Abstract
The central role of CD4+ T cells and the balance between T helper (Th) subpopulations in the pathogenesis of autoimmune diseases have been extensively studied. Proteoglycan (aggrecan)-induced arthritis (PGIA) is a murine model for rheumatoid arthritis (RA), which is characterized by a Th1 dominance at the onset of the disease. In addition to CD4+ T cells, antigen-presenting B cells and autoantibodies seem to play an important role in the development and regulation of PGIA. To identify proteoglycan-specific CD4+ T cell subsets and Th1- and Th2-supported antibody isotypes during the progression of PGIA, spleen cells of proteoglycan-immunized BALB/c mice were harvested at different times of immunization, and at different stages of the disease, and their cytokine production and antigen-specific antibody isotype profiles were determined by enzyme-linked immunospot (ELISPOT) assays. Both Th1 and Th2 cytokine-producing cells, with the predominance of IL-4/IL-5-secreting cells, were detected during the prearthritic stage, and a shift toward a Th1 dominance was observed at the time of onset of arthritis. Tissue homogenates of acutely inflamed joints contained significantly higher levels of interferon-gamma than IL-4. The prearthritic period and both the acute and chronic phases of joint inflammation were characterized by IgG1 dominance in the sera and this correlated with the number of IgG1-secreting B cells in the spleen. However, the ratio of autoreactive IgG1/IgG2a-secreting cells decreased in arthritic animals. These results indicate the activation and possible regulatory roles of both Th1 and Th2 subsets in the autoimmune process, with the necessity of a relative increase of autoreactive Th1 cells for the induction of joint inflammation.
Keywords: proteoglycan, aggrecan, arthritis, Th1/Th2, autoimmune, isotype, BALB/c
INTRODUCTION
Proteoglycan (aggrecan)-induced arthritis (PGIA) is a murine model which shows many similarities to rheumatoid arthritis (RA), as indicated by clinical assessment, laboratory tests and histopathology of the peripheral joints [1,2]. Arthritis can be induced by systemic immunization with a select group of cartilage proteoglycans in BALB/c mice. The development of the disease is based upon cross-reactive immune responses between the immunizing human and self (mouse) cartilage proteoglycans [3,4].
Several lines of evidence indicate that T helper (Th) cells play an important role in PGIA: (i) the susceptibility to PGIA is restricted by MHC (H-2d haplotype of BALB/c mice) [3]; (ii) proteoglycan-specific CD4+ cells are primed during the development of arthritis [5]; (iii) arthritis can be prevented if CD4+ T cells are eliminated in vitro[5] or in vivo[6]; and (iv) a proteoglycan-specific Th1-type T cell hybridoma induces arthritis in BALB/c mice [7]. Although arthritis can not be transferred to naive recipients by autoreactive antibodies against mouse proteoglycan (i.e. from sera of arthritic mice), or with isolated B cells alone, B cells with T cells are required for the successful transfer of PGIA [4,5]. Taking all facts together, antigen-driven T and B cell cooperation appears to play a critical role in the pathogenesis of this autoimmune disease. B cells may serve as antigen-presenting cells (APC) for CD4+ T lymphocytes [4,8] and activated Th cells may determine the isotype of specific antibodies produced by B cells.
Classically, Th cells are divided into two major subsets based upon their cytokine secretion patterns. The Th1 subset produces IL-2, interferon-gamma (IFN-γ) and tumour necrosis factor-beta (TNF-β), and supports a switch toward IgG2a isotype; Th1 dominance is associated with the potential to develop autoimmune disease. In contrast, the Th2 subset that secretes IL-4, IL-5, IL-6, IL-10 and IL-13 inhibits cell-mediated immune responses and promotes IgG1 production [9–11]. While the balance between Th1 and Th2 subpopulations seems to be important in the pathological mechanisms of autoimmune diseases, evidence is accumulating against the belief that an absolute dominance of Th1 or Th2 subset is always critical for disease induction or protection, respectively. For example, while earlier reports suggested that experimental allergic encephalomyelitis (EAE) was mediated by Th1 cells, it was shown recently that Th2 cells could also mediate the disease [10]. Collagen-induced arthritis (CIA) is associated with Th1 dominance, but the highest numbers of IL-4-producing cells were detected just before the onset of arthritis [12].
PGIA is characterized by a progressive disease course with intermittent exacerbations and remissions reminiscent of the clinical appearance of RA. Since this progressive disease is ‘decorated’ with acute inflammatory episodes, it offers a unique tool to follow fluctuations in the activation of the Th subsets over a long period of time. The BALB/c strain has a genetic predisposition to a predominant Th2 response [9], therefore PGIA is of special interest with respect to the involvement of distinct Th subsets. In this study we monitored the production of Th1 and Th2 cytokines, and Th1/Th2-supported antibody isotypes, to gain insight into the regulatory roles of the two Th cell subsets in PGIA.
