Abstract
We examined the in vitro fungicidal activity of human neutrophils against conidia and yeast cells of Penicillium marneffei. Neutrophils showed a small but significant anti-fungal effect against the yeast form of P. marneffei. Treatment of neutrophils with GM-CSF significantly augmented their anti-fungal activity. In contrast, the conidia form resisted killing even by stimulated neutrophils. Neutrophil fungicidal effect was not inhibited by superoxide dismutase (SOD), while the same treatment significantly suppressed the killing of Candida albicans by GM-CSF-stimulated neutrophils. For effective killing of P. marneffei yeast cells by GM-CSF-stimulated neutrophils, direct contact between the two was essential; interference in such interaction by separation using a 0·45-μ m-pored membrane prevented such an effect. Addition of colchicine attenuated GM-CSF-stimulated neutrophil fungicidal activity in a dose-dependent manner. This effect did not appear to be mediated by interference with neutrophil mobility toward yeast cells, because similar results were obtained when the cultures were set in round-bottomed wells which facilitate their direct contact. Finally, granular extracts derived from unstimulated neutrophils significantly suppressed the growth of microorganisms. Pretreatment of neutrophils with GM-CSF markedly enhanced this effect. The fungicidal activity of granular lysates was strongly, but not completely, reduced by heat treatment. Considered together, our results indicate that GM-CSF-stimulated neutrophils killed the yeast form of P. marneffei present in close proximity, probably in a superoxide anion-independent mechanism, but through exocytosis of granular enzymes which were largely heat-labile.
Keywords: Penicillium marneffei, neutrophil, GM-CSF, direct contact, granular lysates
INTRODUCTION
Neutrophils play a major role in host defence against infection with extracellularly growing bacteria and certain fungal pathogens. Previous in vitro studies have shown that neutrophils exert anti-fungal activity against Candida albicans[1], Aspergillus fumigatus[2], Cryptococcus neoformans[3], Histoplasma capsulatum[4] and Blastomyces dermatitidis[5].
Penicillium marneffei is an important opportunistic fungal pathogen known to cause a life-threatening deep-seated systemic infection in patients with AIDS in South-east Asian countries [6–10]. The incidence of P. marneffei infection is rapidly increasing among AIDS patients in Thailand [6]. The latter observation indicates that host defence against this pathogen is mediated by cellular immunity. In fact, this was previously demonstrated experimentally in our laboratory using athymic nude mice [11]. However, little is known about the role of neutrophils in host defence against P. marneffei infection.
GM-CSF is an acidic glycoprotein with a molecular weight of 22 kD. It specifically binds to neutrophil plasma membranes, and stimulates the development of both granulocyte and macrophage colonies and activates neutrophils [12]. In previous studies, GM-CSF was demonstrated to induce superoxide production, lysozyme release, phagocytosis of heat-killed yeast cells by neutrophils and their antibody-dependent cell-mediated cytotoxicity [13], enhance survival [14], promote cell–cell adhesion and alter surface receptor expression of neutrophils [15]. GM-CSF also enhances the killing of Torulopsis glabrata by neutrophils [16].
In the present study we examined the effect of GM-CSF-stimulated human peripheral blood neutrophils on the in vitro growth of P. marneffei and demonstrated that such neutrophils exert a strong killing effect against this fungal pathogen. We also elucidated the role of superoxide anion, a major killing material, in the fungicidal effect of GM-CSF-stimulated neutrophils, as well as the role of physical contact between neutrophils and the fungal pathogen and cytoplasmic granular enzymes in this killing.
MATERIALS AND METHODS
Microorganisms and culture conditions
A clinical strain of P. marneffei, designated H1140 and registered by American Type Culture Collection (ATCC201013), was isolated from a blood sample of a patient with AIDS in Chiang Mai University, Chiang Mai, Thailand, and kindly provided by Dr P. Tharvichitkul. To prepare conidia suspensions, colonies of P. marneffei were flooded with physiological saline and gently scraped with a sterile inoculation wire-loop after 7 days of culture on a potato–dextrose agar (PDA; Eiken, Tokyo, Japan) at 30°C, as reported previously [17]. On the other hand, to prepare inocula of yeast cells, conidia were incubated in brain heart infusion broth (BHI; Eiken) at 37°C for 7–14 days, as described previously by Sekhon et al. [18]. After incubation, broth cultures were filtered through a 10-μ m nylon mesh to remove mixed mycelial fragments and centrifuged at 15 000 g at 4°C for 10 min to separate yeast cells from the culture broth. The obtained yeast cells and conidia preparations contained < 3% and < 5% of hyphae under microscopic examination, respectively. To examine P. marneffei under a light microscope, each fungal element was stained with lactophenol cotton blue (Muto Pure Chemicals Co., Tokyo, Japan). In some experiments we also used C. albicans (strain RK9232), isolated from a clinical specimen from a patient with candidial pneumonia at the Ryukyu University Hospital.
