Abstract
We examined CD4 and major HIV-1 co-receptor expression by trophoblast cells (TC) from early placentas, and the permissiveness of TC for infection by several natural HIV-1 isolates in vitro. Ten early placentas (4–6 weeks of gestation) from HIV− women were obtained after elective abortion. CD4 and HIV-1 co-receptor expression by TC was examined in terms of both mRNA and protein. The same TC were then challenged with three clinical HIV isolates of known phenotype, two originating from mothers who transmitted the virus to their child and one from a vertically infected newborn. TC infection was detected by polymerase chain reaction. CD4 expression was detected in five of the 10 placentas, while membrane protein expression of CCR3, CXCR4 and CCR5 was detected in every case, despite quantitative differences among individuals. Bonzo, GPR1 and ChemR23 mRNAs were detected in all TC preparations. TC from seven out of eight placentas were permissive to HIV entry, but no productive viral replication was detected (reverse transcriptase activity in culture supernatants). Interestingly, the addition of chemokine(s) or a CD4-blocking antibody to the cultures failed to inhibit TC virus entry. These data point to marked interindividual variability in HIV co-receptor expression by trophoblast cells and show that TC from early placentas can be infected in vitro by clinical HIV-1 isolates. They also suggest that viral entry in vitro might occur through a mechanism independent of both CD4 and chemokine receptors.
Keywords: HIV vertical transmission, trophoblast, receptor, virus entry
INTRODUCTION
Most HIV-infected children acquire the virus by direct transmission in utero or during delivery, or through breast feeding. The concept of direct materno–fetal transmission is now accepted, but several questions remain, especially as regards the timing and mechanisms involved. Using a mathematical model, Rouzioux et al. have shown that 95% of cases of transmission in utero occur less than 2 months before delivery [1]. In keeping with this concept, anti-retroviral treatment with zidovudine from the 14th week until the end of pregnancy has markedly reduced the vertical transmission rate in Europe and North America from 22% to only 6%, as shown in the ACTG 076/ANRS 024 trial. When this treatment is associated with elective Caesarean section, transmission rate reduced to < 2% [2]. Shorter anti-retroviral treatment has also significantly reduced the rate of vertical transmission in Africa and south-east Asia, albeit to a lesser degree.
The residual vertical transmission rate might be due to early infection of the feto-placental unit and the fetus itself. This is supported by studies based on PCR and in situ hybridization: for example, Backéet al. detected HIV-1 DNA in fetal tissues as early as week 12 of gestation [3], while Lewis et al. showed that embryonic blood cell precursors were infected by HIV-1 in 8-week fetuses [4]. While these reports demonstrate the possibility of transplacental or transannexial HIV-1 transmission, the precise timing and routes or cell types involved remain to be determined.
The trophoblast, which forms the outer cell layer of the placenta, is directly exposed to maternal blood, and is thus a good candidate for controlling HIV-1 transmission to the fetus. There is evidence that trophoblast cells (TC) can be infected in vivo [4–7], but viral replication in these cells in vivo is controversial [8,9], as are the mechanisms of TC infection. Douglas et al. [10] suggested an endocytosis-mediated mechanism of virus entry in vitro, while David et al. [11] described a clearly CD4-dependent mechanism of infection in their culture system. However, for most authors no CD4 expression has been detected (in terms of mRNA or membrane antigen) on TC from either mature or immature placentas [12–15]. The recent discovery of HIV-1 co-receptors on a variety of cells led us to investigate their possible involvement in the regulation of HIV infection of TC from early and late placentas. The major chemokine receptors are now known to act as HIV co-receptors. More specifically, CXCR4, CCR5 and, to a lesser extent, CCR3, CCR2b, BOB, Bonzo [5,16–21], GPR1 [22], CCR8 [23], APJ [24,25] and ChemR23 [26] play a key role in HIV/SIV entry into lymphocytes and macrophages. CXCR4 and CCR5 are currently recognized as the major HIV-1 co-receptors, being used, respectively, by syncytium-inducing (SI) and non syncytium-inducing (NSI) strains [27]. Some isolates also use, in addition, the other described co-receptors, although no clear correlation was found with their pathogenicity [28].
We have previously reported that TC isolated from term placentas of HIV− women are very slightly susceptible to infection by primary isolates in vitro [29,30]. We also observed no expression of functional CXCR4 or CCR5 on these cells, while CD4 expression was very low or undetectable.
