Abstract
Cellular immune mechanisms resulting in interferon-gamma (IFN-γ) production are essential for protection against cutaneous leishmaniasis. Antigens of the intracellular amastigote form of the parasite, found in mammalian hosts, are likely to be good candidates for the induction of T cell response and protection from development of leishmaniasis. We purified a stage-specific antigen from amastigote soluble antigen (A-SLA) of Leishmania major by immunoaffinity chromatography. The purified protein was characterized as a cysteine proteinase with enzymatic activity which is inhibited by E-64, and it was named the amastigote cysteine proteinase (ACP). BALB/c mice were immunized by two intraperitoneal injections, at a month interval, of 5 μg of ACP or A-SLA in Freund’s complete adjuvant (FCA). Animals were challenged 4 weeks later with 106 L. major promastigotes and examined 4 months after the last injection. The immunized animals developed significantly smaller or no lesions compared with controls. Spleen cells from immunized mice showed a significant proliferative response and produced a high level of IFN-γ in response to ACP, suggesting the induction of Th1 cells after immunization. These results make 24-kD ACP a possible component for an eventual cocktail vaccine against L. major infection.
Keywords: Leishmania, major, amastigote-specific cysteine proteinase, vaccination
Introduction
Protozoan parasites of the genus Leishmania present two forms in their life cycle: the promastigote, which multiplies in the mid gut of the sand fly vector, and amastigote, the obligate intracellular form which lives within phagolysosomes of the vertebrate host [1,2]. Leishmania are associated with a broad spectrum of disease, ranging from simple cutaneous to invasive visceral leishmaniasis [3]. In the murine model of leishmaniasis caused by L. major, a clear cut dichotomy between the two functional T helper subsets, Th1 and Th2, has been observed [4–8]. Th1 cells, which produce interferon-gamma (IFN-γ) and IL-2, mediate protection in resistant mouse strain (C57Bl/6), and Th2 cells, which produce IL-4 and IL-10, promote disease progression in susceptible strain (BALB/c) [9]. Appreciable levels of protection against cutaneous leishmaniasis have been achieved in genetically susceptible mice after immunization with promastigote surface membrane antigens such as LPG, gp63 and gp46 [10–12]. In contrast to the promastigte stage of life, relatively little is known about the possible protective antigens of the amastigote stage.
In a previous study, we isolated fractions of soluble Leishmania antigen (SLA) from both developmental stages of L. major using fast protein liquid chromatography (FPLC). A distinct band of 24 kD, eluted in the first fraction of amastigote SLA, was characterized as an amastigote-specific cysteine proteinase (ACP) because of its enzymatic activity on gelatin-incorporated SDS–PAGE which was inhibited by leupeptin and E-64. The ACP was also shown to be a potent immunogenic component of the amastigote, capable of inducing IFN-γ in vitro by peripheral blood mononuclear cells (PBMC) of immune human individuals [13,14].
In the present study, the 24-kD ACP together with Freund’s complete adjuvant (FCA) was used to immunize the susceptible BALB/c mice. It induced the production of a high level of IFN-γ, indicating a Th1 immune response, and protected the vaccinated animals against a lethal challenge with L. major. We propose that the 24-kD protein may be considered a possible component of a cocktail vaccine against human cutaneous leishmaniasis.
MATERIALS and METHODS
Mice and parasites
Female BALB/c mice 8–12 weeks old were obtained from breeding stock maintained at the Pasteur Institute of Iran. The L. major strain used, MRHO/IR/75/ER, was provided by Dr E. Javadian (School of Public Health, University of Tehran). The parasites were kept virulent by continuous passage in BALB/c mice. Amastigotes were isolated from lesions of infected BALB/c mice according to the methods described by Glaser et al. [15].
Preparation and fractionation of amastigote soluble antigen
Soluble antigens from isolated Leishmania amastigotes and promastigotes were prepared and fractionated according to Scott et al. [16] with some modification [14]. Briefly, purified amastigotes or promastigotes were washed four times in cold PBS and resuspended at 109 parasites/ml in 100 mm Tris–HCl, 1 mm EDTA pH 8 with 50 μg/ml leupeptin, 50 μg/ml antipapain, 50 μg/ml aprotinin and 1·6 mm PMSF (all from Fluka Chemie AG, RdH Laborchemikalien GmbH). The suspension was incubated for 10 min on ice and sonicated at 4°C with two 20-s blasts. The amastigote or promastigote suspensions were then centrifuged (27 000 g for 20 min) and the supernatants collected and recentrifuged (100 000 g, 4 h).