Culture medium and reagents
RPMI 1640 medium was purchased from Gibco BRL (Grand Island, NY), fetal calf serum (FCS) from Whittaker (Walkersville, MD), superoxide dismutase (SOD) and colchicine from Wako Pure Chemical Co. (Osaka, Japan). Recombinant human GM-CSF was kindly provided by Kirin Co. (Tokyo, Japan). Mono-Poly Resolving Medium was purchased from Dainippon Pharmaceutical Co. (Tokyo, Japan).
Preparation of neutrophils
Neutrophils were prepared by the rapid single-step method [19]. In brief, a heparinized peripheral venous blood sample from a healthy volunteer was layered over Ficoll–Hypaque medium of density 1·115 (Mono-Poly Resolving Medium) and centrifuged at 400 g for 30 min at room temperature. Neutrophils in the second band from the top were collected and then contaminating erythrocytes were lysed by osmotic shock with 0·17 m NH4Cl. After washing three times with ice-cold RPMI 1640 supplemented with 5% FCS, the cells were counted with a haemocytometer. May–Giemsa-stained cytospin preparations of the obtained cell suspensions typically showed > 95% neutrophils, < 4% eosinophils and < 3% mononuclear cells. Cell viability, as assessed by trypan blue exclusion, was always > 98%.
Killing assay
Neutrophils were dispensed in triplicate at 1 × 105 cells/well in 200 μ l each in 96-well flat- or round-bottomed microculture plates (Falcon no. 3072; Becton Dickinson, Lincoln Park, NJ). These cells were co-cultured with 1 × 103 colony-forming units (CFU)/well of P. marneffei or C. albicans with or without 10 ng/ml of GM-CSF in a 5% CO2 incubator. In some experiments we investigated the importance of direct contact between microorganisms and neutrophils in neutrophil killing. For this purpose, neutrophils and P. marneffei were cultured in 24-well culture plates (Falcon no. 3047; Becton Dickinson), separated by a 0·45-μ m pore membrane (Millicell-HA; Millipore Corp., Bedford, MA). The microorganisms were harvested 24 h later by washing each well with 1·0 ml of distilled water. The samples were inoculated at 50 μ l on PDA plates after appropriate dilution with distilled water and cultured at 30°C for 2 days. The number of viable colonies was counted and multiplied by the dilution factor. In the present study neutrophils were considered to be the main killers of extracellular microorganisms, because any opsonins such as complement and antibody were not added to the cultures.
Preparation of granular extract
Granular lysate was extracted from human neutrophils using the method described by Panyutich et al. [20]. Briefly, neutrophils were suspended at 108/ml in ice-cold 0·34 m sucrose adjusted to pH 7·4. The cell suspension was sonicated and then unbroken cells and nuclei were removed by centrifugation at 200 g for 10 min. Granular pellets were collected by centrifugation at 13 000 g for 30 min at 4°C. For extraction, granules were resuspended in 0·3 ml of 0·01 m citric acid pH 2·7 and stirred for 2 h at 4°C. In other experiments, to examine the influence of heat treatment, granular lysates were boiled for 10 min after extraction. Granule-free extraction buffer was also used as a control.
Statistical analysis
Data were expressed as mean ± s.d. The unpaired Student’s t-test was used to compare differences between groups. P < 0·05 was considered significant.
