Abstract
IL-10 modulation of human intestinal T lymphocyte functions was studied for the first time. Lymphocyte proliferation was determined by 3H-thymidine incorporation; cytokine production, by ELISA; expression of surface markers, by immunofluorescence and flow cytometric analysis; and cytotoxicity, by lysis of 51Cr-labelled target cells. IL-10 blocked phytohaemagglutinin (PHA)-induced activation and proliferation of CD8+ T cells from the epithelium and lamina propria. It was a greater inhibitor of IL-2, interferon-gamma, and tumour necrosis factor-alpha production than were IL-4 or transforming growth factor-beta. In contrast, IL-10 enhanced IL-2-stimulated proliferation of both CD4+ and CD8+ T cells by increasing cell division after activation. It also augmented IL-2- but not IL-15-induced cytotoxicity of intestinal lymphocytes against colon cancer by a mechanism independent of natural killer cells. In conclusion, IL-10 blocking of proinflammatory cytokine secretion probably reduces intestinal inflammation. IL-10 augmentation of IL-2-induced cytotoxicity may help to maintain host defence.
Keywords: lymphokine-activated killer activity, tumour necrosis factor, interferon-gamma, IL-2, IL-10, inflammatory bowel disease
INTRODUCTION
IL-10 is synthesized by a variety of cell types, such as T cells (including lamina propria lymphocytes (LPL)), B cells, antigen-presenting cells (APC), and malignancies. It is produced 24–48 h after lymphocyte activation, indicating that it regulates the later stages of the immune response. Among the many immunomodulatory events, IL-10 production by T lymphocytes and natural killer (NK) cells is enhanced by IL-12, while synthesis by monocytes is augmented by tumour necrosis factor-alpha (TNF-α) [1–5].
IL-10 affects T cells indirectly through reduction of APC activities, such as production of IL-1 and TNF-α, and expression of costimulatory molecules, such as B7 and MHC class II [6–8]. In certain rare situations, IL-10 alters T cell functions directly [9]. Besides its action on lymphocytes, IL-10 prevents interferon-gamma (IFN-γ)-induced loosening of the colonic epithelial cell tight junctions, thereby permitting entry of foreign antigens from the lumen [10]. The studies of IL-10 vary considerably from one another, so generalizations are often difficult to make. Such variables include: use of rodents versus humans; T cell clones versus fresh T cells; splenocytes versus lymphocytes from peripheral blood; and the presence of monocytes/macrophages versus dendritic cells. No studies have evaluated the actions of IL-10 on human intestinal lymphocytes.
The colon and ileum display IL-10 in the epithelium, lamina propria, and submucosa, while jejunum has IL-10 in the epithelium [11,12]. This cytokine is crucial for suppressing inflammation in the gut. IL-10 knockout animals develop a disease resembling ulcerative colitis (UC) [13]; transforming growth factor-beta (TGF-β) knockouts develop various abnormalities, some of which resemble inflammatory bowel disease (IBD) [14]; whereas IL-4 knockout mice have no intestinal disease [15].
The role of IL-10 in IBD is unclear. The concentrations of IL-10 in pg/ml amounts were elevated in the serum of patients with active disease [16], while in another study, serum levels were normal [17]. Intestinal mRNA levels were reported to be normal or reduced in UC [17,18]. Yet IL-10, given systemically to those with Crohn's disease (CD) and by enema to those with UC, ameliorated symptoms, demonstrating its therapeutic potential [18,19].
The present study evaluates for the first time the effects of IL-10 on intestinal lymphocytes to determine which T cell subsets react to this cytokine, which functions are affected, and whether the changes are inhibitory or enhancing. The increased LAK activity induced by IL-10 in intestinal lymphocytes is a novel finding. These basic studies are important in order to define the role of IL-10 in intestinal diseases and its therapeutic implications.
