Abstract
In a previous study, it was reported that stimulation with a TXA2 receptor agonist, U46619, augments the expression of adhesion molecules by human umbilical vein endothelial cells (HUVEC). In the present study we showed that U46619 augments the expression of MCP-1 in HUVEC, both at the protein and mRNA levels. Pretreatment with TXA2 receptor antagonists greatly diminishes the extent of tumour necrosis factor-alpha (TNF-α)-, platelet-activating factor (PAF)-, or U46619-induced mRNA accumulation and production of MCP-1. Protein kinase C (PKC) inhibitors diminish U46619-induced mRNA accumulation and production of MCP-1. NAC, which inhibits nuclear factor κ B (NF-κ B) activation and activating protein 1 (AP-1) binding activity, inhibits the expression of MCP-1 at the protein and mRNA levels. These results indicate that in HUVEC stimulation via the TXA2 receptors augments MCP-1 production by induction of the NF-κ B and AP-1 binding activity through the PKC system.
Keywords: vascular endothelial cells, thromboxane A2 receptor, MCP-1, PKC, NF-κ B
INTRODUCTION
TXA2, which possesses a series of biological activities such as contraction of vascular smooth muscle, platelet degranulation and aggregation, is an eicosanoid associated with stimulatory effects on lymphocyte proliferation [1] or polymorphonuclear cell adhesiveness [2]. Autoimmune vasculitis or atherosclerosis is a vascular inflammatory response characterized by leucocytic infiltration due to interactions with endothelial chemokines and adhesion molecules [3]. We have previously shown that TXA2, the production of which is enhanced in human umbilical vein endothelial cells (HUVEC) by tumour necrosis factor-alpha (TNF-α) or platelet-activating factor (PAF) stimulation, binds with TXA2 receptors located on the cell surface and augments the expression of endothelial adhesion molecules [4–6].
MCP-1, one of the important chemokines, has a potent and specific chemoattractant activity for monocytes [7] and T lymphocytes [8]. MCP-1 is secreted by various cells, notably endothelial cells, in response to inflammatory cytokines such as TNF-α and IL-1 [9–11]. Martin et al. showed that cytokine-induced MCP-1 gene expression in human endothelial cells depends on the co-operative action of nuclear factor κ B (NF-κ B) and activating protein 1 (AP-1) [12]. In our previous study, it was suggested that intercellular adhesion molecule-1 (ICAM-1) or endothelial leucocyte adhesion molecule‐1 (ELAM-1) expression of HUVEC stimulated via TXA2 receptors is augmented by the induction of NF-κ B and AP-1 binding activity through the protein kinase C (PKC) system [6].
The present study was designed to determine whether or not a TXA2 receptor agonist augments the expression of MCP-1 in HUVEC, both at the protein and mRNA levels. TXA2 receptor antagonists, PKC inhibitors, or inhibitors of transcription factors were used to determine the intracellular signal pathways stimulated by a TXA2 receptor agonist to express MCP-1 on HUVEC.
MATERIALS and METHODS
Reagents
Recombinant human TNF-α was purchased from Genzyme Co. (Boston, MA). Human PAF was purchased from Sigma Chemical Co. (St Louis, MO). U46619 (9,11-dideoxy-9α, 11α-methano-epoxyprostaglandin F2α), an agonist of TXA2 receptor, and SQ29 548 (7-[3-[[2-[(phenylamino)-carbonyl]-hydradino]methyl]-7-axabicyclo[2,2,1]hept-2-yl]-,[1S(1α,2α,(Z),3α,4α)]-5-heptenoic acid), an antagonist of the TXA2 receptor, were purchased from Cayman Chemical Co. (Ann Arbor, MI). BAYu3405 (3R)-3-(4-fluorophenyl-sulphonamido)-1,2,3,4-tetrahydro-9-carbazolepropanoic acid, another antagonist of TXA2 receptor, was provided by Bayer Yakuhin Ltd. (Osaka, Japan). Staurosporine, a PKC inhibitor, was purchased from Seikagaku Co. (Tokyo, Japan). GF 109203X (bis-indolylmaleimide), a potent and specific PKC inhibitor, was purchased from Sigma. 3-Aminobenzamide (3-AB), an inhibitor of AP-1 binding activity; pyrolidine-dithiocarbamate (PDTC), an inhibitor of NF-κ B binding activity; and N-acetylcysteine (NAC), an inhibitor of NF-κ B and AP-1 binding activity, were also purchased from Sigma.
