Abstract
Synovial fluid (SF) levels of soluble CD23 (sCD23) were determined in 96 patients presenting with an inflammatory knee effusion (73 with RA and 23 with reactive arthritis (ReA) serving as a control inflammatory non-erosive group) and were correlated with the degree of joint destruction, with local immune parameters (IL-1β, IL-3, IL-4, IL-6, IL-8, IL-10, IL-12 and sCD25) and with serum markers of inflammation, C-reactive protein and erythrocyte sedimentation rate. RA patients, classified as erosive or not according to Larsen’s grade, were separated as follows: (i) 13 patients with non-erosive RA; (ii) 16 RA patients with erosions in hands but not in knees, matched for disease duration with the first group; (iii) 44 RA patients with hand and knee erosions, matched with the second group for rheumatoid factor positivity but of longer disease duration. SF sCD23 levels were significantly increased in both erosive RA groups compared with non-erosive diseases, whether RA or ReA (P < 0·05), whose SF levels were not different. SF IL-10 showed a similar profile to that of SF sCD23 and was the only other parameter characteristic of erosive RA, but no direct correlation was found between the two. SF sCD23 was significantly correlated with IL-12 (r = 0·65, P = 0·0001) and sCD25 (r = 0·39, P = 0·0019) exclusively in the two erosive RA populations. In conclusion, these data showing that increased levels of sCD23 are not only found in the SF of erosive joints but also in knee SF of patients with erosive RA but without knee x-ray-diagnosed erosions suggest that this parameter might be of predictive value for joint destruction. Longitudinal studies are however needed to confirm its potential clinical interest.
Keywords: soluble CD23, rheumatoid arthritis, erosions, synovial fluid
Introduction
The CD23 antigen, the low-affinity receptor for the Fc portion of IgE (Fcε RII), is expressed mainly on B lymphocytes and monocytes, but can also be expressed by a variety of haematopoietic cells including platelets, eosinophils, T lymphocytes, follicular dendritic cells and natural killer (NK) cells [1]. It is a 45-kD membrane type II glycoprotein whose proteolytic cleavage gives rise to unstable soluble fragments subsequently transformed into a stable 25-kD product referred to as soluble CD23 (sCD23). Cell surface CD23 expression and sCD23 release are up-regulated by IL-4 in all cell types expressing CD23, and inhibited by interferon-gamma (IFN-γ), IFN-α, transforming growth factor-beta (TGF-β) and glucocorticoids on B cells [2]. While sCD23 retains the capacity to bind IgE, it displays many cytokine activities that are IgE-independent [2,3], including inhibition of apoptosis [4].
Increased serum levels of sCD23 have been described mainly in patients with allergy [5], with chronic lymphocytic leukaemia [6,7] as well as in various autoimmune diseases [8–14] including RA [9,15–18]. RA is a chronic inflammatory disease associated with B cell and T cell dysfunction. RA patients display an increased expression of CD23 on B cells [15,16,19] and an increased release of sCD23 by peripheral blood mononuclear cells (PBMC) [16]. Increased serum levels of sCD23 in RA are related to disease status, since unaffected monozygotic twins of RA patients have normal sCD23 values [20].
CD23 expression on lymphocytes has been identified in synovial biopsies from patients presenting chronic synovitis of various origins [21]. However, studies investigating sCD23 levels in the synovial fluid (SF) are scarce [18,22]. Recently, sCD23 has been considered as a proinflammatory cytokine inasmuch as it directly triggers tumour necrosis factor-alpha (TNF-α), IL-1β and IL-6 release by PBMC [23] as well as by monocytes after an interaction with the adhesion molecules CD11b and CD11c [24]. Increased levels of TNF-α and IL-1β have been found in the SF of RA patients compared with other inflammatory arthritides such as psoriatic arthritis, and are thought to reflect the usually more erosive course of the disease [25]. Furthermore, in mouse collagen-induced arthritis, a model for human RA, anti-CD23 treatment has been shown to reduce cartilage and bone destruction as well as the clinical severity of the disease [26]. Therefore, we examined sCD23 levels in the SF of RA patients and compared subpopulations matched for disease duration but differing by the presence or absence of x-ray erosions. We also analysed cytokines that participate in the immune process underlying RA.
PATIENTS and METHODS
Patients
SF samples were obtained from 96 patients undergoing arthrocentesis of the knee for diagnostic or therapeutic purposes. Seventy-three samples were obtained from patients with RA, as defined by the 1987 American College of Rheumatology revised criteria [27]. All patients had clinically active synovitis at the time of arthrocentesis. Twenty-three samples were obtained from patients presenting with knee swelling due to reactive arthritis (ReA) (seronegative oligoarthritis with culture and/or serological evidence for either sexually transmitted disease or enteritis due to Salmonella or Yersinia infection) [28] and served as a control group with an inflammatory non-erosive arthropathy. Patient demographic, clinical and biological data are shown in Table 1. Radiographs of hands, feet and knees were obtained in RA patients within a lapse of 3 months before or at the time of arthrocentesis and allowed classification into three distinct groups according to Larsen’s grade [29] (erosive disease amounting to at least Larsen’s grade 2 in hands/feet and Larsen’s grade 3 in knees): (i) 13 patients without erosions in hands, feet or knees (knee–/hand– or k−/h−); (ii) 16 patients without erosions in the aspirated knee (Larsen’s grade <3) but with erosions visible in their hands and/or feet (Larsen’s grade ≥2) (knee –/hand+ or k−/h+); (iii) 44 patients with erosions in hands and/or feet (Larsen’s grade ≥2) and in knees (Larsen’s grade ≥3) (knee+/hand+ or k+/h +). Patients from the first two groups (non-erosive RA and erosive k−/h+ RA) were matched for disease duration, while patients with erosive k+/h+ RA had a significantly longer disease duration (Table 1). Patients with erosive RA (k−/h+ and k+/h+) were matched for positivity of rheumatoid factor (RF).
