the presence of PPD showed enhancement of TNF-α secretion. The remaining TB plasma samples showed a similar response to PPD and, for three of them, an apparently reduced response.
Fig. 2.
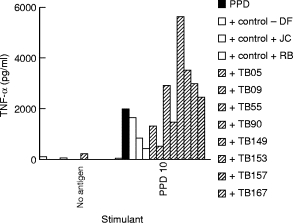
Enhancement of TNF-α release in the presence of a panel of plasma from patients with active pulmonary tuberculosis and healthy PPD skin test donors. Purified adherent cells (1 × 106 cells in 500 μl) were stimulated with PPD at 10 μg/ml. TNF-α release was assessed in antigen stimulated adherent cells in the presence of either control plasma, or plasma from patients with active pulmonary tuberculosis. All plasma samples were heat inactivated and added at a dilution of 1:500. Spontaneous release of TNF-α by adherent cells was also assessed in the presence of plasma samples without any added stimulant, and was <10 pg/ml for all plasma samples tested. □, Control plasma;  , patient plasma.
, patient plasma.
To see if this enhancement was a consistent effect, we tested six additional PPD skin test negative healthy donors. The results are shown in Fig. 3. The TNF-α secretion is expressed mean of the group (± SEM). Consistent enhancement of TNF-α secretion was observed in 4/8 TB plasma samples. The remaining control (DF) and TB plasmas showed activity similar to PPD alone indicating that there was no consistent inhibitory activity in TB plasma samples.
Fig. 3.
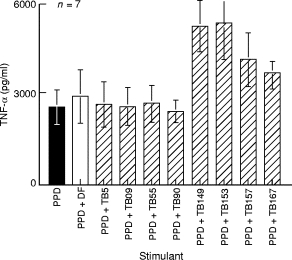
Enhancement of TNF-α secretion in seven PPD skin test donors. Results are expressed as concentration of TNF-α release in the presence of control antibodies or patient antibodies compared to PPD stimulated TNF-α release in the absence of added plasma. Vertical bars indicate mean of the group and horizontal lines indicate the standard error around the group mean. All other parameters were the same as those in Fig. 2. ▪, Stimulation with PPD alone; □, addition of control plasma; hatched bars indicate addition of TB plasma.
Correlation of TNF-α enhancing activity with presence of IgG1 subclass antibody
Although we had excluded participation of complement receptors by heat inactivation of plasma samples, participation of nonopsonic receptors could not be excluded since we had generated immune complexes, which would result in participation of all IgG antibody subclasses. We therefore examined the relationship of IgG subclass antibody concentrations to PPD in each plasma sample with TNF-α secretion. Spearman rank analysis using the two-tailed Fisher exact test was carried out to determine the relationship. Among the four IgG subclasses, only IgG1 showed a significant relationship (rho = 0.762; P = 0.04). No significant relationship was observed with IgG2 (rho = 0.310; P = 0.412), IgG3 (rho = 0.089; P = 0.813) and IgG4 (rh = 0.357; P = 0.344).
This relationship is further depicted as a scattergram in Fig. 4 for IgG1 and IgG3 subclass antibodies, for which high affinity Fc receptors are present on monocytes. Enhancement with IgG1 antibodies was much more marked beyond 2000 U/ml of IgG1 antibody (top panel). There was no clear relationship between IgG3 antibodies and TNF-α secretion (bottom panel)
Fig. 4.