MATERIALS AND METHODS
Isolation of intraepithelial lymphocytes, LPL, peripheral blood mononuclear cells, and T cell subsets
Intestinal lymphocytes were obtained from jejunal mucosa from morbidly obese individuals undergoing gastric bypass operations as described previously [20]. In brief, the minced mucosa was treated for 30 min with 1 mm dithiothreitol, then for three 45-min incubations with 0.75 mm EDTA. The cells in the supernate were separated by a discontinuous Percoll density gradient to obtain intraepithelial lymphocytes (IEL). After removing residual IEL and epithelial cells from the partially treated tissue with three more EDTA treatments, the remaining tissue was digested for 3 h at 37°C in collagenase (20 U/ml), then pressed through a wire mesh sieve to obtain a single-cell suspension of LPL, also purified by Percoll density gradient centrifugation. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation. After separation, IEL were 96 ± 5% CD2+, 8 ± 4% CD4+, 89 ± 2% CD8+, and 1 ± 1% CD14+; LPL were 94 ± 5% CD2+, 65 ± 12% CD4+, 30 ± 1% CD8+, and 1 ± 0% CD14+; and PBMC were 89 ± 4% CD2+ and 8 ± 1% CD14+. T lymphocytes were separated into CD4+ and CD8+ subsets by negative selection with immunomagnetic sorting using antibodies to CD8 and CD4 (Coulter-Immunotech, Hialeah, FL), respectively, followed by goat anti-mouse IgG conjugated to magnetic beads as detailed previously [21]. The purity of the CD4+ T cells was 96 ± 4%, while that of the CD8+ subset was 96 ± 5%.
Depletion of APC
Monocyte depletion was accomplished by immunomagnetic removal of DR+ or CD14+ cells, resulting in < 0.01% monocytes. MHC-DR is expressed on most APC, including dendritic cells. B cells are infrequently found in jejunal LPL (≤ 1%). CD14 is found on macrophages/monocytes but not on the majority of dendritic cells.
Measurement of proliferation
Lymphocytes (2 × 105/0.2 ml) were cultured with one or more of the following agents: phytohaemagglutinin (PHA; 1 μg/ml; Murex Diagnostics, Norcross, GA), IL-2, IL-7, IL-10, IL-15, antibody to IL-10 (all from R&D Systems, Minneapolis, MN), antibody to CD3-ε (1 μg/ml; Immunotech, Westbrook, ME), and antibodies to T112 and T113 (1:500 dilution, kind gift of E. Reinherz, Dana-Farber Cancer Institute, Boston, MA). After a 3-day incubation, proliferation was determined by 3H-thymidine incorporation.
Determination of cytokine levels
The amounts of IL-2, IFN-γ, and TNF-α content in supernates of IEL cultured for 3 days with PHA and IL-4, IL-10, or TGF-β were measured by ELISA (R&D).
Immunofluorescence
Indirect immunofluorescence was performed by incubating mononuclear cells with MoAbs to CD2, CD4, CD8, CD14, CD16, CD56, CD80, CD86, MHC class II (DR), and IL-2 receptors (Immunotech), followed by fluorescein-conjugated goat anti-mouse IgG. Data were analysed on a Coulter Epics Profile analytical flow cytometer.
Cytotoxicity (51Cr-release) assay
IEL were cultured for 3 days with different concentrations of IL-2 or IL-15 with or without IL-10. In some experiments, NK cells expressing CD16 or CD56 were removed by immunomagnetic sorting just prior to the cytotoxicity assay (resulting in < 0.1% NK cells). The cells were washed and incubated for 4 h at various ratios with 51Cr-labelled HT-29 cells (American Type Culture Collection, Rockville, MD). The percentage of cytotoxicity was calculated in relation to the spontaneous and maximal releases of the radiolabel by the target cells in medium or 2% cetrimide solution (Fisher Scientific, Springfield, NJ), respectively, as described previously [21].
Statistical analysis
For each data set, an arithmetic mean and s.e.m. were calculated. Pairs of data sets were compared by Student's t-test for paired or independent variables.
RESULTS
The effects of IL-10 (100 ng/ml) on the proliferation of IEL, LPL, and PBMC in response to various stimuli were measured (Fig. 1). For all three lymphocyte types, IL-10 had similar inhibitory effects with PHA- and anti-CD3-induced events and similar enhancement of IL-2-induced proliferation. A major difference was the reduction in CD2 antibody-induced events by IEL but not by LPL or PBMC. Also, IL-10 had variable effects on IL-7-induced proliferation. The actions of IL-15, the most powerful cytokine for IEL proliferation and cytotoxicity [22], were unaffected by IL-10.