Vascular endothelial cell cultures
Primary cell cultures were established according to procedures already described [13]. The HUVEC used in this experiment were between their first and third passages. When cultures reached confluence, the cells were harvested and re-plated at a density of 1 × 106 cells/dish. To these cells, 2 ml of Medium 199 with or without 10−6 m U46619, 100 U/ml of TNF-α, or 10−7 m PAF were added. In some cases, SQ29 548, BAYu3405, staurosporine, GF 109203X, 3-AB, PDTC or NAC were added 15 min before treatment with U46619, TNF-α, or PAF.
Reverse transcriptase-polymerase chain reaction analysis of mRNA in endothelial cells
U46619, TNF-α, or PAF were added to the endothelial cell cultures for 6 h. Total RNA was isolated by using RNA Zol B (Biotecx Labs. Inc., Houston, TX). Detection and analysis of gene expression at the RNA level was performed in accordance with procedures described earlier [6] by employing a GeneAmp RNA polymerase chain reaction (PCR) kit and a thermal cycler (Perkin Elmer Cetus, Norwalk, CT). After the reverse transcription (RT) reaction, PCR amplification was performed. The sequences of the sense and anti-sense primers for MCP-1 were 5′-ATAGCAGCCACCTTCATTCG-3′ and 5′-TTCCCCAAGTCTCTGTATCT-3′, respectively. Amplification of the same RNA with β-actin primers confirmed that equal amounts of RNA were reverse-transcribed. The respective sequences of primers for β-actin were 5′-TACATGGCTGGGGTGTTGAA-3′ and 5′-AAGAGAGGCATCCTCACCCT-3′. The amplification profile was 30 cycles of denaturation at 95°C for 30 s, primer annealing at 56°C for 30 s, and extension at 72°C for 1 min. The PCR products were run in 2% agarose gels (BioRad, Hemel Hempstead, UK) containing 0·5 mg/ml ethidium bromide (Sigma). The gels were visualized under ultraviolet illumination and photographed with a CCD video camera AE-6911CX (Atto, Tokyo, Japan). Quantitative image analysis of the PCR fragments on the gel was performed using a public domain NIH image program (written by W. Rashard, National Institutes of Health, Bethesda, MD) which was described earlier [6]. The size and intensity of each band were integrated for quantification as a relative amount of PCR products: relative amount of PCR product (integrated density) = band area × band-specific intensity, where band-specific intensity was calculated as the average intensity per pixel detected in the band area.
The total RNA (at concentrations of 0·5, 1·0, and 2·0 μg/μl) that was collected from HUVEC was subjected to RT-PCR by using a β-actin primer and quantitative image analysis. It was found that at each concentration the integrated density increased exponentially between 20 and 30 cycles, reaching a plateau at 35 cycles (Fig. 1a). Similar results were obtained when a MCP-1 primer was used. When the amplification cycle was set at a constant 30 cycles while the total RNA concentration was varied (0·125–4 μg/μl), a significant correlation was noted between the integrated density and the total RNA concentration (r2 = 0·99, P < 0·01; r2 = 0·96, P < 0·01; Fig. 1b), even if the primer was β-actin or MCP-1. Based on these results, we believe that the method employed in the present study is suitable for a quantitative analysis of MCP-1 mRNAs of HUVEC.
Fig. 1.
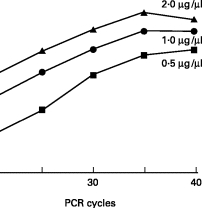
(a) Effects of the polymerase chain reaction (PCR) amplification cycles on the integrated density in quantitative image analysis of PCR fragments. The total RNA (at concentrations of 0·5, 1·0, and 2·0 μg/µl) that was collected from human umbilical vein endothelial cells was subjected to reverse transcription (RT)-PCR by using a β-actin primer and quantitative image analysis described in Materials and Methods. When the PCR amplification cycle was varied (20–40 cycles), the integrated density of PCR products was calculated. Data represent means of three separate experiments. (b) Correlation between total RNA concentration of samples and the integrated density in quantitative image analysis of PCR fragments. When the PCR amplification cycle using a β-actin or MCP-1 primer was set at a constant 30 cycles while the total RNA concentration was varied (0·125–4 μg/µl), a correlation was examined between the integrated density and the total RNA. Data represent means of three separate experiments.