In this study we examined TC from early placentas for expression of CD4 and the main HIV-1 co-receptors by using mRNA assays and specific antibodies, when available. We then also examined whether HIV-1 clinical isolates of known phenotype originating from two mothers who transmitted the virus to their children and from a vertically infected infant could induce productive infection of TC in vitro.
MATERIALS AND METHODS
The study was conducted in accordance with French ethical guidelines.
Isolation of TC
Ten early placentas (A–J) from HIV− women were collected immediately after elective RU 486-induced abortion. Gestational age was 28–42 days.
TC were isolated by using a modification of Kliman's technique [31]. They were further purified by negative selection with anti-human CD3, CD9, CD31 (Immunotech, Marseilles, France), CD14 and CD45RA (Becton Dickinson, Mountain View, CA) antibodies and magnetic beads coated with sheep anti-mouse IgG antibody (Dynabeads M-450; Dynal, Compiegne, France). Purity was checked by FACScan analysis. This procedure regularly yielded a cell population virtually devoid of contaminating cells.
Immunostaining and flow cytometry
A solution of 5% goat serum in PBS (Gibco BRL, Paisley, UK) was used to saturate non-specific sites on immunopurified TC. Twenty micrograms per millilitre of the following MoAbs: MoAb 183 (anti-hCCR5; R&D, Minneapolis, MN), 172 (anti-hCXCR4; R&D), 7B11 (anti-CCR3; NIBSC, Potters Bar, UK), CD3, CD9, CD31 (Immunotech), CD14, CD45RA (Becton Dickinson), OKT4 (anti-CD4; Ortho Diagnostic Systems, Raritan, NJ) and GB25 (a marker of human trophoblasts [32], a kind gift from Aster Biotechnology, La Gaude, France) were used to stain 2 × 105 cells. Labelling was revealed by using an FITC-conjugated goat anti-mouse IgG (Immunotech). Antibody specificity was assessed using transfected U87.CD4 cells expressing human chemokine receptors (kindly provided by Dr D. Littman through the MRC AIDS Reagent Project). Cells (2 × 104 TC for each stain) were analysed on a Becton Dickinson FACScan flow cytometer.
Double staining
TC from placenta J were double-stained. After saturation with goat serum, cells were stained with GB25 (mouse IgG1 isotype) or mouse IgG1 as control, and were revealed with PE-conjugated goat anti-mouse IgG. After saturation with PBS + 2% mouse serum, cells were stained with (i) FITC-conjugated mouse anti-hCCR5 (PharMingen, San Diego, CA), (ii) FITC-conjugated mouse anti-hCXCR4 (R&D) (or mouse FITC–IgG as control), or (iii) 7B11 (anti-hCCR3, IgG2a; MRC AIDS Reagent Project, UK), mouse IgG2a as control and revealed with FITC-conjugated goat anti-mouse IgG.
RNA extraction and reverse transcriptase-PCR
TC were resuspended at 5 × 106/ml in RNA-B lysing solution (Eurobio, Les Ulis, France). Total RNA was extracted by a phenol-chloroform procedure; precipitated RNA was depleted of contaminating DNA by DNase treatment (Boehringer, Mannheim, Germany) followed by a second phenol-chloroform precipitation step. mRNA was reverse-transcribed using an oligo-dT primer (Boehringer) and the M-MLV reverse transcriptase kit (Gibco BRL). Primers used for PCR are described in Table 1. Reverse transcriptase (RT)-PCR conditions for the detection of cellular gene expression were as follows: denaturation, 3 min at 94°C; hybridization, 2 min at 60°C (61.5°C for BOB, 62.5°C for CXCR4); extension, 1 min (1.5 min for CXCR4) at 72°C (one cycle); denaturation, 45 s at 94°C; hybridization, 2 min at 60°C (61.5°C for BOB, 62.5°C for CXCR4); extension, 1 min (1.5 min for CXCR4) at 72°C (40 cycles); extension, 10 min at 72°C (one cycle).
Table 1.