Fig. 1.
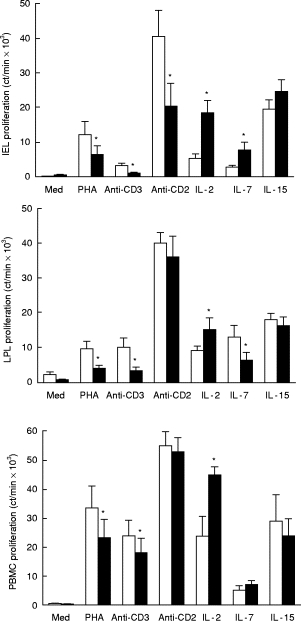
Effects of IL-10 on proliferation of intraepithelial lymphocytes (IEL) (top), lamina propria lymphocytes (LPL) (middle), and peripheral blood mononuclear cells (PBMC) (bottom). Lymphocytes were cultured for 3 days with each stimulus listed on the abscissa with or without IL-10 (100 ng/ml). Proliferation was determined by 3H-thymidine incorporation and expressed as the mean ± s.e.m. Those values with IL-10 that differed significantly from its pair without IL-10 are denoted by asterisks (P < 0.05, n = 6, Student's t-test, paired variables). Additives: □, none; ▪, IL-10.
Dose–response curves were constructed depicting various concentrations of IL-10 and their actions on IEL proliferation to PHA, CD3 ligation, and IL-2. IL-10 concentrations > 1 ng/ml were needed to trigger changes in lymphocyte proliferation (Fig. 2).
Fig. 2.
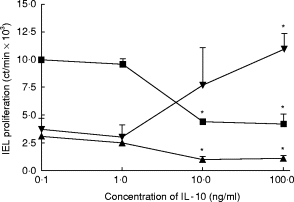
Effects of different concentrations of IL-10 on proliferation of intraepithelial lymphocytes (IEL) stimulated with phytohaemagglutinin (PHA; ▪), antibody to CD3 (▴), or IL-2 (▾). IEL were cultured with each stimulus in the presence of different concentrations of IL-10, and proliferation was determined 3 days later. Those values marked with asterisks differed significantly from those representing cultures without IL-10 (P < 0.05, n = 5, Student's t-test, paired variables).
To determine whether IL-10 affected activation or cell division of IEL, this cytokine (100 ng/ml) was added on days 0, 1, or 2 of a 3-day culture containing PHA, antibody to CD3, or IL-2 (Fig. 3). With the first two stimuli, IL-10 reduced blastogenesis only when added within the first 24 h of culture, indicating that it targets lymphocyte activation. In contrast, IL-10 enhanced IL-2-induced proliferation when added up to 48 h after the culture was started, suggesting that it acts on cell division after activation. To substantiate these findings further, an activation event, IL-2 receptor expression, was measured 24 h after incubating IEL with each stimulus in the presence or absence of IL-10 (Fig. 4). IL-2 receptor expression declined when IL-10 was added with PHA but did not change with medium or IL-2, confirming its effect on lymphocyte activation with PHA but not IL-2.
Fig. 3.
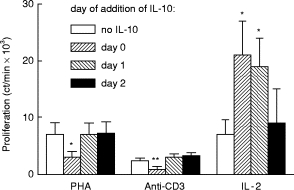
Effects of IL-10 added on different days to intraepithelial lymphocytes (IEL) cultured with phytohaemagglutinin (PHA), antibody to CD3, or IL-2. IEL were cultured with the stimuli listed on the abscissa. IL-10 (100 ng/ml) was added on day 0 (initiation of the culture), day 1, or day 2 of a 3-day culture, and proliferation measured. The asterisks denote those values that differ significantly from IEL proliferation without IL-10 (P < 0.05, n = 6, Student's t-test, paired variables).
Fig. 4.
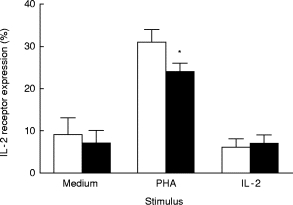
Effects of IL-10 on IL-2 receptor expression by intraepithelial lymphocytes (IEL). IEL were cultured with the stimuli listed for 3 days with (▪) or without IL-10 (□) and then stained by immunofluorescence for IL-2 receptor expression. *Differs significantly from the value without IL-10 (P < 0.05, n = 5, paired Student's t-test).