MATERIALS and METHODS
Antigens and immunization
High density cartilage proteoglycans (aggrecan) were purified by CsCl gradient centrifugation from fetal human, newborn mouse and adult bovine articular cartilages, and from rat chondrosarcoma as described elsewhere. Purified proteoglycans were digested with chondroitinase ABC (Sigma Chemical Co., St Louis, MO) in the presence of proteinase inhibitors [13] and dialysed against PBS pH 7·4. Ovalbumin (OVA) was purchased from Sigma.
Female BALB/c mice (Charles River Breeding Laboratories, Wilmington, MA) were either purchased directly from Kingston, NY (K51 colony) or maintained as an inbred colony in our local animal care facility (Department of Anatomy, University Medical School of Debrecen, Hungary). Mice were tested prior to immunization (non-immunized group) or during and after immunization with various proteoglycans by a standard protocol [1,3,4]. Mice were immunized intraperitoneally with either (i) human fetal articular cartilage proteoglycan (HFPG), (ii) bovine articular proteoglycan (BPG), (iii) rat chondrosarcoma proteoglycan (CSPG), or (iv) an irrelevant protein (OVA) on days 0, 7, 28 and 49. First and fourth antigen injections (100 μg protein) were given in Freund’s complete adjuvant (FCA), and the same dose of antigen was injected as second and third boosts in Freund’s incomplete adjuvant.
Clinical assessment of arthritis and experimental groups
Animals were examined twice a week for signs of arthritis. A scoring system, based upon swelling and redness of the paws, was used for the assessment of the severity of arthritis in the acute phase [7,14]. In PGIA, mice develop swelling and redness in one or more limbs, 7–14 days after the fourth injection of HFPG [1,2,14]. During the period between the last antigen injection and arthritis onset, the animals were considered to be in the ‘prearthritic’ stage, and after the onset of inflammation they were sorted in ‘acutely arthritic’ groups. Two to six weeks after the initial symptoms, mice exhibited joint deformities, stiffness or ankylosis, in the presence or absence of redness and swelling. These animals were considered to be in the chronic phase. Approximately 10–20% of immunized animals remained clinically silent at weeks 8–10 of immunization. If no more antigen was given, these asymptomatic animals developed ankylosis 3–4 months later in their peripheral joints despite the absence of initial symptoms of inflammation. Such animals were referred to as ‘non-arthritic’ immunized controls during the asymptomatic period. Serum samples were collected from the retrobulbar venous plexus twice a week during the first 5 weeks, and then weekly.
ELISA for the detection of the isotypes of antigen-specific antibodies
Maxisorp immunoplates (Nunc Intermed Ltd, Copenhagen, Denmark) were coated with proteoglycans or OVA (1 μg protein/100 μl per well). Free binding capacity of the wells was blocked by 1% gelatin (Reanal, Hungary) in PBS. Sera were applied at increasing dilutions and the isotypes of proteoglycan-specific antibodies were determined and titrated using alkaline phosphatase-conjugated rat anti-mouse IgG1 or IgG2a secondary antibodies (Southern Biotechnology Inc., Birmingham, AL). Colour reaction was developed by p-nitrophenyl phosphate (Sigma) and absorbance measured at 405 nm using a Multiscan ELISA reader (Dynatech Labs, Inc., Alexandria, VA).
ELISPOT assays
Antigen-specific immunoglobulin-synthesizing ‘spot-forming’ plasma cells (SFC) were detected by ELISPOT assay [15]. Nitrocellulose-bottomed 96-well plates (Mahan, Millipore, Vienna, Austria) were coated with chondroitinase ABC-digested HFPG or native mouse proteoglycan (1 μg protein/well) overnight at 4°C. Spleen cells separated on Lympholyte-M gradient (Accurate Chemical and Scientific Co., Westbury, MA) [4] were seeded (5 × 105 cells/well) in triplicates and incubated in a CO2 incubator overnight. The plates were washed and then alkaline phosphatase-conjugated rat anti-mouse IgG1 and IgG2a antibodies and phosphatase substrate BCIP/NBT (Sigma) were used to detect spots counted visually in a Leica stereomicroscope.