Sequences of oligonucleotides used in the reverse transcriptase-polymerase chain reaction analysis and HIV DNA detection

Viral phenotype
The viruses used for in vitro infection experiments (kindly provided by Dr G. Scarlatti, TIBID, Milan, Italy) were isolated from two mothers who transmitted the virus to their children (4538 and 3344) and from a vertically infected child (132); viral stock titration and phenotyping were done as described by Scarlatti et al. [33]. The viral phenotypes were as follows: isolate 4538 uses CCR3, CCR5 and CXCR4; isolate 3344 uses CCR5 exclusively; and isolate 132 uses CXCR4 and, to a lesser extent, CCR3.
TC infection
Before infection, viral suspensions were incubated for 30 min with 200 U/ml DNase (Sigma Chemical Co, St Louis, MO). Four hours after plating in F10 + 20% fetal calf serum (FCS), TC were infected with the equivalent of 2 ng of p24 of each viral suspension (equivalent to 0.05 MOI) and left overnight. The following day, cells were washed repeatedly and fed with fresh culture medium. In all experiments TC were also challenged: (i) with heat-inactivated virus; (ii) in the presence of 10 μg/ml zidovudine. Blocking experiments were performed (i) in the presence of 100 ng/ml SDF-1 and a mixture of 100 ng/ml MIP-α, MIP-β and RANTES (R&D); (ii) in the presence of a blocking anti-CD4 antibody (Q4120, kindly provided by Dr Q. Sattentau through the MRC AIDS Reagent Project); and (iii) a non blocking (OKT4) anti-CD4 antibody (5 μg/ml). Chemokines, zidovudine and anti-CD4 antibodies were renewed in the culture medium at each passage. Cord blood lymphocytes were infected in the same conditions, as controls. Viral expression in cell-free supernatants was monitored by measuring RT activity (Retrosys, RT kit; Innovagen, Sweden).
PCR analysis of HIV-1 DNA
On day 7 post-infection (p.i.), cells were collected, counted and lysed (107 cells/ml) in lysis buffer (10 mm Tris–HCl pH 8.3, 1 mm EDTA, 1% Tween-20, 0.04% proteinase K). After overnight incubation at 37°C followed by proteinase K inactivation at 95°C for 5 min, nested PCR targeting the tat gene was performed in duplicate on 105 lysed cells to detect the proviral genome. The primers are shown in Table 1. The PCR conditions were as follows: denaturation, 3 min at 94°C (one cycle); hybridization, 1 min at 53°C; extension, 1 min at 72°C; denaturation, 0.5 min at 94°C (38 cycles); extension, 10 min at 72°C (one cycle). For inner PCR, the same conditions were applied to 5 μl of the amplicon from the outer PCR. In these conditions one copy was detectable, and a duplicate or triplicate experiment was performed in order to assess the detection of less than one copy for 100 000 cells.
ELISA
ELISA kits were used to measure chemokine production in vitro. Purified TC were seeded at 106 cells/ml and grown for 24 h in the presence or absence of DNase-treated virus. Supernatants were then collected to measure chemokine concentrations. Quantikine MIP-1α, MIP-1β and RANTES ELISA kits (R&D) were used according to the manufacturer's instructions.
RU 43086
RU 43086 (RU 486, a kind gift from Roussel Uclaf, Romainville, France) as well as RU 43044 were added at doses of 2 μg or 20 μg/ml to aliquots of purified trophoblast cell cultures obtained from early or term Caesarean delivery placentae. Those concentrations are equivalent to, or slightly greater than those found in the serum of women undergoing RU 486 induction of abortion.
RESULTS
Purity of cultured trophoblasts
Contaminating cells were screened for in each preparation, at the time of infection, by staining with anti-CD3, CD9, CD14, CD45 and CD45RA and CD31; GB25 was used as a positive marker for trophoblast cells. FACS analysis showed the absence of contamination by fibroblasts, except in placenta F, which contained 3% of fibroblasts. Preparations from placentas D and E contained, respectively, 1% and 2% of CD3+ cells, and were negative for CD4 (see below). In other recent experiments, a lack of contamination was also observed while using in addition anti-CD11b (data not shown).
CD4 expression
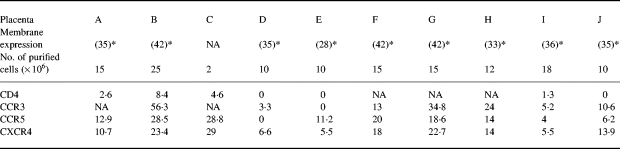
CD4 expression was tested, just prior to infection, by flow cytometric analysis of preparations from placentas A, B, C, D, E, I and J (Fig. 1), and by RT-PCR on preparations from placentas A, B, F, G and H (Fig. 2). CD4 membrane expression was positive, but close to the detection limit, on TC from placentas A and I, while TC from placentas B and C contained 8.4% and 4.6% CD4+ cells, respectively. CD4 was undetectable on TC from placentas D, E and J.