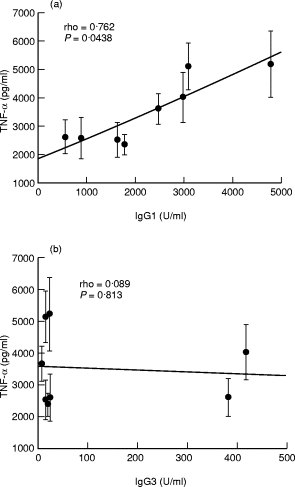
Relationship of antibodies to PPD in plasma samples and enhancement of TNF-α secretion. Spearman Rank correlation analysis (two-tailed exact Fisher test) was carried out to determine the correlation. between TNF-α secretion and the concentrations of IgG1 and IgG3 antibodies to PPD in plasma samples (n = 8). Each • represents the average of results obtained from seven donors (± SEM), shown as vertical lines. Antibody concentration is given as units of activity. (a) Antibody activity for IgG1. (b) Antibody activity for IgG3 anti PPD antibodies. The significance of correlation (P-values) is given for antibody subclasses.
Absorption of IgG antibodies with protein ‘A’
To further confirm that IgG1 antibodies were playing a role in the enhancement of TNF-α secretion in mycobacterial stimulated monocyte cultures, plasma samples were absorbed with protein ‘A’. Absorption of IgG1 and IgG3 PPD-specific antibodies was monitored by ELISA assay. Figure 5 shows the concentrations of pre and post protein ‘A’ absorbed plasma samples. In post absorbtion samples, more than 90% of the antibody was removed. Protein ‘A’ does not bind IgG3 antibodies and only a small proportion of IgG3 antibodies was removed. This could be the result of nonspecific absorbtion to sepharose 4B (to which protein ‘A’ was covalently bound). Unabsorbed and absorbed plasma was then directly compared in the monocyte stimulation assay.
Fig. 5.
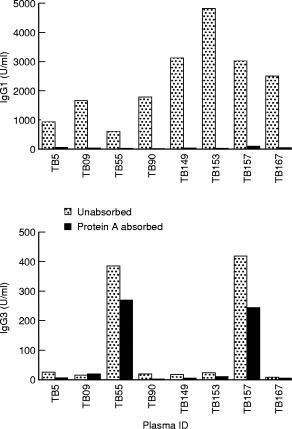
Absorption of IgG1 antibodies from plasma samples obtained from tuberculosis patients. Plasma samples were subjected to protein ‘A’ absorption. IgG1 and IgG3 antibodies were determined in untreated (hatched bars) or protein ‘A’ treated plasma (dotted bars).
Protein A treatment removes TNF-α enhancing activity
Table 2 shows the results with protein ‘A’ absorbed plasma samples. In PPD unstimulated moncyte cultures, none of the plasma samples resulted in TNF-α secretion indicating that the protein A treatment did not result in endotoxin contamination of the samples (data not shown). Treatment with protein ‘A’ resulted in reducing the TNF-α secretion to baseline or below baseline levels. TNF-α secretion occurring below baseline levels may be the result of some inhibitory activity, the reasons for which are as yet unclear. Nevertheless, these results strongly suggest that IgG1 antibodies may be responsible for enhancement of TNF-α secretion from antigen stimulated monocyte cultures in vitro.
Table 2.
Removal of IgG1 antibodies decreases TNF-α secretion from PPD stimulated monocytes
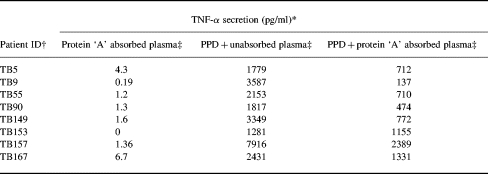
* PPD (10μg/ml) stimulated monocytes (1 × 106) in the absence of plasma resulted in secretion of 1760 pg/ml of TNF-α
† Patient plasmas were absorbed with protein ‘A’ to remove IgG1 antibodies.
‡ Stimulation in monocytes was assessed in the presence of protein ‘A’ treated plasma but without concurrent PPD stimulation. Monocytes were also stimulated with PPD in the presence of either unabsorbed or absorbed plasma at a dilution of 1:500.