Cytokine secretion by single cells was determined by two different ELISPOT assay systems. Lympholyte-separated spleen cells (5 × 105 splenocytes/well) were seeded in triplicate onto nitrocellulose-bottomed plates (Mahan) in serum-free RPMI 1640. After overnight incubation and extensive washing, plates were blocked with 1% bovine serum albumin (BSA; Sigma) in PBS. Goat anti-mouse IL-2 or IL-5 antibodies (both from Sigma) at 1:500 dilutions were detected by peroxidase-labelled rabbit anti-goat immunoglobulin (Sigma). In the second method, Immobilon-P membrane-bottomed 96-well plates (Millipore, Bedford, MA) were coated (5 μg/ml) with rat anti-mouse IL-4 or IFN-γ MoAbs (Biosource Int., Camarillo, CA) and lympholyte-purified spleen cells, previously cultured in the presence or absence of native mouse proteoglycan for 5 days, were seeded in RPMI 1640 with 5% fetal bovine serum (FBS). Wells were washed after overnight incubation and captured cytokines detected with biotinylated anti-mouse IL-4 or IFN-γ (PharMingen, San Diego, CA). Amino-ethyl-carbazole (Sigma) was used as a chromogen and the spots were counted in a stereomicroscope.
Detection of cytokines in tissue homogenates
Non-inflamed and acutely inflamed hind paws from the same mice with acute PGIA (n = 15), and paws from non-immunized BALB/c mice (n = 10) were cut above the plane of the tibio-tarsal joints. After the removal of the skin, the paws were weighed and immediately homogenized on ice in 1 ml of PBS containing enzyme inhibitors [13]. Protein content was determined by Pierce’s bicinchoninic acid assay (Pierce, Rockford, IL) according to the manufacturer’s instructions, and the homogenates were stored at −80°C. IFN-γ and IL-4 contents of tissue homogenates were measured using Quantikine M mouse IFN-γ and IL-4 ELISAs (R&D Systems, Minneapolis, MN).
Immunohistochemistry
Femoral heads of non-immune (n = 4), acutely arthritic (n = 4) and immunized asymptomatic (n = 4) BALB/c mice were frozen and cryosectioned. Sections were air-dried, fixed at −20°C in acetone. Endogenous peroxidase activity was quenched by 1% hydrogen peroxide and non-specific binding sites were blocked with 10% FBS for 20 min as described. After three consecutive washes Tris-buffered saline (TBS, pH 7·4) rat anti-mouse IgG1 or IgG2a antibodies (Southern Biotechnology) were added in 1:50 dilutions for 1 h. Peroxidase-conjugated rabbit anti-rat IgG (Dako A/S, Glostrup, Denmark) was applied in 1:1000 dilution for 1 h as a secondary antibody. The colour reaction was developed using diaminobenzidine (Sigma) and hydrogen peroxide. Normal rat IgG (Sigma) with second antibody, or second antibody without primary antibody served as negative controls.
Statistical analysis
For the ELISA and ELISPOT assays, the means and s.e.m. were calculated for each group. For comparison of animal groups Mann–Whitney rank sum test and Pearson’s product moment correlation were used.
RESULTS
Antigen-specific serum IgG isotype profile in proteoglycan-immunized mice
The kinetics of proteoglycan-specific IgG1 and IgG2a antibody production in sera of proteoglycan-immunized mice were monitored over a period of 15 weeks. Due to the high amino acid sequence homology among core proteins from different species, and essentially identical carbohydrate side chains of cartilage proteoglycans, it was necessary to determine antibody responses to both the immunizing antigens (total antibody) and to mouse self proteoglycan (autoantibody). Antibody responses to immunizing antigen, either IgG1 or IgG2a, were very similar in HFPG- and BPG-immunized animals (Fig. 1a,c). IgG1 levels reached a plateau within the first few weeks and this IgG1 subclass remained the dominant isotype during the entire experimental period (Fig. 1a,c; 1:500 serum dilutions are shown). The serum levels of anti-proteoglycan IgG2a antibodies were significantly lower in both groups and reached a plateau by weeks 6–8 of immunization (Fig. 1a,c). Arthritic symptoms appeared at weeks 8–9 of immunization in the HFPG-immunized group.
Fig. 1.
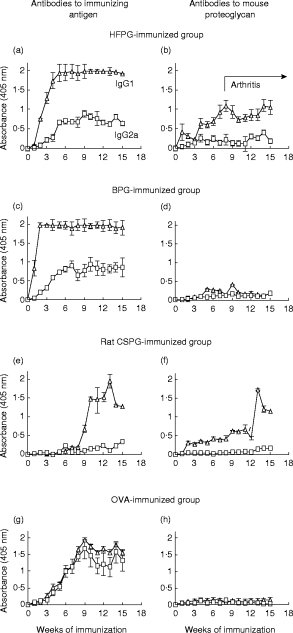
Serum IgG1 (Δ) and IgG2a (□) levels in BALB/c mice immunized with arthritogenic human fetal articular cartilage proteoglycan (HFPG) (a,b) (n = 20), non-arthritogenic bovine articular proteoglycan (BPG) (c,d) (n = 10) or chondrosarcoma proteoglycan (CSPG) (e,f) (n = 8) and with ovalbumin (OVA) (g,h) (n = 10). Left side panels show antibody responses to immunizing antigens and right side panels the antibody levels to mouse (self) proteoglycan. The first case of arthritis was recorded on the seventh week in the HFPG-immunized group and the onset was 10 ± 4·2 weeks (mean ±s.d.). Although serum samples were collected twice a week (during the first 5 weeks), only weekly intervals are shown. Antibody titres were measured at various serum dilutions, but for comparison, only 1:500 (for immunizing antigen) and 1:100 dilutions (antibodies to mouse proteoglycan) are shown. Error bars indicate s.e.m.