RESULTS
Fungicidal activity of GM-CSF-stimulated neutrophils against P. marneffei
To examine the effect of neutrophils on the growth of P. marneffei, two types of microorganisms, yeast cells and conidia, were cultured with neutrophils in the presence or absence of GM-CSF and the number of viable organisms was counted. The number of P. marneffei yeast cells remained constant or slightly decreased in control culture media during the observation period. In contrast, the number started to decrease at 3 h when yeast cells were cultured with neutrophils, and the lowest number was noted at 12–24 h [21]. As shown in Table 1, neutrophils significantly reduced the number of viable yeast cells compared with that of microorganisms in medium alone after 24 h of culture, and GM-CSF markedly enhanced this effect. In contrast, the conidia form of P. marneffei was resistant to the anti-fungal activity of both unstimulated and GM-CSF-stimulated neutrophils. These results indicate that yeast cells were more susceptible to the killing effect of neutrophils than conidia. Therefore, in the following experiments we used the former type of P. marneffei. This form is usually detected in clinical samples from patients with penicilliosis marneffei.
Table 1.
Different susceptibilities of yeasts and conidia of Penicillium marneffei to GM-CSF-activated polymorphonuclear neutrophils (PMN)
Yeasts or conidia of Penicillium marneffei(1 × 103) were cultured with GM-CSF (10 ng/ml). After 24 h, the number of live microorganisms was counted. Results are expressed as mean colony-forming units (CFU) ± s.d. of triplicate cultures.
P < 0·05 compared with no GM-CSF group.
Not significant compared with no GM-CSF group.
Neutrophil fungicidal activity against P. marneffei is largely mediated by a superoxide-independent mechanism
Superoxide anion is well documented as a major effector molecule in neutrophil killing mechanisms against microbial agents. To examine whether superoxide anion is involved in the killing activity of neutrophils, P. marneffei were cultured with GM-CSF-stimulated neutrophils in the presence or absence of SOD, a scavenger of this oxygen radical. As shown in Table 2, GM-CSF-activated neutrophils exerted a significant fungicidal activity against P. marneffei compared with unstimulated neutrophils. This effect was not influenced by the addition of 0·2 mg/ml of SOD. The same treatment also did not show any influence on the anti-fungal capacity of unstimulated neutrophils (data not shown). In striking contrast, the fungicidal effect of GM-CSF-stimulated neutrophils against C. albicans was significantly inhibited by the same dose of SOD.
Table 2.
Effect of superoxide dismutase (SOD) on GM-CSF-induced polymorphonuclear neutrophil (PMN) killing activity against Penicillium marneffei
| P. marneffei | Candida albicans | |
|---|---|---|
| No stimulant | 11 718 ± 242† | 35 332 ± 2298 |
| GM-CSF | 4810 ± 1222* | 1708 ± 673* |
| GM-CSF + SOD | 3828 ± 152** | 20 372 ± 5714*** |
Penicillium marneffei yeast cells or C. albicans (2 × 10 4) were cultured with PMN with or without GM-CSF (10 ng/ml) in the presence or absence of SOD (0·2 mg/ml). After 24 h the number of live microorganisms was counted. Results are expressed as mean colony-forming units (CFU) ± s.d. of triplicate cultures.
P < 0·05 compared with no stimulant group.
Not significant compared with GM-CSF group.
P < 0·05 compared with GM-CSF group.
Importance of direct contact in neutrophil-induced killing of P. marneffei
To investigate further the fungicidal mechanism of neutrophils against P. marneffei, cultures of yeast cells and neutrophils were set in two chambers separated by a membranous septum. The septum acted as a physical barrier to cell movement between the upper and lower chambers but allowed the diffusion of macromolecules as large as 0·45 μ m. As shown in Fig. 1, the number of fungal pathogens significantly decreased when cultured with neutrophils in the same chamber. In contrast, when the two components were cultured separately in the lower and upper chambers, no significant decrease in the number of viable colonies was observed. These results indicate that the killing of P. marneffei by GM-CSF-activated neutrophils was dependent on physical contact or close interaction between the fungus and neutrophils.
Fig. 1.