Determination of MCP-1 synthesis in the cultured endothelial cells
The capacity of the cultured HUVEC to synthesize MCP-1 was evaluated by determining the concentrations of MCP-1 in the culture supernatant, using the appropriate ELISA kit (Biosource Int., Camarillo, CA). The assays were performed by using a rabbit anti-human MCP-1 antibody, human recombinant MCP-1, biotinylated rabbit anti-human MCP-1, and avidin–horseradish peroxidase. The chromogen substrate was added and the reaction was terminated with 50 μl/well of 3 m H2SO4. The absorbance was read at 450 nm in an ELISA plate reader (Nalge Nunc Int., Napierville, IL). This ELISA method consistently detected concentrations above 20 pg/ml, but did not cross-react with IL-1β, IL-6, TNF-α, interferon-gamma (IFN-γ), SLF, RANTES, or granulocyte-macrophage colony-stimulating factor (GM-CSF). The cellular proteins were solubilized with 1% Triton X-100 in 0·9% NaCl and centrifuged at 750 g for 10 min at 4°C. The protein content was determined by the standard procedures [13], with bovine serum albumin as the standard. The MCP-1 contents were normalized to the protein content of the cell layer.
Determination of cell viability in the cultured endothelial cells
Cell viability was assessed by quantification of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Chemicon Int., Termecula, CA) [14] reduction by mitochondrial dehydrogenases. The HUVEC were incubated for 2 h with 1·2 mm MTT in Medium 199. After the cells were washed with PBS, formazan dye was solubilized in 5% formic acid in isopropanol, and the extinction was measured at 550 nm versus 690 nm in a microplate reader (Nalge Nunc Int.).
Determination of protein synthesis in cultured endothelial cells
Protein synthesis in HUVEC was measured by 35S-methionine incorporation. The cells were plated at a density of 106 cells/dish and incubated in 5% CO2 at 37°C for 24 h. The medium was removed and 1·5 ml/dish of methionine-free medium was added. 35S-methionine (37 TBq/mmol; Amersham Int., Aylesbury, UK) was then added to cultures at a final concentration of 1·3 GBq/ml, and the cells were incubated for an additional 24 h under these conditions. The cell layers were then solubilized at 4°C with a lysis buffer (containing 0·5% Triton X-100, 0·25% deoxycholic acid, 10 mm ethylenediamine-tetraacetic acid, 1 mm phenylmethylsulphonyl fluoride, and 50 mm Tris–HCl, pH 8·5). Trichloroacetic acid (10%) was added to cell lysates. After a 20-min incubation on ice, the cell lysates were collected on glass-microfibre filters on a vacuum manifold and washed three times with 5% trichloroacetic acid and then once with ethanol. Filters were transferred to a liquid scintillation cocktail (NEN Research Products, Boston, MA) and counted with a liquid scintillation analyser (LSC-5100; Aloka, Tokyo, Japan).
Statistical analysis
All results were expressed as means ±s.d. Changes with respect to basal values when only two observations were obtained were analysed by a paired or unpaired Student’s t-test.
Comparisons among means of multiple groups were analysed by one-way analysis of variance and Scheffe’s multiple comparisons test. Differences were considered significant at P < 0·05.
RESULTS
Effects of TXA2 receptor agonist (U46619) or antagonists on mRNA expression and the production of MCP-1 in HUVEC
MCP-1 mRNA was barely detectable in unstimulated HUVEC (Fig. 2a,b). U46619 at doses of 10−7 m or 10−6 m induced a dose-dependent increase in the accumulation of MCP-1 mRNA within 6 h. SQ29 548 or BAYu3405 (10−7 m, 10−6 m, or 10−5 m) [15,16] were added to HUVEC prior to adding 10−6 m U46619. SQ29 548 or BAYu3405 alone did not affect cellular viability (Table 1) or the accumulation of MCP-1 mRNA (Fig. 3b) but dose-dependently diminished the accumulation of MCP-1 mRNA in response to U46619 (Fig. 2a,b).
Fig. 2.