Fractionation of amastigote SLA was done by FPLC Mono Q column (Pharmacia HR5/5). The amastigote SLA was dialysed against starting buffer (100 mm Tris–HCl, 1 mm EDTA pH 8) and then passed through a 0·22-μm filter. A total volume of 20 ml dialysed amastigote SLA (4·5 mg/ml) was loaded on the column. Bound molecules were eluted using a 20-ml linear NaCl gradient (0–1 m NaCl) at a flow rate of 0·75 ml/min. The first fraction was concentrated using a centricon 3 (MW-Co 3000).
Preparation of antiserum to and immunoaffinity purification of ACP
The first fraction of amastigote SLA was separated by 12% SDS–PAGE and the 24-kD band (ACP) was cut and homogenized. The homogenized band at a concentration of 100 μg/ml was emulsified in an equal volume of FCA and injected intramuscularly into a rabbit. An additional 100 μg protein in Freund’s incomplete adjuvant (FIA) was used as booster after 4 weeks, and the rabbit was bled after an additional week. Purified rabbit antibody bound to cyanogen bromide (CNBr)-activated Sepharose 4B (Pharmacia LKB Biotechnology, Uppsala, Sweden) was used as affinity columns for further isolation of ACP from first fraction of amastigote SLA. The purity of the eluted ACP was assessed by SDS–PAGE. The purified ACP was dialysed against PBS and filtered (0·22 μm) for further utilization in lymphocyte culture.
Immunization of mice
Four groups of 10 female BALB/c mice were injected intraperitoneally with a volume of 100 μl per mouse as follows: group I, 5 μg of ACP in FCA; group II, 5 μg of amastigote soluble antigen (A-SLA) in FCA; group III, 100 μl of FCA; group IV, 100 μl of PBS. One month later mice were boosted (FCA was replaced by FIA) and 4 weeks after the last injection they were challenged with 106 stationary phase promastigotes of L. major. The course of infection was monitored by weekly measurement of the infected footpad with a metric caliper compared with the non-infected footpad.
T cell proliferation assay
Spleens from each group were removed 30 days after first injection, 30 days after booster (day 60) and 3 months after infection (day 150). Cells were isolated, counted, and after evaluation of cell viability by trypan blue exclusion they were frozen in liquid nitrogen. When needed, cells were rapidly thawed and washed three times in RPMI. Lymphocytes were cultured in complete medium containing RPMI 1640, 5% fetal calf serum (FCS), 2 mm glutamine, 10 mm HEPES, 5 ×10−5 mm 2-mercaptoethanol (2-ME), 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were incubated in U-bottomed 96-well microtitre plates (Costar, Cambridge, MA) at a density of 2 ×105 cells/well in the presence of either 15 μg/ml A-SLA, 15 μg/ml of promastigote‐SLA (P-SLA), 16 μg/ml ACP or 8 μg/ml concanavalin A (Con A) and incubated for 3 days at 37°C in an atmosphere of 5% CO2. Then 1 μCi of 3H-thymidine (Amersham, Aylesbury, UK) was added to each well and incubated overnight. Cells were collected on filters with a harvester and 3H-thymidine incorporation was determined by liquid scintillation counting. All tests were performed in triplicate, and proliferative responses were expressed as their stimulation indices (SI), which represent the ratio of mean proliferation after stimulation to the mean proliferation of medium controls.
Detection of IFN-γ and IL-5
IFN-γ and IL-5 were measured in supernatants of cell proliferation assays of the four groups by the ELISA method [17]. In the case of IFN-γ, the reaction was performed by using protein G-purified rat anti-mouse IFN-γ MoAb R4-6A2 [18], biotin-labelled rat anti-mouse IFN-γ MoAb AN18.17.24 [19] and horseradish peroxidase-conjugated biotin–streptavidin (BRL, Gaithersburg, MD). For IL-5 the reagents were anti-mouse IL-5 rat MoAb TRFK4 as the coating antibody and TRFK5 (biotin-labelled) as the second antibody (all cell lines kindly provided by DNAX Research Institute, Palo Alto, CA) [20]. Plates were analysed on an ELISA reader at 490 nm. The specific cytokine concentrations were calculated on the basis of standard curves of recombinant cytokines. Concentrations from the supernatants higher than minimal values obtained from the respective standards were considered positive. Minimal values considered for IFN-γ were 30 pg/ml and for IL-5 were 80 pg/ml.