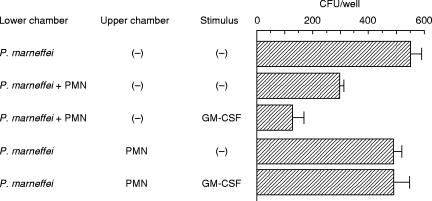
Contact-dependent killing of Penicillium marneffei by neutrophils. The culture was set in 24-well double chambers separated by a membrane of 0·45-μ m pores. Penicillium marneffei yeast cells (1 × 103) were cultured in the presence of 10 ng/ml of GM-CSF in the lower chamber with or without neutrophils (1 × 105) in the lower or upper chamber in a volume of 1·0 ml for 24 h, and then the number of live microorganisms was counted. Each bar represents the mean ± s.d. of triplicate cultures. PMN, Polymorphonuclear neutrophils.
Inhibition of neutrophil fungicidal activity against P. marneffei by colchicine
In the next experiments, we elucidated further the mechanism of fungicidal activity by examining the effect of colchicine treatment on the killing of P. marneffei by GM-CSF-stimulated neutrophils. As shown in Fig. 2, such treatment significantly inhibited the fungicidal activity of neutrophils against P. marneffei in a dose-dependent manner. This inhibition was not due to the cytolytic effect of this agent, because the viability of neutrophils remained > 93% 24 h after treatment with the highest dose (0·5 mm) of colchicine, and there was no significant difference in the viability of neutrophils between colchicine-treated and untreated groups (data not shown). These results suggest that exocytosis of neutrophil granular enzymes may be involved in the killing of P. marneffei by GM-CSF-stimulated neutrophils. Alternatively, colchicine might hinder neutrophil migration toward the yeast cells in culture wells. This conclusion is based on previous studies by Valerius [22], who demonstrated inhibition by colchicine of cell locomotion through the blockade of cytoskeleton function. To examine this possibility, we conducted similar experiments in round-bottomed culture wells. Such wells facilitate direct contact between neutrophils and microorganisms and lessen the importance of cell migration. As shown in Fig. 3a,b, colchicine treatment significantly inhibited the fungicidal activity of both unstimulated and GM-CSF-stimulated neutrophils against P. marneffei in the round-bottomed culture wells, in a manner similar to that seen in flat-bottomed culture wells. These results indicate that the inhibitory effect of colchicine was not due to interference with neutrophil locomotion toward microorganisms.
Fig. 2.
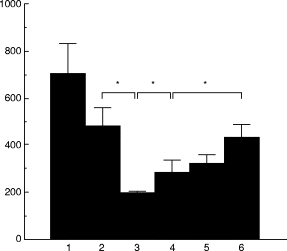
Effect of colchicine on the killing of Penicillium marneffei by GM-CSF-stimulated neutrophils. Penicillium marneffei yeast cells (1 × 103/well) were cultured in 96-well flat culture plates with or without neutrophils (1 × 105/well), pretreated with or without the indicated concentrations of colchicine for 30 min, in the presence or absence of 10 ng/ml of GM-CSF. After 24 h the number of live microorganisms was counted. Each bar indicates the mean ± s.d. of triplicate cultures. 1, Medium alone; 2, neutrophils; 3, neutrophils + GM-CSF; 4, neutrophils + GM-CSF + colchicine (0·1 mm); 5, neutrophils + GM-CSF + colchicine (0·25 mm); 6, neutrophils + GM-CSF + colchicine (0·5 mm). *P < 0·05.
Fig. 3.
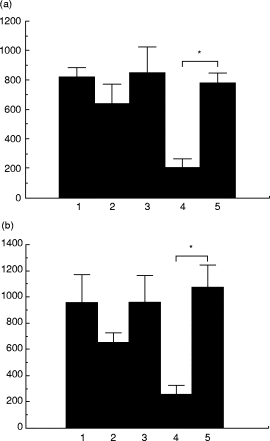
Effect of colchicine on neutrophil fungicidal activity. In 96-well flat- (a) or round- (b) bottomed culture plates, Penicillium marneffei yeasts (1 × 103/ml) were cultured with or without neutrophils (1 × 105/ml) and GM-CSF (10 ng/ml) in the presence or absence of 0·5 mm colchicine. After 24 h, the number of live microorganisms was counted. Each bar indicates the mean ± s.d. of triplicate cultures. 1, Medium alone; 2, neutrophils; 3, neutrophils + colchicine; 4, neutrophils + GM-CSF; 5, neutrophils + GM-CSF + colchicine. *P < 0·05.