IgG1 was also the predominant IgG isotype to mouse proteoglycan, accompanied by a very low level of mouse proteoglycan-specific IgG2a, even in HFPG-immunized arthritic BALB/c mice (Fig. 1b). These auto(reactive) IgG1 anti-mouse proteoglycan antibodies reacted with core protein epitopes of mouse proteoglycan [16]. Less than 10% of the antibodies reacted with carbohydrate epitopes common in both HFPG and mouse proteoglycan (data not shown). Essentially no, or very low levels of auto(reactive) antibodies to mouse proteoglycan were detected in BPG-immunized non-arthritic animals (Fig. 1d).
Anti-proteoglycan antibodies could be detected only after the fourth antigen injection of rat CSPG-immunized mice, usually from weeks 9–12 of immunization (Fig. 1e,f). IgG1 subclass was the only proteoglycan-specific isotype in rat CSPG-immunized mice. Interestingly, these IgG1 antibodies reacted with both the immunizing rat (Fig. 1e) and native mouse proteoglycan (Fig. 1f). The antibody profile was completely different in OVA-immunized mice. Antibody production was delayed, but equally strong IgG1 and IgG2a responses to OVA were detected (Fig. 1g). As expected, OVA-immunized mice had no antibodies to cartilage proteoglycans (Fig. 1h), and sera from proteoglycan-immunized mice did not react with OVA (data not shown).
Auto- and heteroantigen-specific antibody secreting SFC in the spleens of immunized mice
To assess the synthesis of Th1- and Th2-supporting antibodies in antigen-specific systems, we determined the number of hetero- and autoantigen-specific IgG1- and IgG2a-producing SFC in spleen using an ELISPOT assay (Fig. 2a,b). The spot numbers were compared in a control (non-immunized) and five HFPG-immunized groups: (i) mice primed with a single dose (100 μg) of HFPG in FCA (8 days after priming); (ii) immunized yet non-arthritic mice at week 9 (‘prearthritic’ stage); (iii) mice with acute arthritis at week 9 of immunization; (iv) mice with chronic arthritis at weeks 18–20 of immunization; and (v) identically immunized non-arthritic, possibly ‘arthritis-resistant’ BALB/c mice (also at weeks 18–20).
Fig. 2.
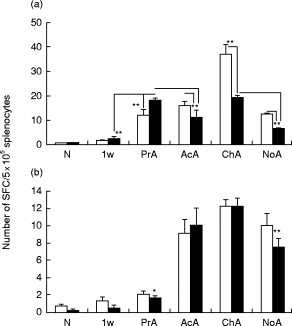
Antigen-specific spot-forming plasma cells (SFC) in spleen. The nitrocellulose bottoms of wells were coated by chondroitinase ABC-digested human fetal articular cartilage proteoglycan (HFPG) (a) or native mouse proteoglycan (b). □, IgG1-secreting cells; ▪, IgG2a-secreting cells. Note different scales of the two panels. N, Non-immunized BALB/c mice (n = 5); 1w, mice tested 1 week after HFPG priming in Freund’s complete adjuvant (FCA) (n = 5); PrA, HFPG-immunized, yet non-arthritic (‘prearthritic’) mice (n = 5); AcA, acutely arthritic mice (n = 3); ChA, chronic arthritic mice (n = 3); NoA, asymptotic/proteoglycan (aggrecan)-induced arthritis (PGIA)-resistant BALB/c mice. Animals (n = 3) of this latter group were immunized identically with arthritic mice but they failed to develop arthritis even after five HFPG injections. Error bars indicate s.e.m. Level of significance: *P < 0·01; **P < 0·001.
The number of mouse proteoglycan-specific SFC was significantly lower (Fig. 2b) than HFPG-specific SFC (Fig. 2a) (note different scales). The arthritic state (acute or chronic) was characterized by increased numbers of both hetero- and autoreactive IgG1 and IgG2a antibody-secreting splenocytes (Fig. 2), and the SFC number was usually higher in chronic than acute arthritis, irrespective of IgG subclass or antigen used for the coating of membranes. Moreover, a diversification between anti-human and anti-mouse proteoglycan response was detected. In acute, and even more in chronic arthritis there was a striking increase in the number of HFPG-specific IgG1-secreting cells (Fig. 2a) not seen in the case of autoantibody-secreting cells (Fig. 2b). Since autoantigen-specific cells that cross-react with both heterologous and autologous proteoglycans are believed to be pathogenically involved in joint inflammation in PGIA, the mouse proteoglycan-specific response is of special interest. While immunization with arthritogenic HFPG clearly resulted in significant increases of both IgG1 and IgG2a antibody-producing cells, the number of autoreactive IgG2a- and IgG1-secreting SFC (Fig. 2b) suggests that arthritic mice (in either acute or chronic phase, but not in identically immunized asymptomatic controls) exhibit increasing preference toward the production of Th1-supported IgG2a isotype (Fig. 2b).