T cell subsets from both IEL and LPL were analysed separately for IL-10-induced changes in proliferation (Fig. 5). For both intestinal lymphocyte types, IL-10 reduced PHA stimulation of the CD8+ subset and increased IL-2 effects on both the CD4+ and CD8+ T cell subsets.
Fig. 5.
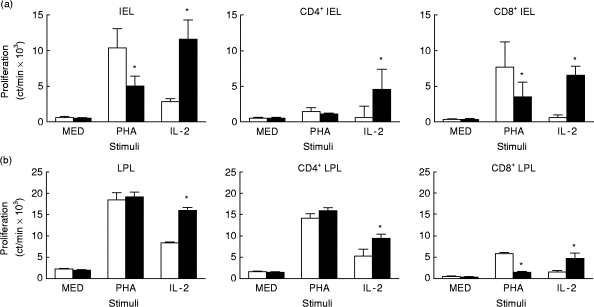
Effects of IL-10 on proliferation of CD4+ and CD8+ intraepithelial lymphocytes (IEL) (a) and lamina propria lymphocytes (LPL) (b). Unseparated T cells and T cell subsets of IEL and LPL were cultured for 3 days with phytohaemagglutinin (PHA) or IL-2, with or without IL-10, and proliferation determined by 3H-thymidine incorporation. The asterisks mark those values significantly different from their pair (P < 0.05, n = 4, paired Student's t-test). □, Medium; ▪, IL-10.
The ability of IL-10 to block the production of IL-2, IFN-γ, or TNF-α by PHA-activated LPL and PBMC was compared with the suppressive abilities of IL-4 and TGF-β (Fig. 6). IL-10 caused the greatest reduction in proinflammatory cytokine synthesis.
Fig. 6.
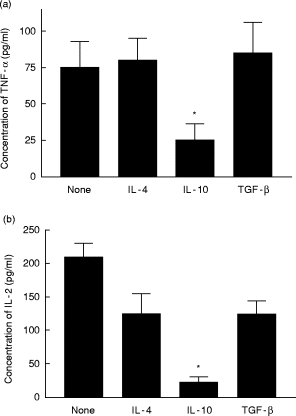
Effects of IL-4, IL-10, and transforming growth factor-beta (TGF-β) on lamina propria lymphocyte (LPL) synthesis of tumour necrosis factor-alpha (TNF-α) (a) and IL-2 (b). LPL were cultured with phytohaemagglutinin (PHA) and each of the three suppressive cytokines for 3 days. The culture supernate was tested for TNF-α and IL-2 content by ELISA. The values marked with asterisks are significantly lower than those generated with PHA alone (‘none’) (P < 0.05, n = 4, Student's t-test, paired variables).
To determine the role of APC on IL-10 effects, IEL were depleted of CD14- or MHC class II (DR)-expressing cells by immunomagnetic removal (n = 4 and n = 5, respectively) and then tested for proliferation to PHA or IL-2 with or without IL-10 (Fig. 7). This manipulation reduced but did not eliminate the PHA response, suggesting either that a small number of APC remaining after depletion triggered PHA-induced T cell proliferation, or that part of the stimulation, like IL-2 produced by activated T cells, could stimulate T cells without APC. Removal of APC did not affect IL-2-induced proliferation, suggesting that this stimulus does not require APC. The IL-10 effect was the same whether or not APC were removed.
Fig. 7.
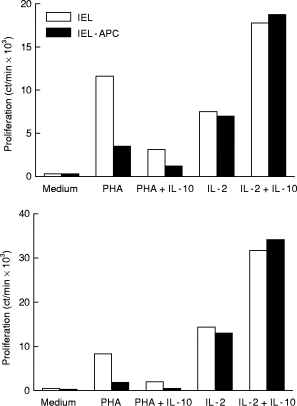
Effects of IL-10 on intraepithelial lymphocytes (IEL) depleted or not of antigen-presenting cells (APC) (two experiments). IEL were depleted of DR+ cells and tested for proliferative responses to phytohaemagglutinin (PHA) and IL-2 with or without IL-10. In both cases IL-10 effect on PHA-induced proliferation required APC, while that on IL-2-induced proliferation did not.