Fungicidal activity of granular extracts against P. marneffei
Finally, to confirm the involvement of exocytosed material from neutrophil granules in the fungicidal activity of neutrophils, the effect of granular extracts obtained from neutrophils against P. marneffei was examined. As shown in Table 3, the number of live yeast cells significantly decreased when they were exposed to lysates derived from unstimulated neutrophils, compared with that of microorganisms incubated with the lysing buffer (vehicle) only. This effect was further enhanced by stimulation of neutrophils with GM-CSF prior to extraction of granular lysates. In addition, the fungicidal activity of granular extracts from GM-CSF-stimulated neutrophils was strongly but not completely attenuated by heat treatment (Table 4). These results indicate that the fungicidal activity of GM-CSF-stimulated neutrophils against P. marneffei was mediated largely by heat-labile and in part by heat-stable granular enzymes.
Table 3.
Killing effect of granular lysates from human polymorphonuclear neutrophils (PMN)
| CFU/well† | |
|---|---|
| Vehicle‡ | 1286 ± 314 |
| Lysate from non-activated PMN | 326 ± 43* |
| Lysate from PMN activated by GM-CSF | 24 ± 13** |
Penicillium marneffei yeast cells (1 × 103) were exposed to lysate extracted from PMN with or without 24 h preactivation by GM-CSF (10 ng/ml). After 24 h the number of live microorganisms was counted. Results are expressed as mean colony-forming units (CFU) ± s.d. of triplicate cultures.
The vehicle of lysate, 0·01 m citric acid solution, was added as a control.
P < 0·05 compared with vehicle group.
P < 0·05 compared with non-activated group.
Table 4.
Influence of heat treatment against granular lysates on the killing effect
| CFU/well† | |
|---|---|
| Vehicle‡ | 1717 ± 160 |
| Lysate from non-activated PMN | 54 ± 8 |
| Heat-treated lysate from non-activated PMN | 853 ± 47* |
| Lysate from PMN activated by GM-CSF | 29 ± 2 |
| Heat-treated lysate from PMN activated by GM-CSF | 884 ± 46** |
Penicillium marneffei yeast cells (1 × 10 3) were exposed to lysate with or without boiling for 10 min after extraction from polymorphonuclear neutrophils (PMN) in the presence or absence of 24 h preactivation by GM-CSF (10 ng/ml). After 24 h the number of live microorganisms was counted. Results are expressed as mean colony-forming units (CFU) ± s.d. of triplicate cultures.
The vehicle of lysate, 0·01 m citric acid solution, was added as a control.
P < 0·05 compared with lysate from non-activated PMN group.
P < 0·05 compared with lysate from PMN activated by GM-CSF group.
DISCUSSION
Penicillium marneffei is a dimorphic fungus which grows in a hyphal form and bears conidia under culture conditions at 30°C, but converts to a yeast form at 37°C [23]. Although infection is acquired by inhalation of the conidia, the yeast cell is usually found in clinical specimens because of its conversion at the sites of infection [24]. In the present study we compared the susceptibility of conidia and yeast cells with the fungicidal effect of GM-CSF-stimulated neutrophils. Our data confirm that yeast cells, but not conidia, were highly susceptible to the killing of these cells. Similar results were obtained in our previous studies [25,26] demonstrating the differential susceptibility of these two forms of P. marneffei to the nitric oxide-dependent fungicidal effect by mouse macrophages stimulated with interferon-gamma (IFN-γ). Conidia and yeast cells have different cell surface structural characteristics, as demonstrated in previous studies using electron microscopy [27,28]. Chan & Chow [27] demonstrated the presence of a rough outer surface in the yeast form of P. marneffei, but not in conidia. Furthermore, they also identified the presence of a thin cell wall in the yeast form compared with a thick wall in conidia. Thus, the different susceptibilities of these fungal forms to neutrophils may be due to these structural differences.
In previous studies [29] GM-CSF was shown to extend the survival time of neutrophils. These findings suggest that the enhanced neutrophil anti-fungal activity by GM-CSF may be mediated by this non-specific mechanism rather than by increasing their fungicidal activity. To exclude the role of such mechanisms in our study, we examined the viability of these cells in the presence or absence of this cytokine using the trypan blue exclusion method. GM-CSF did not increase the number of neutrophils during 24-h culture. In addition, the viability of these cells was > 96% at 24 h after culture and treatment with this cytokine did not influence survival (unpublished data). These results indicate that cytokine-associated enhancement of neutrophil anti-fungal activity is not mediated by prolongation of the survival time of these cells.