Cartilage-bound antibodies
Tissue/cartilage-bound autoantibodies can be detected during the entire course of PGIA [3]. To determine the isotype of the cartilage-bound antibodies, in this study sections of femoral heads from mice with or without acute PGIA were immunoperoxidase-stained for mouse IgG1 (Fig. 3A) and IgG2a (Fig. 3D). There was an intensive accumulation of both IgG1 and IgG2a in the superficial layer of articular cartilage from mice with acute PGIA. Immunostaining revealed slight immunostaining for mouse IgG1 in cartilage, but no tissue-bound IgG2a antibody was detected in immunized, as yet asymptomatic mice (Fig. 3B,E). Immunostaining of cartilage from non-immunized mice was negative for either isotypes (Fig. 3C,F).
Fig. 3.
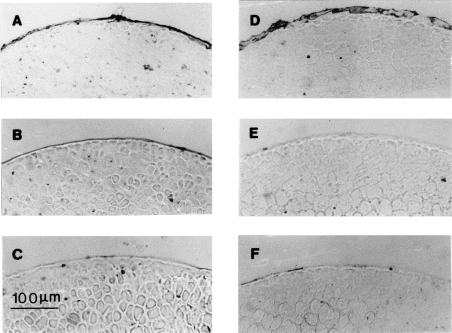
Immunostaining of femoral heads for the detection of surface-bound IgG1 (A,B,C) and IgG2a (D,E,F) in arthritic (A,D), immunized but asymptotic (B,E) and normal (C,F) mice (original mag. ×100).
Cytokine-secreting SFC from spleen
To characterize further the distribution of Th subsets in spleens of mice with PGIA, we determined the number of splenic IL-2- and IL-5-secreting SFC (Fig. 4). Unlike the IgG1/IgG2a ELISPOT assay, this cytokine ELISPOT was not based on antigen recognition, thus the results reflected the proportion of IL-2 (Th1) and IL-5 (Th2)-producing spleen cells in the absence of in vitro stimulation. In these experiments, groups of non-immune, acutely arthritic, and identically immunized asymptomatic (prearthritic) mice were compared. The number of IL-5-secreting SFC in all groups exceeded the number of IL-2-producing splenocytes. This finding suggested a Th2 predominance in both non-immune and immune mice. While the numbers of IL-5-producing cells were similar in both HFPG-immunized (arthritic and non-arthritic) groups, and the IL-5 (Th2-type) and IL-2 (Th1-type) cytokine ratio was comparable in all animals (either immunized or non-immunized), the numbers of IL-2-producing SFC were significantly higher (P < 0·05) in mice with acute arthritis (Fig. 4). A preference to develop a Th1 response at the onset of arthritis was evident when the number of SFC from non-immune, prearthritic and arthritic animals was tested for IFN-γ and IL-4 production in the presence of antigen (Fig. 5).
Fig. 4.
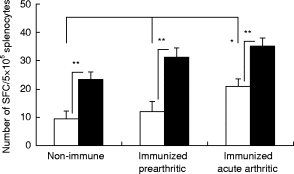
Cytokine-secreting spot-forming plasma cells (SFC) in spleen. □, Number of IL-2-secreting cells; ▪, number of IL-5-secreting cells on week 9 of immunization (n = 5). Note, while the number of IL-5-producing cells was significantly higher in each group (**P < 0·01), the IL-2-producing cell number increased (*P < 0·05) after the onset of arthritis. Error bars indicate s.e.m.
Fig. 5.

Cytokine-secreting spot-forming plasma cells (SFC) after in vitro proteoglycan stimulation. □, Number of IFN-γ-secreting cells; ▪, number of IL-4-secreting cells after 5-day stimulation in the presence of mouse proteoglycan. As in Fig. 4, animals were killed on week 9 of immunization (n = 5). The number of IFN-γ-producing cells was significantly higher (**P < 0·01) in acutely arthritic animals. Error bars indicate s.e.m.