Results
Purification of 24-kD ACP from amastigote soluble antigens
In a previous study, fractionation of amastigote SLA by FPLC on an anion exchange Mono-Q column had revealed, in the first peak (eluted before starting the NaCl gradient), the presence of a 24-kD polypeptide, specific to the amastigote stage, with cysteine proteinase activity (ACP) [14]. Figure 1, lane 1, shows the first fraction of A-SLA analysed on SDS–PAGE with its major band at 24 kD. Rabbit antiserum (see MATERIALS and METHODS) raised against the homogenized 24-kD band was used for its purification. Immunoabsorption of the first fractions on ACP antibodies coupled to CNBr-activated Sepharose resulted in the isolation of 24-kD polypeptide (Fig. 1, lane 2). The Western blot analysis of the purified ACP is presented in Fig. 1, lane 3
Fig. 1.
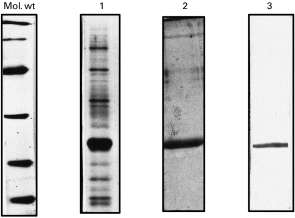
SDS–PAGE analyses of 24-kD amastigote cysteine proteinase (ACP) purification after fractionation of amastigote soluble antigen (A-SLA) extracted by fast protein liquid chromatography (FPLC) anion exchange chromatography. Lane 1, analysis of the first fraction; lane 2, immunoaffinity-purified ACP; lane 3, Western blot of purified ACP with specific rabbit antiserum. Mol. wt, Molecular weight standard proteins (BioRad).
Protection of BALB/c mice against L. major infection
The efficiency of BALB/c mice immunized with purified L. major ACP or whole A-SLA was evaluated by measuring footpad swelling weekly after challenge with 106 stationary phase promastigotes. As shown in Fig. 2, mice in group III, injected with FCA alone or those of group IV injected with PBS alone, developed a progressive infection in the infected foot. A similar pattern in the rate of disease progression was observed when mice were immunized with ACP alone (data not shown). However, immunization with either ACP (group I) or A-SLA (group II) plus adjuvant presented foot lesions significantly smaller than those of groups III and IV. There was a small insignificant rise in lesion size of animals in group I compared with group II. After the last footpad measurement (Fig. 2), mice were killed and their spleen size recorded. Animals immunized in groups I and II, immunized with ACP and A-SLA in combination with adjuvant, respectively, showed normal size spleens which were markedly smaller than those in groups III and IV (data not shown).
Fig. 2.
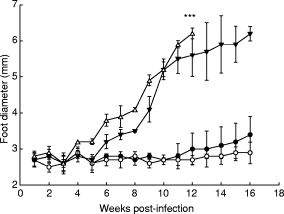
Evaluation of protection against infection with Leishmania major, as represented by footpad swelling in BALB/c mice immunized with purified amastigote cysteine proteinase (ACP) or amastigote soluble antigen (A-SLA). Four groups of 10 mice were injected intraperitoneally with 5 μg of pure ACP (group I; •); 5 μg of A-SLA (group II; ○); 100 μl Freund’s complete adjuvant (FCA) (group III; ▾); or 100 μl PBS (group IV; Δ). Animals were boosted 4 weeks later, and challenged 8 weeks after the first injection with 106 stationary phase promastigotes L. major into their left hind foot. ***Destruction of footpad and mice were killed.
Lymphoproliferative responses and cytokine production
Spleen cell proliferative responses and the production of IFN-γ to ACP and A-SLA, in each of the four groups of injected mice, were measured at 30 days after the first injection, 30 days after the booster injection (day 60) and 3 months (day 150) after infection with promastigotes.
Lymphocytes from ACP +Adj (group I)- and A-SLA +Adj (group II)-immunized mice exhibited significant responses in contact with corresponding antigen. We chose to show (Fig. 3a,b) the results obtained for mice in group I. The lymphoproliferative response of these animals to purified ACP was the highest at all three time points compared with their response to whole A-SLA. These cells responded much less, however, to stimulation with P-SLA at both time intervals before challenge, indicating that the response to ACP is mostly amastigote-specific.
Fig. 3.