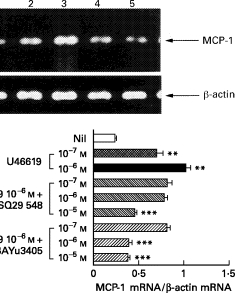
Effects of TXA2 receptor agonist or antagonists on mRNA expression and the production of MCP-1 in human umbilical vein endothelial cells (HUVEC). (a) HUVEC were not stimulated (lane 1), or stimulated with U46619 (10−7 m or 10−6 m) (lanes 2 and 3) and incubated for 6 h. SQ29 548 (10−5 m) or BAYu3405 (10−5 m) were pretreated before the addition of 10−6 m U46619 (lanes 4 and 5). Reverse transcription-polymerase chain reaction (RT-PCR) was done with specific primers for MCP-1 or β-actin on mRNA extracted from 107 cells as described in Materials and Methods. Shown is a gel photograph representative of three similar experiments. (b) The PCR products were quantified by quantitative image analysis as the ratio in relation to β-actin mRNA (1·0). (c) The production of MCP-1 was determined as the concentrations of MCP-1 in the culture supernatants after 24 h stimulation using ELISA. Data represent means ±s.d. of three separate experiments. *P < 0·05 compared with those without stimulation; **P < 0·01 compared with those without stimulation; ***P < 0·01 compared with those stimulated by U46619 (10−6 m).
Table 1.
Effects of TXA2 receptor antagonists, protein kinase C (PKC) inhibitors, or inhibitors of transcription factors on cellular viability of human vascular endothelial cells
| Cell viability (%) | ||
|---|---|---|
| 6 h after treatment | 24 h after treatment | |
| SQ29 548, 10−5m | 104·6 ± 2·6 | 102·5 ± 0·7 |
| BAYu3405, 10−5m | 100·4 ± 2·0 | 108·5 ± 1·6 |
| Staurosporine 3 nm | 102·9 ± 1·6 | 95·1 ± 3·2 |
| GF 109203X 5 μm | 104·2 ± 2·0 | 96·2 ± 1·2 |
| 3-AB 1 mm | 107·3 ± 1·2 | 102·5 ± 1·2 |
| PDTC 100 μm | 102·4 ± 2·0 | 97·6 ± 1·6 |
| NAC 30 mm | 95·1 ± 5·0 | 94·1 ± 6·5 |
Human umbilical vein endothelial cells were treated with SQ29 548, BAYu3405, staurosporine, GF 109203X, 3-AB, PDTC, or NAC, in the concentrations indicated. Then the cells were incubated for 6 h or 24 h, followed by a 2-h treatment with 1·2 mm MTT and photometric determination of formazan dye concentration. Data represent means ±s.d. of three separate experiments as percentage of control.
Fig. 3.
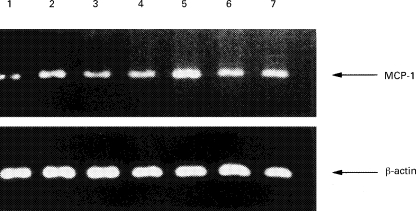
Effects of TXA2 receptor antagonists on the mRNA expression of MCP-1 in human umbilical vein endothelial cells (HUVEC) stimulated by tumour necrosis factor-alpha (TNF-α) or platelet-activating factor (PAF). (a) HUVEC were not stimulated (lane 1), or stimulated with PAF (10−7 m) (lane 2) or TNF-α (100 U/ml) (lane 5) for 6 h. SQ29 548 (10−5 m) or BAYu3405 (10−5 m) were pretreated before the addition of PAF (10−7 m) (lanes 3 and 4) or TNF-α (100 U/ml) (lanes 6 and 7). Shown is a gel photograph representative of three similar experiments. (b) The polymerase chain reaction products were quantified by quantitative image analysis as described in Materials and Methods. Results are expressed as the ratio in relation to β-actin mRNA. Data represent means ±s.d. of three separate experiments. *P < 0·01 compared with those without stimulation; **P < 0·01 compared with those stimulated by TNF-α or PAF.
Twenty-four hours after the addition of 10−6 m U46619, MCP-1 production was significantly intensified (P < 0·05) (Fig. 2c). SQ29 548 or BAYu3405 alone did not affect protein synthesis (Table 2) or the production of MCP-1 by HUVEC (Fig. 4), but both suppressed the potentiation of MCP-1 production dose-dependently (Fig. 2c).
Table 2.