Cytokine composition of inflamed tissues from mice with PGIA
Most of the animals developed arthritis first in joints of one or two paws, and subsequent joints and limbs became involved later during the course of the disease. While IFN-γ, IL-2, IL-4 or IL-5 could not be detected in tissue/joint extracts of normal, non-immunized or OVA/FCA-injected BALB/c mice (data not shown), these cytokines reached detectable levels in both arthritic and clinically non-afflicted joints. In these experiments we compared cytokine amounts of inflamed versus non-inflamed paws from the same arthritic animal. Both IFN-γ and IL-4 could be detected in both inflamed and non-inflamed paws of the same mice (Fig. 6). The cytokine content of the paws varied widely, and on average higher amounts were found in the absence than in the presence of inflammation. Inflamed paws however were characterized by a strong predominance of IFN-γ over IL-4 (Fig. 6).
Fig. 6.
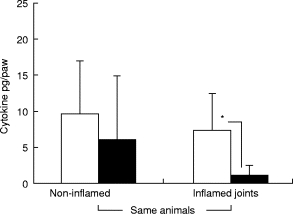
IFN-γ (□) and IL-4 (▪) contents measured in tissue extracts of non-inflamed and inflamed paws of the same animals (n = 15). The extract of one paw was centrifuged, supernatant adjusted to 1 ml, and cytokine levels measured in serial dilutions of paw extracts. Level of significance: *P < 0·05. Error bars indicate s.e.m.
DISCUSSION
A number of human autoimmune diseases [17,18] and corresponding animal models [19] are characterized by the dominance of Th1 subset of T helper cells, although Th2 involvement, even in the same disease, has also been reported [20–24]. For example, RA was earlier considered to be associated with Th1-type responses [24–26], but recent data suggest a significant Th2 contribution [27–29]. Similarly, both Th subsets were found to be participating in the flare-up of arthritis in murine antigen- [30] and collagen-induced arthritis [12, 20, 21, 31]. Thus, the pathological roles of Th1 versus Th2 subsets in the regulation of autoimmune disease are unclear [32]. The kinetics of Th1 response, a balance between Th1 and Th2 subsets or their products, an antibody switch supported by Th1 cells, or these factors together, may play far more important roles in the onset of an autoimmune disease than the actual dominance of antigen-specific Th1 cells [33].
As a first approach, we assessed the activation of the Th subset by determining Th2-supported IgG1, and Th1-supported IgG2a proteoglycan-specific antibody levels in serum. We found IgG1 dominance in all proteoglycan-immunized groups, suggesting a bias towards a Th2-type response upon immunization in BALB/c mice. Changes were anticipated in proteoglycan-specific IgG2a/IgG1 antibody isotype levels around week 10 of immunization, in correlation with clinical changes (onset of arthritis). In contrast to pristane- [34] or collagen-induced arthritis [21,35], the hetero- and autoantibody subclass levels stayed constant around this time, thus there was no indication of disease-related activation of Th subsets, based on circulating antibody subclass levels. Nonetheless, we found a clear difference between responses elicited by arthritogenic HFPG and non-arthritogenic proteoglycans: the presence of IgG2a autoantibodies (suggesting self proteoglycan-specific Th1 activation) was detected only in the HFPG-immunized group.
Our next question was whether circulating antibody subclass levels reflected immunoglobulin synthesis realistically, or some autoreactive immunoglobulins from the serum were removed due to their binding to the target tissues. Indeed, we demonstrated the presence of autoantibodies (both IgG1 and IgG2a) bound to cartilage in arthritic animals (Fig. 3). Therefore, the IgG1/IgG2a composition and levels in serum samples may not necessarily reflect antigen-specific Th1/Th2 activation. Although a preferential increase in the number of proteoglycan-specific IgG2a autoantibody-synthesizing cells was observed in arthritic mice, arthritis was also associated with an increased number of IgG1 heteroantibody-secreting cells. While neither tissue-bound nor circulating autoantibodies to type II collagen were measured, the kinetics of IgG1- and IgG2a-type heteroantibody profiles in CIA [21] were comparable to our observations.
To determine if there was a change in Th1/Th2 ratios during the development of arthritis, the numbers of Th1- and Th2-type cytokine-secreting spleen cells were compared. In correlation with the antibody isotype profile, a massive Th2 dominance was characteristic of BALB/c mice, in either the non-immunized or immunized state. Cytokine profile (IL-2 versus IL-5) of in vivo-primed T cells from HFPG-immunized mice (measured by direct ELISPOT) showed that the only difference between prearthritic and acutely arthritic animals was a moderate increase of IL-2-secreting cells in the acute phase of the disease. Preference toward a Th1-type response seemed to be more evident when mononuclear spleen cells were stimulated with autologous proteoglycan for 5 days and the numbers of IFN-γ− and IL-4-producing cells were compared. When we determined Th1 and Th2 cytokine levels (IFN-γ and IL-4) in inflamed paws, and compared these with those found in non-inflamed paws of the same animals, we found reduced levels of both cytokines in joints afflicted with inflammation. However, inflamed joint tissues were characterized by a strong disequilibrium of Th1 and Th2 cytokines, where IFN-γ vastly dominated over IL-4.