Since IL-10 is produced by NK cells [4] and increases cytotoxic T lymphocyte precursor frequency [23], it may affect IL-2-induced cytotoxicity (LAK activity). Lymphocytes were cultured with different concentrations of IL-2 or IL-15 in the presence or absence of a constant amount of IL-10 (100 ng/ml). They were then tested for lytic activity against HT-29 target cells. Using the three lymphocyte types, IL-10 increased cytotoxicity induced by IL-2 but not IL-15 (not shown). To determine whether NK cells were involved, cultured IEL were depleted of CD16+ and CD56+ lymphocytes, and then tested for LAK activity (Fig. 8). In a representative of three experiments, IEL LAK activity was partially mediated by NK cells, since it declined by 37% with NK cell depletion, as shown previously [21]. IL-10 enhanced LAK activity whether or not NK cells were present, indicating that it targeted IEL lacking NK markers. IL-10 added to IL-2-stimulated IEL did not alter the percentage of CD16+ and CD56+ lymphocytes (n = 4, not shown).
Fig. 8.
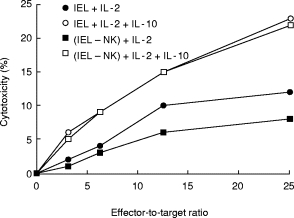
Effects of IL-10 on LAK activity of intraepithelial lymphocytes (IEL) with or without depletion of natural killer (NK) cells. IEL were cultured for 3 days with IL-2 (1 ng/ml) in the presence or absence of IL-10 (100 ng/ml). A portion of the cells was then depleted of NK cells expressing CD16 and CD56. Lytic activity was tested by a 4-h 51Cr-release assay against HT-29 cells. This is a representative of four experiments.
DISCUSSION
Although IL-10 is thought to be predominantly inhibitory, this study shows that it can either reduce or enhance intestinal lymphocyte functions, depending upon the stimulus and the lymphocyte subset. On the one hand, IL-10 blocked PHA-activated proliferation and cytokine production (IL-2, IFN-γ, and TNF-α) by intestinal lymphocytes. This IL-10 effect on proliferation occurred during activation, required APC, and mainly affected CD8+ T cells. The inhibitory effects were indirect, reducing APC functions which, in turn, blocked T cell activation [6–8]. On the other hand, IL-10 enhanced IL-2-induced proliferation and LAK activity by intestinal lymphocytes, acting on cell division of both T cell subsets probably without APC. This may result from a direct action of T lymphocytes with IL-10 [9].
The effect of IL-10 on intestinal lymphocytes was analysed in this study since these cells function quite differently from PBMC and since IL-10 may be important in the treatment of IBD [19]. Normally, the proliferative responses of IEL and LPL to mitogens, CD3 ligation, and alloantigens are quite low due to incomplete activation, although their responses to CD2 ligation are brisk [20,24]. Intestinal lymphocytes, unlike those in the periphery, are chronically activated memory cells. Furthermore, the type of APC in the intestine is probably a mixture of macrophages and dendritic cells, two APC types that differ in surface markers and response to IL-10. Despite these distinctions between lymphocytes in the intestine and peripheral blood, IL-10 proved to have similar effects when using certain stimuli (e.g. PHA, anti-CD3, IL-2) but not others (anti-CD2 and IL-7). This cytokine may serve to reduce further the already low PHA- and CD3/TCR-induced activation by IEL. CD8+ T cells are more sensitive to IL-10-induced inhibition than CD4+ T cells, probably because they become more quickly starved of IL-2 as IL-10 sharply reduces production of this cytokine. The prominence of the IL-10 action on the CD8+ T cell subsets was found previously using circulating lymphocytes [6,8,25].
IL-10 down-regulates certain surface markers on APC, particularly CD80, CD86, and MHC class II [7]. This IL-10 effect may have less impact in the intestine since expression of CD28 on intestinal lymphocytes is low [26]. IL-10 reduction in the actions of dendritic cells, which often do not express B7, may lead to tolerization of T cells [27]. This laboratory has found that human jejunal lymphocyte preparations contain about 1% APC, as determined by fluorescence staining with CD14, HLA-DR, CD80, or CD86.