Fig. 1.
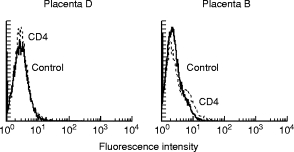
Analysis of CD4 expression on trophoblastic cells (TC). Membrane expression of CD4 on TC isolated from placentas B and D, measured by immunostaining with anti-CD4 MoAb (OKT4) and flow cytometric analysis.
Fig. 2.
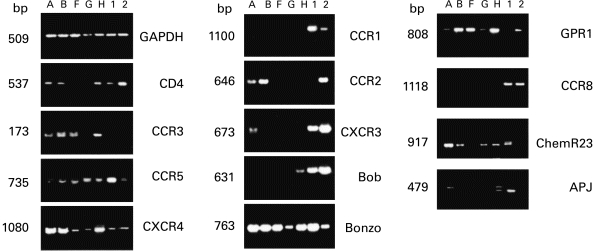
CD4 and β-chemokine receptor mRNAs in trophoblastic cells isolated from early placentas. Reverse transcriptase-polymerase chain reaction detection in placentas A, B, F, G and H, and in appropriate positive samples: human peripheral blood lymphocytes (1) not stimulated or (2) stimulated with phytohaemagglutinin in all experiment except for CCR8 (1) and (2) are thymocytes; for APJ (1) is astrocytes, (2) macrophages; for ChemR23 (1) is macrophages.
No CD4 mRNA was found in TC from placentas F and G, while a positive signal was obtained with TC from placentas A, B and H (Fig. 1, Table 2).
Table 2.
Membrane expression of the main HIV-1 receptors and co-receptors on trophoblastic cells isolated from 10 early placentas. 3a (Table 3): Membrane expression is indicated as the percentage of positive cells after immunostaining for CD4 and for the β-chemokine receptors CCR3, CXCR4 and CCR5, as shown by immunostaining using MoAb and FACS analysis
NA, Not available.
*Gestational age indicated as days after conception.
HIV-1 co-receptor expression
Just prior to infection, CCR3, CXCR4 and CCR5 expression was screened for by flow cytometry. All the samples tested expressed at least two of the three co-receptors, with individual quantitative differences (Table 2). No clear link was found between the pattern of co-receptor expression and gestational age. HIV-1 co-receptor expression on TC was confirmed by double staining with GB25 (Fig. 3). TC from placentas A, B, F, G and H were also screened for mRNA encoding the HIV-1 co-receptors CCR1, CCR2, CCR3, CCR5, CXCR4, CXCR3, BOB (GPR15), Bonzo (STRL33), GPR1, CCR8, APJ and ChemR23 (Fig. 2). Each placenta showed a particular pattern of mRNA expression but no clear correlation with gestational age. CCR8 and CCR1 were not detected by RT-PCR in any of the samples, while they were detected in thymocytes and peripheral blood monocyte samples used as positive controls.
Fig. 3.
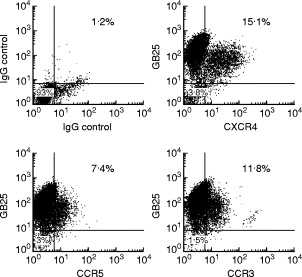
Membrane expression of the main HIV-1 co-receptors in trophoblastic cells isolated from a representative early placenta. Double staining of cells purified from placenta J using GB25 and anti-CCR3, CXCR4 and CCR5, respectively. Results are expressed as the percentage of double-positive cells (GB25+ cells are 92 ± 5% of total cells).
TC infection
The viral genome was screened for by PCR on day 7 of TC infection. Infection experiments were not possible with TC from placentas E and J, as cell numbers were inadequate. In all experiments we used a limited viral inoculum in order to assess permissiveness in near-suboptimal conditions. All the TC preparations except one (placenta D) were infected by at least one virus (Table 3). Infection was inhibited by heating the virus and by adding 10 μg/ml zidovudine to the culture medium, but not by a cocktail of chemokines (RANTES, MIP-1α, MIP-1β and SDF-1), or by the anti-CD4 antibody Q4120 (data not shown). In the very same conditions we blocked infection of peripheral blood mononuclear cells (PBMC). No viral replication was detected in TC culture supernatants by measuring RT activity (detection limit 1 pg/ml), while increasing RT activity was detected from day 4 onward in cord blood lymphocytes infected in the same conditions (data not shown).