Taken together, results presented in this study suggest that both Th1 and Th2 cells, as in RA, may contribute to the pathological changes in PGIA. Interestingly, Th2 dominance is characteristic of peripheral blood mononuclear cells in patients with RA [28], whereas synovial mononuclear cells seem to be mostly Th1 type [36,37]. In our model system, Th2- and Th1-type responses however differed with respect to the antigen specificity, which is unknown in human RA. Arthritis-associated Th2 activation appears to be predominantly directed to the immunizing heterologous proteoglycan, and presumably serves to help HFPG-specific B cells. In addition to specific antibody production, these B cells, in turn, may present self-antigen (proteoglycan) to an autoreactive Th1 population that will gain effector function in arthritis induction. Our results are in line with the concept that autoreactive Th1 cells may play a key role in arthritis induction, in that a preferential increase in the number of autoreactive Th1 cells appears to be associated with the arthritic state. This study however also demonstrates the complexity and dynamic nature of the activation pattern of Th1 and Th2 subsets during the course of autoimmune arthritis.
Acknowledgments
This work was supported in part by NIH grants R01 AR40310 and P01 AR45652, and by a Hungarian National Research Foundation (OTKA) grant TO20253.
REFERENCES
- 1.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–12. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 2.Glant TT, Cs-Szabó G, Nagase H, Jacobs JJ, Mikecz K. Progressive polyarthritis induced in BALB/c mice by aggrecan from human osteoarthritic cartilage. Arthritis Rheum. 1998;41:1007–18. doi: 10.1002/1529-0131(199806)41:6<1007::AID-ART7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987;30:306–18. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- 4.Glant TT, Buzás EI, Finnegan A, Negroiu G, Cs-Szabó G, Mikecz K. Critical role of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 1998;160:3812–9. [PubMed] [Google Scholar]
- 5.Mikecz K, Glant TT, Buzás E, Poole AR. Proteoglycan-induced polyarthritis and spondylitis adoptively transferred to naive (nonimmunized) BALB/c mice. Arthritis Rheum. 1990;33:866–76. doi: 10.1002/art.1780330614. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee S, Webber C, Poole AR. The induction of arthritis in mice by the cartilage proteoglycan aggrecan: roles of CD4+ and CD8+ T cells. Cell Immunol. 1992;144:347–57. doi: 10.1016/0008-8749(92)90250-s. [DOI] [PubMed] [Google Scholar]
- 7.Buzás EI, Brennan FR, Mikecz K, et al. A proteoglycan (aggrecan)-specific T cell hybridoma induces arthritis in BALB/c mice. J Immunol. 1995;155:2679–87. [PubMed] [Google Scholar]
- 8.Brennan FR, Mikecz K, Buzás EI, et al. Antigen-specific B cells present cartilage proteoglycan (aggrecan) to an autoreactive T cell hybridoma derived from a mouse with proteoglycan-induced arthritis. Clin Exp Immunol. 1995;101:414–21. doi: 10.1111/j.1365-2249.1995.tb03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosmann TR, Cherwinski H, Bond MA, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 10.Kelso A. Th1 and Th2 subsets: paradigms list? Immunol Today. 1995;16:374–9. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 11.Kiely PD. The Th1-Th2 model—what relevance to inflammatory arthritis? Ann Rheum Dis. 1998;57:328–30. doi: 10.1136/ard.57.6.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doncarli A, Stasiuk LM, Fournier C, Abehsira-Amar O. Conversion in vivo from an early dominant Th0/Th1 response to a Th2 phenotype during the development of collagen-induced arthritis. Eur J Immunol. 1997;27:1451–8. doi: 10.1002/eji.1830270623. [DOI] [PubMed] [Google Scholar]
- 13.Glant TT, Mikecz K, Poole AR. Monoclonal antibodies to different protein-related epitopes of human articular cartilage proteoglycans. Biochem J. 1986;234:31–41. doi: 10.1042/bj2340031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikecz K, Brennan FR, Kim JH, Glant TT. Anti-CD44 treatment abrogates tissue edema and leukocyte infiltration in murine arthritis. Nature Med. 1995;1:558–63. doi: 10.1038/nm0695-558. [DOI] [PubMed] [Google Scholar]
- 15.Czerkinsky C, Nilsson LA, Nygren H, Ouchterlony O, Tarkowsky A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 16.Negroiu G, Roughley PJ, Cs-Szabó G, Brennan FR, Glant TT. Identification of autoimmune/arthritogenic epitopes of cartilage proteoglycans (PGs) using autoreactive antibodies to mouse aggrecan. Arthritis Rheum. 1995;38:S295. (Abstr.) [Google Scholar]