Neutrophils exert their fungicidal effect through two main mechanisms [30]. The first is mediated by reactive oxygen intermediates, such as superoxide anion, produced by activated phagocytes, while the second is due to the action of anti-microbial components exocytosed from the cytoplasmic granules, such as cationic proteins, lysozyme, lactoferrin, acid phosphatase, β-glucuronidase and elastase. Our results indicate that the killing mechanism of GM-CSF-stimulated neutrophils on P. marneffei was not mediated by a superoxide-dependent mechanism, because SOD failed to inhibit the anti-fungal activity of GM-CSF-stimulated neutrophils. In striking contrast, the fungicidal activity of GM-CSF-stimulated neutrophils against C. albicans was strongly associated with the former mechanism, because this activity was strongly inhibited by treatment with SOD. Similar results were reported by Diamond and co-workers [31]. These results suggest the involvement of oxygen-independent mechanisms in the GM-CSF-induced fungicidal activity of neutrophils against P. marneffei.
We have recently reported the important role of direct contact between neutrophils and P. marneffei yeast cells on the anti-fungal effect of neutrophils [21]. Using a phase-contrast microscope, we found that GM-CSF-stimulated neutrophils were in close proximity to yeast cells and aggregated around them during the killing process. These observations suggest the importance of close contact between neutrophils and P. marneffei for the expression of fungicidal effect. This observation was compatible with our present data indicating that the anti-fungal activity of GM-CSF-stimulated neutrophils was abrogated by interfering with the direct contact between these two cellular components using a 0·45-μ m pored membrane. Taken together, these results indicate that neutrophils exert their fungicidal activity through a direct binding or migration towards the yeast cells. Although the precise mechanism of this phenomenon remains to be elucidated, physical interaction of neutrophils with the fungal surface might be important for expressing their fungicidal effect, because early responses of neutrophils, such as membrane depolarization, increase in cytosolic calcium and phosphoinositide turnover, have been reported to occur after triggering of their surface molecules by certain fungal components, a process usually followed by the release of both fungicidal granule constituents and oxidant from the respiration burst [32].
To characterize further neutrophil fungicidal mechanisms, we used colchicine, which binds to tubulin and inhibits its polymerization [33]. Our data indicated that this agent significantly suppressed the fungicidal activity of GM-CSF-stimulated neutrophils against P. marneffei in a dose-dependent manner. There are two possible mechanisms that might explain this effect: (i) inhibition of neutrophil secretion of granular cytolytic material, and/or (ii) suppression of neutrophil migration towards target cells. In the present study we provided direct evidence in support of the former mechanism and that the latter mechanism was unlikely to explain the observed colchicine effect. Thus, a similar suppression of neutrophil fungicidal activity by colchicine was noted when cultures were set in flat- and round-bottomed wells. These findings suggest the involvement of granular material in the fungicidal activity of GM-CSF-stimulated neutrophils. We also provided evidence which strongly supports the notion that granular lysates prepared from neutrophils strongly inhibit the growth of fungal pathogens.
Neutrophil cytoplasmic granules store numerous anti-microbial components of the oxygen-independent system, which include both cytotoxic proteins and peptides, such as lactoferrin, serprocidins, protegrins, lysozyme, calprotectin and defensins [34]. These proteinaceous cytotoxic molecules are known to have anti-fungal capacity. Diamond et al. reported that lysozyme damaged hyphae of A. fumigatus and Rhizopus oryzae[35]. Defensins, rich in the azurophilic granules, were shown to express a potent killing activity against C. neoformans[36]. Interestingly, several investigators demonstrated that GM-CSF induced lysozyme secretion by neutrophils [13], promoted cell–cell adhesion through enhanced expression of β2-integrins on their surface [15] and enhanced the neutrophil fungicidal activity against T. glabrata[16]. In our study the fungicidal activity of granular lysate was significantly abrogated by heat treatment, but was partially resistant to this treatment, indicating that the granular fungicidal materials consisted largely of heat-labile molecules. At the moment however it remains to be determined which molecules are responsible for the neutrophil anti-fungal activity against P. marneffei.