Effects of TXA2 receptor antagonists, protein kinase C (PKC) inhibitors, or inhibitors of transcription factors on protein synthesis of human vascular endothelial cells
| 35S-methionine counts (dpm/μg protein) | |
|---|---|
| Nil | 3216·0 ± 103·0 |
| SQ29 548 10−5m | 3364·0 ± 269·0 |
| BAYu3405 10−5m | 3228·0 ± 168·0 |
| Staurosporine 3 nm | 3229·0 ± 235·0 |
| GF 109203X 5 μm | 3018·0 ± 294·0 |
| 3-AB 1 mm | 3126·0 ± 170·0 |
| PDTC 100 μm | 3062·0 ± 336·0 |
| NAC 30 mm | 3015·0 ± 367·0 |
| Cycloheximide 10 μg/ml | 397·0 ± 111·0* |
Human umbilical vein endothelial cells were treated with or without SQ29 548, BAYu3405, staurosporine, GF 109203X, 3-AB, PDTC, NAC, or cycloheximide, in the concentrations indicated. Then the cells were treated with 1·3 GBq/ml 35S-methionine for 24 h, and measured as 35S-methionine incorporation as described in Materials and Methods. Data represent means ±s.d. of three separate experiments.
P < 0·01 compared with those without stimulation.
Fig. 4.

Effects of TXA2 receptor antagonists on the production of MCP-1 in human umbilical vein endothelial cells (HUVEC) stimulated by tumour necrosis factor-α (TNF-α) or platelet-activating factor (PAF). HUVEC were pretreated with or without SQ29 548 or BAYu3405 (10−7, 10−6, or 10−5 m) and stimulated with TNF-α (100 U/ml) or PAF (10−7 m) for 24 h. The production of MCP-1 was determined as the concentrations of MCP-1 in the culture supernatants using ELISA. Data represent means ±s.d. of three separate experiments. *P < 0·05 compared with those without stimulation; **P < 0·01 compared with those without stimulation; ***P < 0·01 compared with those stimulated by TNF-α (100 U/ml) or PAF (10−7 m).
Effects of TXA2 receptor antagonists on mRNA expression and the production of MCP-1 in stimulated HUVEC
SQ29 548 or BAYu3405 (10−7 m, 10−6 m, or 10−5 m) were added to HUVEC before adding 100 U/ml of TNF-α or 10−7 m PAF. Six hours after the addition, TNF-α-induced MCP-1 mRNA accumulation was dose-dependently diminished by SQ29 548 or BAYu3405 (Fig. 3a,b). PAF-induced MCP-1 mRNA accumulation was also dose-dependently diminished by SQ29 548 or BAYu3405.
Twenty-four hours after the addition, the enhancement of TNF-α-stimulated MCP-1 production was dose-dependently suppressed by SQ29 548 or BAYu3405 (Fig. 4). Potentiation of MCP-1 production by PAF was also dose-dependently suppressed by SQ29 548 or BAYu3405.
Effects of PKC inhibitors on mRNA expression and the production of MCP-1 in HUVEC
Staurosporine (1 or 3 nm) or GF 109203X (1 or 5 μm), which inhibits PKC activation [17,18], were added to HUVEC prior to the addition of 10−6 m U46619. Staurosporine or GF 109203X alone did not significantly affect cellular viability, protein synthesis, or the expression of MCP-1 in HUVEC at both protein and mRNA levels (Tables 1 and 2, Fig. 5). Pretreatment with staurosporine dose-dependently reduced the expression of MCP-1 in response to U46619 at both protein and mRNA levels. GF 109203X (1 μm) significantly suppressed the extent of U46619-induced expression of MCP-1 at both protein and mRNA levels (P < 0·05, P < 0·01).
Fig. 5.
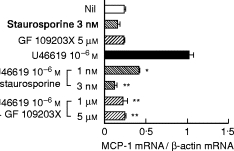
Effects of protein kinase C (PKC) inhibitors on mRNA expression and the production of MCP-1 in human umbilical vein endothelial cells (HUVEC). HUVEC were pretreated with or without staurosporine (1 or 3 nm) or GF109203X (1 or 5 μm), and stimulated with U46619 (10−6 m). (a) The mRNA expression of MCP-1 was analysed by reverse transcription-polymerase chain reaction (RT-PCR). Results are expressed as the ratio in relation to β-actin mRNA. (b) The production of MCP-1 was determined as the concentrations of MCP-1 in the culture supernatants using ELISA. Data represent means ±s.d. of three separate experiments. *P < 0·05 compared with those stimulated by U46619 (10−6 m); **P < 0·01 compared with those stimulated by U46619 (10−6 m).
Effects of inhibitors of transcription factors on mRNA expression and the production of MCP-1 in HUVEC
3-AB, an inhibitor of poly ADP-ribosylation, was used to target AP-1 since it inhibits oxidant-induced AP-1 binding activity [19]. The anti-oxidant PDTC, a selective inhibitor of NF-κ B activation, was used because it acts without affecting the induction of AP-1 binding activity [20]. NAC, a general anti-oxidant and precursor of glutathione, was used because it inhibits NF-κ B and AP-1 binding activity [21,22].