DISCUSSION
The dominant symptoms in tuberculosis patients with advanced disease are cachexia and weight loss. These effects are associated with the biological activity of the pro-inflammatory cytokine TNF-α in circulation. When several mycobacterial proteins were shown to directly stimulate TNF-α secretion from blood monocytes, this observation was considered a rational explanation for the chronic release of TNF-α in tuberculosis patients. However, host factors that modulate cytokine secretion remain largely unknown. Our previous observation that opsonising antibodies (IgG1 and IgG3), for which monocytes have high affinity receptors, are augmented in advanced disease led us to investigate the role of Fc receptors in antigen uptake and subsequent modulation of cytokine expression. Our results strongly suggest that uptake of antigen in the presence of IgG1 antibodies enhances the secretion of TNF-α and that this is directly related to the concentrations of IgG1 antibody activity providing, for the first time, a link between the presence of antibodies and pathogenesis in tuberculosis.
The current study was carried out using adherent cell population from healthy PPD skin test donors to minimize the confounding role of endogenous antibodies that may be present on monocytes in PPD positive donors. In fact, very low background concentrations of IgG1 antibodies was detected in healthy donors. Since monocytes also have receptors for complement, all plasma samples were heat inactivated to minimize the effect of antigen uptake mediated via the complement receptors. As shown previously [13, 14], both PPD, containing secreted and cytosolic antigens, and sonicated H37Rv, derived from the virulent strain of M. tuberculosis containing predominantly cell associated antigens (data not shown), were able to directly induce secretion of TNF-α from the adherent cell population. Enhancement was not related to nonspecific factors in plasma samples, since plasma sample control donors as well as TB patients containing low concentrations of IgG1 antibodies did not augment TNF-α secretion. No association was observed with any of the other IgG subclasses, including IgG3. This precludes a role for immune complexes generated during the in vitro incubation. The absence of a relationship with IgG3 antibodies was surprising since IgG3 antibodies also bind to opsonic receptors with high affinity. This may be due to the considerably low level of antibody activity in the IgG3 subclass compared to IgG1. IgG3 may be more efficient via the complement receptors since it is the most efficient complement-binding antibody among the four IgG subclasses [19]. We excluded any participation by complement receptors by heat inactivating the plasma samples. Activity of IgG3 via the complement receptors may require much lower concentrations. This issue needs to be investigated further. The suggestion that IgG1 is playing a major role was further confirmed by absorption of plasma samples with protein ‘A’ that can bind to the Fc portion of human IgG1 but not to IgG3 antibodies [20, 21]. Even with IgG1-depleted but IgG3-containing plasma (TB 55 and Tb157), enhancing activity was reduced by more than 90%.
One other interesting observation was that although a significant correlation (P = 0.04) was observed between the concentrations of IgG1 antibodies and enhancement of TNF-α secretion, there was still considerable variation between donor to donor with the same concentration of IgG1 antibodies. The reason for this observation may be the complexity of the antigen mixtures present in PPD and more consistent results may be obtained with more purified antigen preparations. We are extending these observations with partially purified fractions from culture filtrates of M. tb and to other pro-inflammatory and downregulatory cytokines. Nevertheless, these studies provide, for the first time, a direct link between the augmented humoral antibody response and disease pathogenesis. Further studies may provide important insights into the role of IgG1 antibodies and chronic release of TNF-α and other pro-inflammatory cytokines in mycobacterial diseases.
Acknowledgments
This work was supported through a NIH grant (A1–35240 PI: Dr Robert Wallis). I would like to thank Dr Ghaffar Dawood for providing patient material, and Mrs Maqboola Dojki and Mrs Firdaus Shahid for the cataloging of information and determination of PPD specific antibodies in the plasma samples.