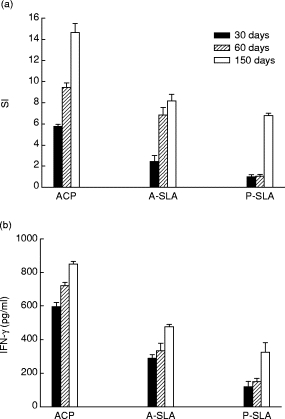
Proliferation index (a) as determined by 3H-thymidine uptake, and IFN-γ production (b) as measured by ELISA, for spleen cells of BALB/c mice immunized with purified amastigote cysteine proteinase (ACP) (group I). Cells were obtained from spleen removal at day 30, 60 or 150, and cultivated in the presence of indicated antigens. Each data point corresponds to the mean value obtained for triplicate tests. SI, Stimulation index. A‐SLA, Amastigote‐SLA; P‐SLA, promastigote‐SLA.
Lymphocytes from groups III and IV injected with FCA and PBS, respectively, failed to respond to either purified ACP or to A-SLA, and their SIs were near or below 1 (Tables 1 and 2). The non-responsiveness of animals in groups III and IV seemed to be parasite-specific, since these cells exhibited proliferative responses to Con A to the same extent as cells from mice in groups I and II (Table 2).
Table 1.
The level of IFN-γ production, determined for spleen cells of mice in group I (amastigote cysteine proteinase (ACP)), group II (amastigote soluble antigen (A-SLA)), and group III (Freund’s complete adjuvant (FCA)) at days 30 and 60, in the presence of the indicated antigens
| Group I(ACP) | Group II (A-SLA) | Group III (FCA) | ||||
|---|---|---|---|---|---|---|
| Antigen | Day 30 | Day 60 | Day 30 | Day 60 | Day 30 | Day 60 |
| ACP | 595 ± 23·50 | 720 ± 25·8 | 203 ± 25·8 | 310 ± 9·4 | UD | UD |
| A-SLA | 290 ± 20·50 | 334 ± 44·60 | 551 ± 13·4 | 780 ± 18·5 | UD | UD |
UD, Under detection limit.
Group IV had similar result to group III. Each data point corresponds to the mean value obtained for triplicate tests.
Table 2.
Stimulation index (SI) values representing the proliferative responses and level of IFN-γ production, determined for spleen cells of mice in group I, group II and group III at day 150, in the presence of the indicated antigens
| Group I (ACP) | Group II (A-SLA) | Control (FCA) | ||||
|---|---|---|---|---|---|---|
| Antigen | SI | IFN-γ (pg/ml) | SI | IFN-γ (pg/ml) | SI | IFN-γ (pg/ml) |
| ACP | 14·58 ± 0·91 | 850 ± 16·10 | 8·10 ± 0·78 | 400 ± 11·4 | 1·01 ± 0·6 | 63 ± 6 |
| A-SLA | 8·1 ± 0·65 | 475 ± 14·8 | 15·1 ± 3·4 | 900 ± 58 | 1·2 ± 0·2 | 58 ± 2 |
| Con-A | 24·2 ± 12·4 | >3000 | 21·6 ± 4·5 | >3200 | 29·5 ± 3·2 | >3000 |
Group IV had similar result to group III. Each data point corresponds to the mean value obtained for triplicate tests.
ACP, amastigote cysteine proteinase; A-SLA, amastigote soluble antigen.
To understand whether the protection from leishmaniasis, as demonstrated in Fig. 2, of mice immunized with ACP or A-SLA, correlates with a specific pattern of lymphokine production, culture supernatants from cell proliferation assays of the four groups were collected for measurement of IFN-γ and IL-5. Significant levels of IFN-γ were detected in the supernatant of spleen cells from groups I and II in response to ACP or A-SLA at various times after immunization or after infection (Tables 1 and 2). No measurable levels of IFN-γ were detected in the supernatant of cells from group III or IV (Tables 1 and 2). Figure 3b shows the results of IFN-γ production for animals immunized with ACP. High levels of IFN-γ were detected in response to both ACP and A-SLA, but very low levels to P-SLA. These results indicate a Th1 response to amastigote-associated antigens. No IL-5 production was detected in the supernatants of cell proliferation after stimulation with ACP, A-SLA or P-SLA, at any time in immunized mice at any stage during infection.