In conclusion, the fungicidal effect of GM-CSF-stimulated neutrophils is produced by physical interaction of these cells with the yeast form of this microorganism. The effect is mediated by exocytosis of the granular cytolytic molecules from neutrophils rather than by oxygen radical-dependent mechanisms. Further studies to define the precise mechanism of this effect might contribute to the development of effective therapy against intractable penicilliosis marneffei in patients with AIDS.
Acknowledgments
This work was supported in part by a Grant-in-aid (C) (09670292) from the Ministry of Education, Science and Culture, by Grants from the Ministry of Health and Welfare, Japan and by the Japan Health Science Foundation. The authors thank Dr F. G. Issa (Word-Medex, Sydney, Australia) for critical reading and editing of this manuscript.
References
- 1.Diamond RD, Krzesicki R, Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophil in the absence of serum in vitro. J Clin Invest. 1978;61:349–59. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rex JH, Bennett JE, Gallin JI, Malech HL, Melnick DA. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J Infect Dis. 1990;162:523–8. doi: 10.1093/infdis/162.2.523. [DOI] [PubMed] [Google Scholar]
- 3.Diamond RD, Root RK, Bennett JE. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–76. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- 4.Kurita N, Brummer E, Yoshida S, Nishimura K, Miyaji M. Antifungal activity of murine polymorphonuclear neutrophils against Histoplasma capsulatum. J Med Vet Mycol. 1991;29:133–43. [PubMed] [Google Scholar]
- 5.Drutz DJ, Frey CL. Intracellular and extracellular defenses of human phagocytes against Blastomyces dermatitidis conidia and yeasts. J Lab Clin Med. 1985;105:737–50. [PubMed] [Google Scholar]
- 6.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirithanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–3. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 7.Dixon DM, McNeil MM, Cohen ML, Gellin BG, La Montagne JR. Fungal infections: a growing threat. Public Health Report. 1996;111:226–35. [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont B, Denning DW, Marriott D, Sugar A, Viviani MA, Sirisanthana T. Mycoses in AIDS patients. J Med Vet Mycol. 1994;32(Suppl. 1):65–77. doi: 10.1080/02681219480000731. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JE, Hu DJ, Holmes KK, Jaffe HW, Masur H, De Cock KM. Preventing opportunistic infections in human immunodeficiency virus-infected persons: implications for the developing world. Am J Trop Med Hyg. 1996;55:1–11. [PubMed] [Google Scholar]
- 10.Hilmarsdottir I, Meynard JL, Rogeaux O, et al. Disseminated Penicillium marneffei infection associated with human immunodeficiency virus: a report of two cases and review of 35 published cases. J Acquir Immune Defic Syndr. 1993;6:466–71. [PubMed] [Google Scholar]
- 11.Kudeken N, Kawakami K, Kusano N, Saito A. Contribution of cell-mediated immunity in host resistance against infection caused by Penicillium marneffei. J Med Vet Mycol. 1996;34:371–8. doi: 10.1080/02681219680000671. [DOI] [PubMed] [Google Scholar]
- 12.Gasson JC, Weisbart RH, Kaufman SE, Clark SC, Hewick RM, Wong GG. Purified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophils. Science. 1984;226:1339–42. doi: 10.1126/science.6390681. [DOI] [PubMed] [Google Scholar]
- 13.Burgess AW, Begley CG, Johnson GR, et al. Purification and properties of bacterially synthesized human granulocyte-macrophage colony stimulating factor. Blood. 1987;69:43–51. [PubMed] [Google Scholar]
- 14.Lopez AF, Williamson DJ, Gamble JR, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–8. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnaout MA, Wang EA, Clark SC, Sieff CA. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins of mature granulocytes. J Clin Invest. 1986;78:597–601. doi: 10.1172/JCI112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowanko IC. Granulocyte-macrophage colony-stimulating factor augments neutrophil killing of Torulopsis glabrata and stimulates neutrophil respiratory burst and degranulation. Clin Exp Immunol. 1991;83:225–30. doi: 10.1111/j.1365-2249.1991.tb05619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudeken N, Kawakami K, Saito A. CD4 + T cell-mediated fatal hyperinflammatory reactions in mice infected with Penicillium marneffei. Clin Exp Immunol. 1997;107:468–73. doi: 10.1046/j.1365-2249.1997.d01-945.x. [DOI] [PubMed] [Google Scholar]