Table 3.
Infection of purified trophoblastic cells challenged with primary clinical HIV-1 isolates. HIV-DNA was detected by polymerase chain reaction (PCR) targeting the tat gene. Results are expressed as the number of positive PCR runs relative to all PCR runs performed

*p.i., Post-infection.
†Cells were collected on day 14.
NA, Not available.
No RANTES production was detected in culture supernatants of either infected or uninfected TC. Slight MIP-1α and MIP-1β production, close to the detection limit, was found in culture supernatants of TC from placentas A and B. Only TC from placenta C produced significant amounts of MIP-1α and MIP-1β (1450 pg/ml and 260 pg/ml, respectively). No change in chemokine production was induced by HIV-1 challenge (data not shown).
DISCUSSION
Transplacental HIV passage might occur through physical breaches [34] or successive infection of placental cells. In this latter case, the trophoblast would have to be crossed by HIV-1 in order to infect underlying placental macrophages and endothelial cells lining the fetal capillaries.
The feto-placental unit can be infected as little as 8 weeks after conception [4]. Our data are consistent with this observation, as TC from seven of the eight early placentas studied here could be infected by primary clinical isolates in vitro. However, this high rate of successful early trophoblast infection in vitro conflicts with the natural materno–fetal transmission rate of only 22%.
This apparent discrepancy might be explained by the fact that we failed to observe viral replication in any of our TC cultures. Previous reports have shown little if any viral replication in trophoblasts or choriocarcinoma cells in vivo [3,8,9].
It is conceivable, however, that productive infection of trophoblasts might occur in defined, transient conditions under the influence of growth factors and/or cytokines.
We have previously reported that TC isolated from term placentas could not be infected in vitro in the same culture conditions. This difference between early and term placentas was not due to exposure to the abortifacient RU 486, because (i) adding RU 486, or the control RU 43044 (which binds specifically to corticosteroid receptors but does not bind to Pg receptor) to term trophoblast cultures failed to induce co-receptor expression or modify their permissiveness for HIV; (ii) similarly, addition of RU 486 or RU 43044 to early TC cultures did not alter co-receptor expression (data not shown).
The difference in HIV permissiveness between early and late TC may reflect a difference in cell differentiation according to the developmental stage. This in turn might depend on TC exposure to different concentrations of cytokines and growth factors; indeed, the concentrations of at least some such factors in pregnant women's blood are known to vary according to gestational age [35].
Contrary to late TC, all early TC expressed CCR3, CXCR4 and CCR5, and 50% of samples also expressed CD4.
Little information is available on the role played by chemokines and chemokine receptors in normal placental development. Chemokines are chemoattractants for lymphomyeloid cells. The presence of several chemokine receptors on TC during the time of decidua invasion suggests the possible involvement of these molecules in TC migration, but this requires further studies.
Our results do not suggest an absolute requirement of CD4 for viral entry into early primary trophoblast cells, as three TC preparations with undetectable CD4 mRNA or membrane protein expression were permissive to virus entry. Moreover, the CD4-blocking antibody Q4120 did not prevent TC infection, although it partially blocks HIV-1 entry into B cells, which have undetectable membrane CD4 expression [36]. CD4 is not involved in gp120 uptake by trophoblasts, pointing to another, non-saturable and probably non-specific mechanism [15].
These data suggest that, despite the presence of HIV-1 co-receptors on TC, viral entry might occur through a mechanism different from the classical CD4–co-receptor pathway. Previous work has suggested that, in vitro, both the trophoblastic cell line BeW0 [37] and TC isolated from term placentas uptake HIV-1 through an endocytotic mechanism [10]. Moreover, cell-to-cell TC infection has been shown in vitro to be much more efficient than TC infection by cell-free virus [38].
The different permissiveness of early and late TC for HIV-1 infection might imply that placenta can play an active role in controlling transmission of the virus to the fetus throughout gestation. Furthermore, the lack of virus production in trophoblastic cells after contact with cell-free viruses indicates a post-entry restriction of replication. Elucidation of the mechanisms involved will be crucial for understanding the route and mode of perinatal HIV-1 transmission and for improving strategies aimed at preventing materno–fetal HIV-1 transmission.