Discussion
The protective potential of Leishmania amastigote cysteine proteinase with apparent molecular weight of 24 kD was investigated. This polypeptide was detected in the first fraction of amastigote-soluble antigens. Its enzymatic activity and specific expression, limited to the amastigote stage of the parasite (ACP), have been demonstrated previously [14]. The components of this fraction were also revealed as strong IFN-γ inducers of PBMC of humans that had recovered from cutaneous leishmaniasis [13]. In this study, we proceeded to the purification of the 24-kD ACP, to the preparation of rabbit anti-ACP and to mouse immunization with ACP.
The results of BALB/c mice immunization with purified ACP or with whole A-SLA, in combination with Freund’s adjuvant, followed by injection of 106 live promastigotes into footpads, indicated a long-term protection from leishmaniasis as evaluated by reduced footpad swelling (Fig. 2). A high index of in vitro spleen cell proliferative response to antigen stimulation was accompanied by an elevated level of IFN-γ production. In light of the fact that ACP is specific to amastigotes, the results obtained from mice immunized with purified ACP presented in Fig. 3a,b lead to the following interpretations: (i) the high level of cell proliferation index and IFN-γ production, in response to purified ACP stimulation, can be attributed to specific response to this antigen. The absence of IL-5, and the high level of IFN-γ implicate the host Th1 subset which resulted in protection from leishmaniasis (Fig. 2). They also confirm the results obtained by Scott et al. [21,22] for mice immunization using promastigote SLA. These authors described correlation between Th1 activity and resistance to the development of leishmaniasis, on one hand, and between Th2 activity and susceptibility to development of leishmaniasis, on the other; (ii) the corresponding, but relatively smaller, responses observed after stimulation with whole A-SLA can be attributed to the presence of ACP antigen at a smaller concentration; (iii) the relatively small proliferation index and IFN-γ production after P-SLA stimulation observed at day 150 might be attributed to some common antigens between A-SLA and P-SLA, other than ACP.
It has been demonstrated that the complex mixture of soluble antigens derived from whole parasites can provide excellent protection only when it is administrated together with some adjuvant [23]. Similar to ACP, other studies with different antigens such as LACK, TSA or PT3 of gp63 have shown that the antigen by itself is not protective unless administrated with IL-12, FIA or poloxamer 407, respectively [24–26].
Some of the recent studies proposed that cysteine proteinases (CP) play a role in induction of Th2 and disease progression. These are shown by L. mexicana mutants lacking cysteine proteinase genes such as cpa, cpb or both cpa and cpb [27]. Although CP may be involved in modulating the host’s immune responses to favour parasite survival and proliferation, they can be immunogenic also. With respect to the protective capacity of cysteine proteinases, data obtained by Wolfram et al. [28] using a recombinant L. mexicana amastigote cysteine proteinase expressed in Escherichia coli showed that CP is a T cell immunogen, resulting in the development of a potentially protective Th1 cell line. A CP of L. pifanoi, however, provides little protection for the host against infection with parasite; although more recently a similar L. amazonesis CP provided some protection against subsequent challenge through inducing a Th1-associated response [29]. These different results presumably reflect the complexity of the immune response to Leishmania parasite. The nature and dose of the antigen, the route of immunization, the use of adjuvant and finally the strain of parasites used for challenge, are the most important factors to be considered for vaccine development.
In our hands, BALB/c mice immunized with purified 24-kD ACP in the presence of FCA developed resistance to leishmaniasis after challenge with 106 live promastigotes. This resistance was accompanied by production of high levels of IFN-γ, indicating the induction of a specific Th1 cell response. These results may provide some basis for the use of L. major 24-kD ACP in a native or in a recombinant form as a component of a cocktail vaccine against L. major infection.
Acknowledgments
The authors wish to thank Dr Irandokht Fischberg, Dr Farrokh Modabber and Dr Anis Jafari for their help and critical reading of the manuscript. This investigation received financial support from UNDP/World Bank/WHO Special Program for Research and Training in Tropical Disease (TDR), ID no. 970556.