- 17.Adorini L, Guery JC, Trembleau S. Manipulation of the Th1/Th2 cell balance: an approach to treat human autoimmune diseases? Autoimmunity. 1996;23:53–68. doi: 10.3109/08916939608995329. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8:837–42. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 19.Yu M, Johnson JM, Tuohy VK. Generation of autonomously pathogenic neo-autoreactive Th1 cells during the development of the determinant spreading cascade in murine autoimmune encephalomyelitis. J Neurosci Res. 1996;45:463–70. doi: 10.1002/(SICI)1097-4547(19960815)45:4<463::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Mauri C, Williams RO, Walmsley M, Feldman M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–8. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 21.De Franco M, Gille-Perramant MF, Mevel JC, Couderc J. T helper subset involvement in two high antibody responder lines of mice (Biozzi mice): HI (susceptible) and HII (resistant) to collagen-induced arthritis. Eur J Immunol. 1995;25:132–6. doi: 10.1002/eji.1830250123. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima A, Hirose S, Yagita H, Okumura K. Roles of IL-4 and IL-12 in the development of lupus in NZB/W F1 mice. J Immunol. 1997;158:1466–72. [PubMed] [Google Scholar]
- 23.Ausubel LJ, Krieger JI, Hafler DA. Changes in cytokine secretion induced by altered peptide ligands of myelin basic protein peptide 85–99. J Immunol. 1997;159:2502–12. [PubMed] [Google Scholar]
- 24.Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci USA. 1994;91:8562–6. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Roon JAG, van Roy JL, Duits A, Lafeber FP, Bijlsma JW. Proinflammatory cytokine production and cartilage damage due to rheumatoid synovial T helper-1 activation is inhibited by interleukin-4. Ann Rheum Dis. 1995;54:836–40. doi: 10.1136/ard.54.10.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolhain RJ, van der Heiden AN, tel Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 27.van Roon JAG, Verhoef CM, van Roy JL, et al. Decrease in peripheral type 1 over type 2 T cell cytokine production in patients with rheumatoid arthritis correlates with an increase in severity of disease. Ann Rheum Dis. 1997;56:656–60. doi: 10.1136/ard.56.11.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haddad A, Bienvenu J, Miossec P. Increased production of a Th2 cytokine profile by activated whole blood cells from rheumatoid arthritis patients. J Clin Immunol. 1998;18:399–403. doi: 10.1023/a:1023278606036. [DOI] [PubMed] [Google Scholar]
- 29.Isomäki P, Luukkainen R, Lassila O, Toivanen P, Punnonen J. Synovial fluid T cells from patients with rheumatoid arthritis are refractory to the T helper type 2 differentiation-inducing effects of interleukin-4. Immunology. 1999;96:358–64. doi: 10.1046/j.1365-2567.1999.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs MJM, Van Den Hoek AEM, van Lent PLEM, van de Loo FAJ, van de Putte LBA, van den Berg WB. Role of IL-2 and IL-4 in exacerbations of murine antigen-induced arthritis. Immunology. 1994;83:390–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Müssener Å, Litton MJ, Lindroos E, Klareskog L. Cytokine production in synovial tissue of mice with collagen-induced arthritis (CIA) Clin Exp Immunol. 1997;107:485–93. doi: 10.1046/j.1365-2249.1997.3181214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson CI, McDevitt HO. Redirecting Th1 and Th2 responses in autoimmune disease. Curr Opin Microbiol Immunol. 1999;238:79–122. doi: 10.1007/978-3-662-09709-0_5. [DOI] [PubMed] [Google Scholar]
- 33.Van der Graaff WL, Prins APA, Dijkmans BAC, van Lier RAW. Prognostic value of Th1/Th2 ratio in rheumatoid arthritis. Lancet. 1998;351:1931. doi: 10.1016/s0140-6736(05)78615-3. [DOI] [PubMed] [Google Scholar]
- 34.Beech JT, Siew LK, Ghoraishian M, Stasiuk LM, Elson CJ, Thompson SJ. CD4+ Th2 cells specific for mycobacterial 65-kilodalton heat shock protein protect against pristane-induced arthritis. J Immunol. 1997;159:3692–7. [PubMed] [Google Scholar]
- 35.Kageyama Y, Koide Y, Yoshida A, et al. Reduced susceptibility to collagen-induced arthritis in mice deficient in IFN-gamma receptor. J Immunol. 1998;161:1542–8. [PubMed] [Google Scholar]
- 36.Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1998;25:1466–71. [PubMed] [Google Scholar]
- 37.Van der Graaff WL, Prins APA, Niers TMH, Dijkmans BAC, van Lier RAW. Quantitation of interferon gamma- and interleukin-4-producing T cells in synovial fluid and peripheral blood of arthritis patients. Rheumatology. 1999;38:214–20. doi: 10.1093/rheumatology/38.3.214. [DOI] [PubMed] [Google Scholar]