3-AB (100 μm or 1 mm), PDTC (10 or 100 μm), or NAC (5 or 30 mm) were added to HUVEC cultures before adding 10−6 M U46619. 3-AB or PDTC alone did not affect cellular viability, protein synthesis, or the expression of MCP-1 in HUVEC at either protein or mRNA levels (Tables 1 and 2, Fig. 6). NAC alone did not affect cellular viability or protein synthesis (Tables 1 and 2), but significantly suppressed the accumulation of MCP-1 mRNA and the production of MCP-1 (Fig. 6). U46619-induced MCP-1 expression was dose-dependently reduced by 3-AB or PDTC at both protein and mRNA levels. NAC almost completely suppressed mRNA accumulation and the production of MCP-1 induced by U46619.
Fig. 6.
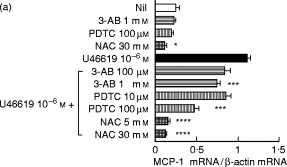
Effects of inhibitors of transcription factors on mRNA expression and the production of MCP-1 in human umbilical vein endothelial cells (HUVEC). HUVEC were pretreated with or without 3-AB (100 μm or 1 mm), PDTC (10 or 100 μm), or NAC (5 or 30 mm) and stimulated with U46619 (10−6 m). (a) The mRNA expression of MCP-1 was analysed by reverse transcription-polymerase chain reaction (RT-PCR). Results are expressed as the ratio in relation to β-actin mRNA. (b) The production of MCP-1 was determined as the concentrations of MCP-1 in culture supernatants using ELISA. Data represent means ±s.d. of three separate experiments. *P < 0·05 compared with those without stimulation; **P < 0·01 compared with those without stimulation; ***P < 0·05 compared with those stimulated by U46619; ****P < 0·01 compared with those stimulated by U46619.
DISCUSSION
The results of the present study elucidate the following: (i) treatment of HUVEC with a TXA2 receptor agonist, U46619, induces mRNA accumulation and production of MCP-1 (Fig. 2); (ii) pretreatment with TXA2 receptor antagonists significantly diminishes the extent of TNF-α-, PAF-, or U46619-induced mRNA accumulation and production of MCP-1 (Figs 2, 3 and 4); (iii) pretreatment with PKC inhibitors greatly diminishes the extent of U46619-induced mRNA accumulation and production of MCP-1 (Fig. 5); (iv) pretreatment with NAC (an inhibitor of NF-κ B and AP-1 binding activity) almost completely diminishes U46619-induced mRNA accumulation and production of MCP-1 (Fig. 6).
In the human MCP-1 gene, TPA-responsive elements (TRE), which are recognized by the AP-1 transcription factor heterodimers c-jun and c-fos, and κ B enhancer element in the 5′ upstream region of the gene were found [23]. Simultaneous site-directed mutagenesis of both the NF-κ B and the AP-1 binding sites blocked the induction of the MCP-1 promoter by IL-1β or TNF-α[12]. TPA, a potent PKC activator, induced MCP-1 gene expression in HUVEC. This signal transduction is the transcription activation promoted by binding of AP-1 to TRE [24]. PKC has also been shown to engage in the activation of NF-κ B through phosphorylation of its inhibitor Iκ B in the cytoplasm [25]. The PKC activity of the human vascular endothelial cells increased when stimulated with U46619, a TXA2 receptor agonist [4]. ICAM-1 or ELAM-1 expression of HUVEC via TXA2 receptors is augmented by induction of NF-κ B and AP-1 binding activity through the PKC system [5]. The present study suggests that MCP-1 expression is also augmented by intracellular signal transmission through PKC-dependent induction of NF-κ B and AP-1 binding activity in the stimulation via TXA2 receptors in HUVEC.
Subconfluent endothelial cells, known to express elevated levels of endogenous IL-1 in vitro[26], express elevated levels of MCP-1 mRNA [27]. The expression of MCP-1 in unstimulated HUVEC was suppressed by NAC at both protein and mRNA levels (Fig. 6). Martin et al. suggested that NF-κ B and AP-1 are activated in response to IL-1 stimulation in HUVEC [12]. These findings suggest a possibility that basal MCP-1 expression in HUVEC may depend on the action of NF-κ B and AP-1 through the production of endogenous IL-1.