- 18.Sekhon AS, Garg AK, Padhye AA, Hamir Z. In vitro susceptibility of mycelial and yeast forms of Penicillium marneffei to amphotericin B, fluconazole, 5-fluorocytosine and itraconazole. Eur J Epidemiol. 1993;9:553–8. doi: 10.1007/BF00209535. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante A, Thong YH. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque–Ficoll method. J Immunol Methods. 1980;36:109–17. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- 20.Panyutich A, Shi J, Boutz PL, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun. 1997;65:978–85. doi: 10.1128/iai.65.3.978-985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudeken N, Kawakami K, Saito A. Cytokine-induced fungicidal activity of human polymorphonuclear leukocytes against Penicillium marneffei. FEMS Immunol Med Microbiol. 1999;26:115–124. doi: 10.1111/j.1574-695X.1999.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 22.Valerius NH. In vitro effect of colchicine on neutrophil granulocyte locomotion. Assessment of the effect of colchicine on chemotaxis, chemokinesis and spontaneous motility, using a modified reversible Boyden chamber. Acta Pathol Microbiol Scand. 1978;86:149–54. [PubMed] [Google Scholar]
- 23.Garrison RG, Boyd KS. Dimorphism of Penicillium marneffei as observed by electron microscopy. Can J Microbiol. 1973;19:1305–9. doi: 10.1139/m73-209. [DOI] [PubMed] [Google Scholar]
- 24.Deng ZL, Connor DH. Progressive disseminated penicilliosis caused by Penicillium marneffei. Am J Clin Pathol. 1985;84:323–7. doi: 10.1093/ajcp/84.3.323. [DOI] [PubMed] [Google Scholar]
- 25.Kudeken K, Kawakami K, Saito A. Different susceptibilities of yeasts and conidia of Penicillium marneffei to nitric oxide-mediated fungicidal activity of murine macrophages. Clin Exp Immunol. 1998;112:287–93. doi: 10.1046/j.1365-2249.1998.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudeken K, Kawakami K, Saito A. Role of superoxide anion in the fungicidal activity of murine peritoneal exudate macrophages against Penicillium marneffei. Microbiol Immunol. 1999;43:323–30. doi: 10.1111/j.1348-0421.1999.tb02412.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan YF, Chow TC. Ultrastructural observations on Penicillium marneffei in natural human infection. Ultrastruct Pathol. 1990;14:439–52. doi: 10.3109/01913129009007223. [DOI] [PubMed] [Google Scholar]
- 28.Cogliati M, Roverselli A, Boelaert JR, Taramelli D, Lombardi L, Viviani MA. Development of an in vitro macrophage system to assess Penicillium marneffei growth and susceptibility to nitric oxide. Infect Immun. 1997;65:279–84. doi: 10.1128/iai.65.1.279-284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez AF, Williamson DJ, Gamble JR, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–8. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klebanoff SJ. Antimicrobial mechanisms in neutrophilic polymorpho-nuclear leukocytes. Semin Hematol. 1975;12:117–42. [PubMed] [Google Scholar]
- 31.Diamond RD, Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978;61:360–9. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diamond RD. Fungal surfaces: effects of interactions with phagocytic cells. Rev Infect Dis. 1988;10(Suppl. 2):S428–S431. doi: 10.1093/cid/10.supplement_2.s428. [DOI] [PubMed] [Google Scholar]
- 33.Zurier RB, Weissmann G, Hoffstein S, Kammerman S, Tai HH. Mechanisms of lysosomal enzyme release from human leukocytes: II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974;53:297. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy O. Antibiotic proteins of polymorphonuclear leukocytes. Eur J Haematol. 1996;56:263–77. doi: 10.1111/j.1600-0609.1996.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 35.Diamond RD, Krzesicki R, Epstein B, Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. Am J Pathol. 1978;91:313–28. [PMC free article] [PubMed] [Google Scholar]
- 36.Ganz T, Selsted ME, Szklarek D, Harwig SSL, Daher K, Bainton DF, Lehrer RI. Defensins;. natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]