The reported effects of IL-10 on cytokine production are variable. In most studies (including the present one), IL-10 reduced production of IFN-γ, TNF-α, and IL-2 [5,28,29]; another study, however, demonstrated no effect [9]. These reports are hard to compare. The present study used primary stimulation of fresh T lymphocytes rather than T cell clones, and showed that IL-10 blocked proinflammatory cytokine synthesis to a greater extent than did two other suppressive cytokines, IL-4 and TGF-β. This may be partly due to reduced IL-12 synthesis by IL-10. Since such proinflammatory cytokines are essential in the activity of IBD, the greater suppressive ability of IL-10 may explain why a disease with the closest resemblance to IBD is found in knockout mice lacking IL-10 rather than IL-4 or TGF-β.
A novel finding in the present study was the IL-10 up-regulation of IL-2-induced LAK activity by IEL, LPL, and PBMC against colon cancer. Other studies showed that IL-10 does not alter LAK activity by murine splenocytes [23] but reduces allospecific cytotoxic activity by human PBMC [8]. A few reports indicated that IL-10 either augmented or did not change the IL-2-driven proliferation of preactivated T cells and T cell clones [30–35], while susceptibility of tumour to lysis is modulated by this cytokine [37]. IL-10, synthesized by LPL, epithelial cells, and colon cancer, affects various aspects of tumour cell lysis [2,11,12,30].
This is the first study to show the enhancing effect of IL-10 on IL-2-stimulated proliferation and cytotoxicity by intestinal lymphocytes. Unlike most IL-10 effects, this one probably does not involve APC. A few reports showed that IL-10 augmented or left unchanged the IL-2-driven proliferation of preactivated T cells and T cell clones [32–36], although no one looked into this using intestinal lymphocytes. IL-10 also decreased the already low proliferation to PHA and anti-CD3, through a mechanism dependent on APC. This IL-10 enhancement of IL-2-induced LAK activity by IEL may increase lysis of colon cancer or infected epithelial cells, serving to strengthen the host immune response. Of the inhibitory cytokines, IL-10 blocks proinflammatory cytokine synthesis the most, thereby reducing inflammation.
Acknowledgments
This project was supported by NIH grant no. DK42166. The author would like to thank Robert Brolin, MD for jejunal specimens and Arthur I. Roberts for technical expertise.
References
- 1.Cohen SBA, Parry SL, Feldmann M, Foxwell B. Autocrine and paracrine regulations of human T cell IL-10 production. J Immunol. 1997;158:5596–602. [PubMed] [Google Scholar]
- 2.Braunstein J, Qiao L, Autschbach F, Schurmann G, Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997;41:215–20. doi: 10.1136/gut.41.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-β enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155:4926–32. [PubMed] [Google Scholar]
- 4.Mehrotra PI, Donnelly RP, Wong S, et al. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J Immunol. 1998;160:2637–44. [PubMed] [Google Scholar]
- 5.Parry SL, Sebbag M, Feldmann M, Brennan FM. Contact with T cells modulates monocyte IL-10 production: role of T cell membrane TNF-α. J Immunol. 1997;158:3673–81. [PubMed] [Google Scholar]
- 6.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–9. [PubMed] [Google Scholar]
- 7.Ding L, Linsley PS, Huang L-Y, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 8.Groux H, Bigler M, de Vries J, Roncarolo M-G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–93. [PubMed] [Google Scholar]
- 9.de Waal Malefyt R, Yssel H, de Vries J. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. J Immunol. 1993;150:4754–65. [PubMed] [Google Scholar]