In an earlier study [4] we suggested that TXA2, the production of which was enhanced through stimulation by TNF-α or PAF, is secreted into the extracellular space and bound to the TXA2 receptor on HUVEC, resulting in augmented expression of ICAM-1. The study revealed that HUVEC may possess a pathway where TXA2, which has been produced by the cells by TNF-α or PAF stimulation, binds with TXA2 receptors and activates PKC. The present study suggests the presence of a system that augments MCP-1 expression via TXA2 receptors following TNF-α or PAF stimulation.
MCP-1 can promote an avidity of β1-integrins VLA-4 and VLA-5 in monocytes. Therefore MCP-1 can increase β1-integrin-mediated binding of monocytes to vascular cell adhesion molecule-1 (VCAM-1) [28]. MCP-1 also induces transendothelial migration of both resting and activated T cells. MCP-1-stimulated transmigration of T cells was inhibited by antibodies against CD11a, thereby confirming the importance of β2-integrins in the transmigration process [29,30]. The activation of β1- and β2-integrins by MCP-1 may follow adhesion and transmigration of monocytes and T cells through binding with ICAM-1 or VCAM-1. The present study shows that stimulation of TXA2 receptors in HUVEC may result in not only augmented expression of adhesion molecules but also an increase in the avidity of monocytes and T cells through MCP-1. TXA2 receptor antagonists may inhibit the production of MCP-1 in human vascular endothelial cells and prevent exacerbation of inflammation by blocking these responses.
Acknowledgments
We wish to thank Dr K. Suzuki (The First Department of Internal Medicine) and E. Takayama (The Department of Parasitology) for their valuable advice.
REFERENCES
- 1.Ceuppens JL, Vertessen S, Deckmyn H, Vermylen J. Effects of thromboxane A2 on lymphocyte proliferation. Cell Immunol. 1985;90:458–63. doi: 10.1016/0008-8749(85)90210-2. [DOI] [PubMed] [Google Scholar]
- 2.Spagnuolo PJ, Ellner JJ, Hassid A, Dunn MJ. Thromboxane A2 mediates augmented polymorphonuclear leukocyte adhesiveness. J Clin Invest. 1980;66:406–14. doi: 10.1172/JCI109870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard OM, Ben-Baruch A, Oppenheim JJ. Chemokines: progress toward identifying molecular targets for therapeutic agents. Trends Biotechnol. 1996;14:46–51. doi: 10.1016/0167-7799(96)80920-6. [DOI] [PubMed] [Google Scholar]
- 4.Ishizuka T, Suzuki K, Kawakami M, Kawaguchi Y, Hidaka T, Matsuki Y, Nakamura H. DP-1904, a specific inhibitor of thromboxane A2 synthesizing enzyme, suppresses ICAM-1 expression by stimulated vascular endothelial cells. Eur J Pharmacol. 1994;262:113–23. doi: 10.1016/0014-2999(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 5.Ishizuka T, Suzuki K, Kawakami M, Hidaka T, Matsuki Y, Nakamura H. Thromboxane A2 receptor blockade suppresses intercellular adhesion molecule-1 expression by stimulated vascular endothelial cells. Eur J Pharmacol. 1996;312:367–77. doi: 10.1016/0014-2999(96)00478-5. [DOI] [PubMed] [Google Scholar]
- 6.Ishizuka T, Kawakami M, Hidaka T, Matsuki Y, Takamizawa M, Suzuki K, Kurita A, Nakamura H. Stimulation with thromboxane A2 receptor agonist enhances ICAM-1, VCAM-1 or ELAM-1 expression by human vascular endothelial cells. Clin Exp Immunol. 1998;112:464–70. doi: 10.1046/j.1365-2249.1998.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valente AJ, Graves DT, Vialle-Valentin CE, Delgado R, Schwartz CJ. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988;27:4162–8. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- 8.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YS, Shyy YJ, Wright JG, Valente AJ, Cornhill JF, Kolattukudy PE. The expression of monocyte chemotactic protein (MCP-1) in human vascular endothelium in vitro and in vivo. Moll Cell Biochem. 1993;126:61–68. doi: 10.1007/BF01772208. [DOI] [PubMed] [Google Scholar]
- 10.Sica A, Wang JM, Colotta F, et al. Monocyte chemotactic and activation factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990;144:3034–8. [PubMed] [Google Scholar]