REFERENCES
- 1.Bates PA. The developmental biology of Leishmania promastigotes. Exp Parasitol. 1994;79:215–8. doi: 10.1006/expr.1994.1084. [DOI] [PubMed] [Google Scholar]
- 2.Chang KP, Chaudhuri G. Molecular determinates of Leishmania virulance. Annu Rev Microbiol. 1990;44:499–529. doi: 10.1146/annurev.mi.44.100190.002435. [DOI] [PubMed] [Google Scholar]
- 3.Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 4.Muller I, Pedrazzini T, Farrell JP, Louis JA. T cell responses and immunity to experimental infection with Leishmania major. Annu Rev Immunol. 1989;7:561–78. doi: 10.1146/annurev.iy.07.040189.003021. [DOI] [PubMed] [Google Scholar]
- 5.Fruth U, Solioz N, Louis JA. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993;150:1857–64. [PubMed] [Google Scholar]
- 6.James SL, Nacy C. Effector functions of activated macrophages against parasites. Curr Opin Immunol. 1993;5:518–23. doi: 10.1016/0952-7915(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 7.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann TR, Coffman RL. TH1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Liew FY. Protection against Leishmania major infection in genetically susceptible BALB/c mice by gp63 delivered orally in attenuated Salmonella typhimurium. Immunology. 1995;85:1–7. [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon-Pratt D, Rodriguez D, Rodriguez JR, Zhang Y, Esteban M. Recombinant vaccinia viruses expressing GP46/M-2 protect against Leishmania infection. Infect Immun. 1993;61:3351–9. doi: 10.1128/iai.61.8.3351-3359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelleher M, Bacic A, Handman E. Identification of a macrophage-binding determinant on lipophosphoglycan from Leishmania major promastigotes. Proc Natl Acad Sci USA. 1992;89:6–10. doi: 10.1073/pnas.89.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafati S, Couty-Jouve S, Alimohammadian MH, Dowlati Y. Evaluation of cellular immune responses to amastigote soluble Leishmania major antigens in recovered cases of cutaneous leishmaniasis. Med J Islamic Rep Iran. 1997;11:33–38. [Google Scholar]
- 14.Rafati S, Couty-Jouve S, Alimohammadian MH, Louis JA. Biochemical analysis and immunogenicity of Leishmania major amastigote fractions in cutaneous leishmaniasis. Clin Exp Immunol. 1997;110:203–11. doi: 10.1111/j.1365-2249.1997.tb08318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser TA, Humphris DC, Mukkada AJ. Leishmania major and L. donovani: a method for rapid purification of amastigote. Exp Parasitol. 1990;71:343–5. doi: 10.1016/0014-4894(90)90039-f. [DOI] [PubMed] [Google Scholar]
- 16.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in murine model II. Immunological properties of protective and non protective subfractions of a soluble promastigotes extract. J Immunol. 1987;139:3118–25. [PubMed] [Google Scholar]
- 17.Slade SJ, Langhorne J. Production of interferon gamma during infection of mice with Plasmodium chabaudi. Immunobiol. 1989;179:353–65. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann TR, Schumacher JH, Fiorentino DF, Leverah J, Moore KW, Bone MW. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6 and a new Th2 specific cytokine, by solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–45. [PubMed] [Google Scholar]
- 19.Prat M, Gribaudo G, Comoglio PM, Cavallo G, Landolfo S. Monoclonal antibodies against murine γ interferon. Proc Nat Acad Sci USA. 1984;81:4515–9. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher JH, O’Garra A, Shrader B, Vankimmenade A, Bond MW, Mosmann TR, Coffman RL. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme linked immunsorbent. J Immunol. 1988;141:1576–81. [PubMed] [Google Scholar]
- 21.Scott P, Casper P, Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990;144:1075–9. [PubMed] [Google Scholar]
- 22.Scott P, Pearce P, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–7. [PubMed] [Google Scholar]
- 23.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–55. [PubMed] [Google Scholar]
- 24.Gurunathan S, Sacks D, Glaichenhaus N, Seder RS. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–47. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb JR, Campose-Neto A, Reed SG. Human and murine immune responses to novel Leishmania major recombinant protein encoded of a multicopy gene family. Infect Immun. 1998;66:3279–89. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spizer N, Jardim A, Lippert D, Olafson RW. Long-term protection of mice against Leishmania major with a synthetic peptide vaccine. Vaccine. 1999;17:1298–300. doi: 10.1016/s0264-410x(98)00363-6. [DOI] [PubMed] [Google Scholar]
- 27.Alexander J, Coombs GH, Mottram JC. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J Immunol. 1998;161:6794–801. [PubMed] [Google Scholar]
- 28.Wolfram M, Ilg T, Mottram JC, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T cells specific for amastigote cysteine proteinases requires intracellular killing of the parasites. Eur J Immunol. 1995;25:1094–100. doi: 10.1002/eji.1830250435. [DOI] [PubMed] [Google Scholar]
- 29.Soong L, Monroe Duboise S, Kima P, McMahan-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63:3559–66. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