Acknowledgments
We thank Pr Françoise Barré-Sinoussi and Dr Gabriel Gras for helpful comments and Dr Laurent Chêne for providing mRNA from human thymic epithelial cells. B.M. personally thanks Dr Sophie Lebel-Binay for invaluable assistance in FACS analysis. Financial support was provided by French Agency for AIDS Research (ANRS) and by a Biomed 2 contract (no. PL951509) from the European Community. B.M. is a recipient of a doctoral fellowship from ANRS. E.M. was supported by SIDACTION (France).
References
- 1.Rouzioux C, Costagliola D, Bragard M, et al. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. Am J Epidemiol. 1995;412:1330–7. doi: 10.1093/oxfordjournals.aje.a117601. [DOI] [PubMed] [Google Scholar]
- 2.Mandelbrot L, Le Chadenec J, Berrebi A, et al. Perinatal HIV transmission. Interaction between Zidovudine prophylaxis and mode of delivery in the French perinatal cohort. JAMA. 1998;280:55–60. doi: 10.1001/jama.280.1.55. [DOI] [PubMed] [Google Scholar]
- 3.Backé E, Jimenez E, Unger M, et al. Demonstration of HIV-1 infected cells in human placenta by in situ hybridisation and immunostaining. J Clin Pathol. 1992;45:871–4. doi: 10.1136/jcp.45.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis SH, Reynolds-Kohler C, Fox HE, et al. HIV-1 in trophoblastic and villous Hofbauer cells and haematologic precursors in eight week fetuses. Lancet. 1990;335:565–8. doi: 10.1016/0140-6736(90)90349-a. [DOI] [PubMed] [Google Scholar]
- 5.Zachar V, Thomas RA, Jones T, et al. Vertical transmission of HIV-1: detection of proviral DNA in placental trophoblast. AIDS. 1994;8:129–30. [PubMed] [Google Scholar]
- 6.De Andreis C, Simoni G, Rossella F, et al. HIV-1 proviral DNA polymerase chain reaction detection in chorionic villi after exclusion of maternal contamination by variable number of tandem repeats analysis. AIDS. 1996;10:711–5. doi: 10.1097/00002030-199606001-00004. [DOI] [PubMed] [Google Scholar]
- 7.Menu E, Mbopi KFX, Lagaye S, et al. Selection of maternal HIV-1 variants in human placenta. J Infect Dis. 1999;179:44–51. doi: 10.1086/314542. [DOI] [PubMed] [Google Scholar]
- 8.Mattern C, Murray K, Jensen A, et al. Localization of HIV core antigen in term human placenta. J Clin Pathol. 1992;45:871–4. [Google Scholar]
- 9.Martin AW, Brady K, Smith SI, et al. Immunohistochemical localization of human immunodeficiency virus p24 antigen in placental tissue. Hum Pathol. 1992;23:411–4. doi: 10.1016/0046-8177(92)90088-k. [DOI] [PubMed] [Google Scholar]
- 10.Douglas CG, Fry GN, Thirkill T, et al. Cell-mediated infection of human placental trophoblast with HIV in vitro. AIDS Res Hum Retrovir. 1991;7:735–40. doi: 10.1089/aid.1991.7.735. [DOI] [PubMed] [Google Scholar]
- 11.David F, Autran B, Tran HC, et al. Human trophoblast cells express CD4 and are permissive for production infection with HIV-1. Clin Exp Immunol. 1992;88:10–16. doi: 10.1111/j.1365-2249.1992.tb03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lairmore ML, Cuthbert PS, Utley L. Cellular localization of CD4 in the human placenta. J Immunol. 1993;151:1673–81. [PubMed] [Google Scholar]
- 13.Zachar V, Spire B, Hirsch I, et al. Human transformed trophoblast derived cells lacking CD4 receptor exhibit restricted permissiveness for human immunodeficiency virus type 1. J Virol. 1991;65:2102. doi: 10.1128/jvi.65.4.2102-2107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mano H, Chermann JC. Fetal human immunodeficiency virus type 1 infection of different organs in the second trimester. Res Virol. 1991;142:95–104. doi: 10.1089/aid.1991.7.83. [DOI] [PubMed] [Google Scholar]
- 15.Esterman AL, Finlay TH, Lee JD, et al. Uptake of human immunodeficiency virus envelope protein gp120 by human trophoblast in culture. Am J Obstet Gynecol. 1996;174:49–54. doi: 10.1016/s0002-9378(96)70372-1. [DOI] [PubMed] [Google Scholar]