References
- 1.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–30. [PubMed] [Google Scholar]
- 2.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptor C3. J Immunol. 1990;144:2771–80. [PubMed] [Google Scholar]
- 3.Huber HS, Douglas D, Nusbacher J, Kochwa S, Rosenfield RE. IgG subclass specificity of human monocyte receptor sites. Nature. 1971;229:419–20. doi: 10.1038/229419a0. [DOI] [PubMed] [Google Scholar]
- 4.Hussain R, Kifayet A, Chiang TJ. Immunologulin G (IgG1) and IgG3 antibodies are markers of progressive disease in leprosy. Infect Immun. 1995;63:410–5. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain R, Dawood G, Abrar N, Toossi Z, Minai A, Dojki M, Ellner JJ. Selective increases in antibody isotypes and IgG subclass responses to secreted antigens in tuberculosis patients and household contacts. Clin Diagn Lab Immunol. 1995;2:726–32. doi: 10.1128/cdli.2.6.726-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shakib F. New York: S. Karger Publications; Basic and clinical aspects of IgG subclasses. [Google Scholar]
- 7.Beutler B, Mahoney LeTrang N, Pekala P, Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264-cells. J Exp Med. 1985;161:984–95. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA, Cannon JG, Wolff SM, et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–50. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisa P, Gennene M, Soder O, Ottenhoff T, Hansson M, Kiessling R. Serum tumor necrosis factor levels and disease dissemination in leprosy and leishmaniasis. J Inf Dis. 1990;161:988–91. doi: 10.1093/infdis/161.5.988. [DOI] [PubMed] [Google Scholar]
- 10.Santos D, Suffys O, Bonifacio PN, Marques K, Sarno EN. In vitro tumor necrosis factor production by mononuclear cells from lepromatous leprosy patients and from patients with erythema nodosum leprosum. Clin Imm Immunopathol. 1993;67:199–203. doi: 10.1006/clin.1993.1065. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor production in patients with leprosy. Infect Immun. 1992;60:1441–6. doi: 10.1128/iai.60.4.1441-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedland JS, Hartley JC, Hartley CGC, Shattock RJ, Griffin GE. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis. Infect Clin Exp Immunol. 1995;100:233–8. doi: 10.1111/j.1365-2249.1995.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valone SE, Rich EA, Wallis RS, Ellner JJ. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with while Mycobacterium bovis BCG and Mycobacterial antigens. Infect Immun. 1988;56:3313–5. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis RS, Amir-Tahmesseb M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte Western blot. PNAS. 1990;87:3348–52. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 16.Hussain R, Hasan R, Khurshid M, Sturm AW, Ellner JJ, Dawood G. Pulmonary tuberculosis in a BCG vaccinated area: relationship of disease severity with immunological and haematological parameters and drug resistance patterns. SE Asian J Trop Med Pub Health. 1996;27:257–62. [PubMed] [Google Scholar]
- 17.Hussain R, Poindexter RW, Wistar R, Reimer CB. Use of monoclonal antibodies to quantify subclasses of human IgG. I. Development of two site immuno enzymometric assays for total IgG subclass determinations. J Immunol Meth. 1986;93:89–96. doi: 10.1016/0022-1759(86)90437-0. [DOI] [PubMed] [Google Scholar]
- 18.Hussain R, Poindexter RW, Reimer CB. Use of monoclonal antibodies to quantify subclasses of human IgG. II. Enzyme immunoassay to define filaria specific IgG subclass antibodies. J Immunol Meth. 1986;94:73–80. doi: 10.1016/0022-1759(86)90217-6. [DOI] [PubMed] [Google Scholar]
- 19.Feinstein A, et al. IgG flexibility in complement activation. Immunol Today. 1986;7:169. doi: 10.1016/0167-5699(86)90168-4. [DOI] [PubMed] [Google Scholar]
- 20.Hjelm H, Hjelm K, Sjoquist J. Protein ‘A’ from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972;28:73–6. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- 21.Recht B, Frangione B, Franklin E, van Loghem E. Structural studies of a human 3 myeloma protein (GOE) that binds to Staph protein A. J Immunol. 1981;127:917–23. [PubMed] [Google Scholar]