- 10.Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterol. 1997;113:151–9. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 11.Autschbach F, Braunstein J, Helmke B, et al. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121–30. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckett CG, Dell'olio D, Kontakou M, Przemioslo RT, Rosen-Bronson S, Ciclitira PJ. Analysis of interleukin-4 and interleukin-10 and their association with the lymphocytic infiltrate in the small intestine of patients with coeliac disease. Gut. 1996;39:818–23. doi: 10.1136/gut.39.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni AB, Karlsson S. Transforming growth factor-β1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143:3–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn R, Rajewsky K, Muller W. Generation and analysis of IL-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 16.Kucharzik T, Stoll R, Lugering N, Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease. Clin Exp Immunol. 1995;100:452–6. doi: 10.1111/j.1365-2249.1995.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen OH, Koppen T, Rudiger N, Horn T, Eriksen J, Kirman I. Involvement of interleukin-4 and -10 in inflammatory bowel disease. Dig Dis Sci. 1996;41:1786–93. doi: 10.1007/BF02088746. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber S, Heinig T, Thiele H-G, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterol. 1995;108:1434–44. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 19.Van Deventer SJH, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Gastroenterol. 1997;113:383–9. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- 20.Ebert EC. Proliferative responses of human intraepithelial lymphocytes to various T-cell stimuli. Gastroenterol. 1989;97:1372–81. doi: 10.1016/0016-5085(89)90379-x. [DOI] [PubMed] [Google Scholar]
- 21.Ebert EC, Roberts AI. Lymphokine-activated killing by human intestinal lymphocytes. Cell Immunol. 1993;146:107–16. doi: 10.1006/cimm.1993.1010. [DOI] [PubMed] [Google Scholar]
- 22.Ebert EC. Interleukin-15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterol. 1998;115:1439–45. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 23.Chen W-F, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;147:528–34. [PubMed] [Google Scholar]
- 24.Qiao L, Schurmann G, Betzler M, Meuer SC. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterol. 1991;101:1529–36. doi: 10.1016/0016-5085(91)90388-2. [DOI] [PubMed] [Google Scholar]
- 25.Nishijima K-I, Hisatsune T, Minai Y, Kohyama M, Kaminogawa S. Anti-IL-10 antibody enhances the proliferation of CD8+ T cell clones: autoregulatory role of murine IL-10 in CD8+ T cells. Cell Immunol. 1994;154:193–201. doi: 10.1006/cimm.1994.1068. [DOI] [PubMed] [Google Scholar]
- 26.Ebert EC, Roberts AI. Costimulation of CD3 pathway by CD28 ligation in human intestinal lymphocytes. Cell Immunol. 1996;171:211–6. doi: 10.1006/cimm.1996.0195. [DOI] [PubMed] [Google Scholar]
- 27.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk A. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 28.Macatonia SE, Doherty TM, Knight SC, O'garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-γ production. J Immunol. 1993;150:3755–65. [PubMed] [Google Scholar]
- 29.Clarke C, Hales A, Hunt A, Foxwell BMJ. IL-10-mediated suppression of TNF-α production is independent of its ability to inhibit NFκB activity. Eur J Immunol. 1998;28:1719–26. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Cohen SBA, Katsikis PD, Feldmann M, Londei M. IL-10 enhances expression of the IL-2 receptor α chain on T cells. Immunology. 1994;83:329–32. [PMC free article] [PubMed] [Google Scholar]
- 31.Gastl GA, Abrams JS, Nanus DM, et al. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer. 1993;55:96–101. doi: 10.1002/ijc.2910550118. [DOI] [PubMed] [Google Scholar]
- 32.MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167. [PubMed] [Google Scholar]
- 33.Schlaak JF, Hermann E, Gallati H, Meyer Zum Buschenfelde K-H, Fleischer B. Differential effects of IL-10 on proliferation and cytokine production of human γ/δ and α/β cells. Scand J Immunol. 1994;39:209. doi: 10.1111/j.1365-3083.1994.tb03362.x. [DOI] [PubMed] [Google Scholar]
- 34.Taga K, Cherney B, Tosato G. IL-10 inhibits apoptotic cell death in human T cells starved of IL-2. Int Immunol. 1993;5:1599. doi: 10.1093/intimm/5.12.1599. [DOI] [PubMed] [Google Scholar]
- 35.Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci USA. 1985;82:4230–4. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert EC, Brolin RE, Roberts AI. Characterization of activated lymphocytes in colon cancer. Clin Immunol Immunopathol. 1989;50:72–81. doi: 10.1016/0090-1229(89)90223-7. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda M, Salazar F, Petersson M, et al. Interleukin-10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–6. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]