- 16.Alkhatib G, Combardiere C, Broder CC, et al. CC CKR5: a Rantes, MIP1-alpha, MIP1-beta receptor as a fusion cofactor for macrophage tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 17.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokines receptor CCR3-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 18.Choe H, Farzan M, Sun Y, et al. The b-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 19.Deng HK, Liu R, Ellmeier W, et al. Identification of a major coreceptor for primary isolates of HIV-1. Nature. 1996;381:661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 20.Deng HK, Unutmaz D, Kewal-Ramani VN, et al. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 21.Liao F, Alkhatib G, Peden K, et al. A novel chemokine receptor-like protein functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–23. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu N, Soda Y, Kanbe K, et al. An orphan G protein-coupled receptor, GPR1, acts as a coreceptor to allow replication of human immunodeficiency virus types 1 and 2 in brain-derived cells. J Virol. 1999;73:5231–9. doi: 10.1128/jvi.73.6.5231-5239.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horuk R, Hesselgesser J, Zhou Y, et al. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV- 1 strains. J Biol Chem. 1998;273:386–91. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 24.Choe H, Farzan M, Konkel M, et al. The orphan seven-transmembrane receptor apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–8. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edinger AL, Hoffman TL, Sharron M, et al. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–40. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samson M, Edinger AL, Stordeur P, et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol. 1998;28:1689–700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 27.Berger EA, Doms RW, Fenyö EM, et al. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 28.Edinger AL, Hoffman TL, Sharron M, et al. Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology. 1998;249:367–78. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- 29.Mognetti B, Menu E, Nardese V, et al. Studies on permissivity of human trophoblastic cells to in vitro infection by several natural HIV-1 isolates; 2nd European conference on experimental AIDS research, Stockholm, Sweden; May 1997. [Google Scholar]
- 30.Mognetti B, Menu E, Colognesi C, et al. Studies on permissivity of human trophoblastic cells to in vitro infection by several natural HIV-1 isolates. Keystone symposia: HIV pathogenesis and treatment, Park City, Utah. March 1998 [Google Scholar]
- 31.Kliman HJ, Nestler JE, Sermasi E, et al. Characterization and in vitro differentiation of cytotrophoblast from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 32.Hsi BL, Yeh CJ. Monoclonal antibody GB25 recognizes human villous trophoblasts. Am J Reprod Immunol Microbiol. 1986;12:1–3. doi: 10.1111/j.1600-0897.1986.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 33.Scarlatti G, Tresoldi E, Bjôrndal A. In vivo evolution of HIV-1 coreceptor usage and sensitivity to chemokine-mediated suppression. Nature Med. 1997;3:1259–65. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 34.Burton GJ, O'shea S, Rostron T. Physical breaks in the placental trophoblastic surface: significance in vertical transmission of HIV. AIDS. 1996;10:1294–6. doi: 10.1097/00002030-199609000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Trancho-Diallo J, Gras G, Parnet-Mathieu F, et al. Modulation of cytokine expression in pregnant women. Am J Reprod Immunol. 1997;37:215–26. doi: 10.1111/j.1600-0897.1997.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 36.Legendre C, Gras G, Krzysiek R, et al. Mechanisms of opsonized HIV entry in normal B lymphocytes. FEBS. 1996;381:227–32. doi: 10.1016/0014-5793(96)00040-3. [DOI] [PubMed] [Google Scholar]
- 37.Phillips DM, Tan X. HIV-1 infection of the trophoblastic cell line BeW0: a study of virus uptake. AIDS Res Hum Retrovir. 1992;8:1683–91. doi: 10.1089/aid.1992.8.1683. [DOI] [PubMed] [Google Scholar]
- 38.Fazely F, Fry GN, Thirkill TL, et al. Kinetics of HIV-1 infection of human placental syncytiotrophoblast cultures: an ultrastructural and immunocytochemical study. AIDS Res Hum Retrovir. 1995;11:1023–30. doi: 10.1089/aid.1995.11.1023. [DOI] [PubMed] [Google Scholar]