- 11.Brown Z, Gerritsen ME, Carley WW, Strieter RM, Kunkel SL, Westwick J. Chemokine gene expression and secretion by cytokine-activated human microvascular endothelial cells. Am J Pathol. 1994;145:913–21. [PMC free article] [PubMed] [Google Scholar]
- 12.Martin T, Cardarelli PM, Parry GCN, Felts KA, Cobb RR. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kB and AP-1. Eur J Immunol. 1997;27:1091–7. doi: 10.1002/eji.1830270508. [DOI] [PubMed] [Google Scholar]
- 13.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–60. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimeric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–70. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999;59:4574–7. [PubMed] [Google Scholar]
- 16.Rosentreter U, Boshagen H, Senter F, Perzborn E, Fiedler VB. Synthesis and absolute configuration of the new thromboxane antagonist (3R)-3-(4-fluorophenyl-sulfonamido)-1,2,3, 4-tetrahydro-9-carbazolepropanoic acid and comparison with its enantiomer. Arzneim–Forsch/Drug Res. 1989;39:1519–21. [PubMed] [Google Scholar]
- 17.Ritchie AJ, Johnsohn DR, Ewenstein BM, Pober JS. Tumor necrosis factor induction of endothelial cell surface antigens is independent of protein kinase C activation or inactivation. Studies with phorbol myristate acetate and staurosporine. J Immunol. 1991;146:3056–62. [PubMed] [Google Scholar]
- 18.Villard E, Alonso A, Agrapart M, Challah M, Soubrier F. Induction of angiotensin I-converting enzyme transcription by a protein kinase C-dependent mechanism in human endothelial cells. J Biol Chem. 1998;273:25191–7. doi: 10.1074/jbc.273.39.25191. [DOI] [PubMed] [Google Scholar]
- 19.Amstad PA, Krupitza G, Cerutti PA. Mechanism of c-fos induction by active oxygen. Cancer Res. 1992;52:3952–60. [PubMed] [Google Scholar]
- 20.Schreck R, Meier B, Mannuel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J Exp Med. 1992;175:1181–94. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roederer M, Staal W, Raju PA, Ela SW, Herzenberg LA. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-l-cysteine. Proc Natl Acad Sci USA. 1990;87:4884–8. doi: 10.1073/pnas.87.12.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergelson S, Pinkus R, Daniel V. Intracellular glutathione levels regulate Fos/Jun induction and activation of glutathione S-transferase gene expression. Cancer Res. 1994;54:36–40. [PubMed] [Google Scholar]
- 23.Shyy Y-J, Li Y-S, Kolattukudy PE. Activation of MCP-1 gene expression is mediated through multiple signaling pathways. Biochem Biophys Res Commun. 1993;192:693–9. doi: 10.1006/bbrc.1993.1470. [DOI] [PubMed] [Google Scholar]
- 24.Shirakawa F, Mizel SB. In vitro activation and nuclear translocation of NF-kB catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Moll Cell Biol. 1989;9:2424–30. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh S, Baltimore D. Activation in vitro of NF-kB by phosphorylation of its inhibitor IkB. Nature. 1990;344:678–82. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 26.Borish L, King MS, Mascali JJ, Johnsohn S, Coll B, Rosenwassen LJ. Transthyretin is an inhibitor of monocyte and endothelial cell interleukin-1 production. Inflammation. 1992;16:471–84. doi: 10.1007/BF00918973. [DOI] [PubMed] [Google Scholar]
- 27.Wempe F, Lindner V, Augustin HG. Basic fibroblast growth factor regulates the expression of the CC chemokine monocyte chemoattractant protein-1 in autocrine-activated endothelial cells. Arterioscler Thromb Vasc Biol. 1997;17:2471–8. doi: 10.1161/01.atv.17.11.2471. [DOI] [PubMed] [Google Scholar]
- 28.Weber C, Alon R, MoSeries B, Springer TA. Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–73. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai J-P, Hudson S, Ye M-W, Chin Y-H. The intracellular signaling pathways involved in MCP-1-stimulated T cell migration across microvascular endothelium. Cell Immunol. 1996;167:269–75. doi: 10.1006/cimm.1996.0035. [DOI] [PubMed] [Google Scholar]
- 30.Weber C, Lu C-F, Casanovas JM, Springer TA. Role of αLβ2 integrin avidity in transendothelial chemotaxis of mononuclear cells. J Immunol. 1997;159:3968–75. [PubMed] [Google Scholar]