Table 1.
Patient demographic, clinical and biological data
| Rheumatoid arthritis | Reactive arthritis | ||||
|---|---|---|---|---|---|
| Non-erosive | Erosive knee−/hand+ | Erosive knee+/hand+ | Non-erosive | ||
| Female/male | n | 6/7 | 13/3 | 29/15 | 11/12 |
| Age (years) | Mean ±s.e.m. | 46·8 ± 2·2 | 54·8 ± 2·2 | 60·9 ± 2·2* | 42·7 ± 2·1 |
| Disease duration (years) | Mean ±s.e.m. | 3·1 ± 0·8 | 5·1 ± 1·2 | 10·1 ± 1·4* | NA |
| Positive rheumatoid factor | n (%) | 4 (30·7)* | 11 (68·7) | 32 (72·7) | 0 |
| Number of previous DMARDs | Mean ±s.e.m. | 1·3 ± 0·2 | 1·6 ± 0·5 | 3·2 ± 0·3* | 0 |
| Concomitant DMARD | n (%) | 6 (46) | 9 (56) | 26 (59) | 0 |
| Concomitant corticosteroids | n (%) | 3 (23) | 8 (50) | 19 (43·2) | 0 |
| Prednisolone daily dose (mg) | Mean ±s.e.m. | 8·4 ± 0·8 | 8·7 ± 1·5 | 10·5 ± 1·3 | 0 |
| Concomitant NSAIDs | n (%) | 13 (100) | 16 (100) | 44 (100) | 22 (96) |
| CRP (mg/l) | Median | 16 (5–21)* | 42 (23·5–61·5) | 33 (10·2–63·2) | 43 (24–103) |
| (interquartile range) | |||||
| ESR (mm/h) | Median | 20 (15·2–43·8) | 31 (23–61) | 49·5 (30–74) | 50 (27·5–85·2) |
| (interquartile range) | |||||
DMARD, Disease-modifying anti-rheumatic drug; NSAID, non-steroidal anti-inflammatory drug; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; NA, not applicable.
P < 0·05 versus erosive knee−/hand+ RA (Mann–Whitney test for age, disease duration, prednisolone daily dose, CRP and ESR levels; χ 2 test for all other data).
SF sampling and immunological parameters
SF samples were aspirated from the knee joint under aseptic conditions, and centrifuged at 670 g for 20 min at 4°C to remove all cells and debris. The supernatants were stored at −80°C prior to assay. SF levels of sCD23, IL-1β, IL-3, IL-4, IL-6, IL-8, IL-10, IL-12 (Biosource Europe (formerly Medgenix), Fleurus, Belgium) and soluble CD25 (sCD25; Boehringer, Mannheim, Germany) were simultaneously determined by ELISA using specific MoAbs and according to the manufacturer’s recommendations. No interference with RF was found. Sera were collected simultaneously to the arthrocentesis in 73 patients (11 k−/h−RA, 16 k−/h+ RA, 31 k+/h+ RA and 15 ReA). Erythrocyte sedimentation rate (ESR) was determined by the Westergren method and C-reactive protein (CRP) levels were measured by nephelometry using specific antisera. RF was determined by immunonephelometry (Dade Behring Inc., Newark, NJ).
Statistical analysis
SF parameters are expressed as median values (with the 25–75% ‘interquartile’ range). Between-group differences were analysed by Mann–Whitney U-test or χ2 test. Correlations were sought by linear regression, after logarithmic transformation. P <0·05 was considered significant.
Results
Since the clinical analysis of the three RA subgroups showed a longer disease duration in the erosive k+/h+ RA group, we compared the parameters obtained in the two subgroups matched for disease duration: non-erosive RA and erosive k−/h+ RA. SF sCD23 levels were significantly lower in non-erosive RA compared with erosive RA (Fig. 1). sCD23 levels were also lower in the non-erosive inflammatory control group, ReA, and were comparable to those observed in non-erosive RA (Table 2). sCD23 levels in non-erosive inflammatory arthropathies were not significantly different from those obtained in osteoarthritis, a degenerative arthropathy (median 1 U/ml, interquartile range 0·9–1·6 U/ml (n = 16)) or from patients with meniscus pathology and whose SF may be considered as ‘normal’ (median 1·1 U/ml, interquartile range 0·7–1·4 U/ml (n = 15)). sCD23 levels were similar in the two erosive RA groups, k−/h+ and k+/h+ (Table 2). Within the RA group, no differences were found between patients according to their treatment (data not shown). Of interest, SF IL-10 had a similar profile to that of sCD23. Indeed, IL-10 levels were lower in non-erosive RA compared with erosive RA, similar in the two non-erosive arthropathies—non-erosive RA and ReA—and similar in the two erosive RA groups (Table 2). SF IL-12 levels were significantly lower in non-erosive RA compared with erosive k−/h+ RA (Table 2). However, IL-12 levels were also significantly lower in the longer disease duration erosive k+/h+ RA group compared with erosive k−/h+ RA, and were not significantly different from those of non-erosive RA. SF levels of sCD25, the soluble IL-2 receptor, were lower in non-erosive RA compared with both erosive RA populations. However, elevated levels of sCD25 were not specific for erosive RA since they were also found in ReA, a non-erosive arthropathy (Table 2). No significant differences were found in SF levels of IL-1β, IL-3, IL-4, IL-6, and IL-8 between non-erosive and erosive k−/h+ RA (Table 2).
Fig. 1.
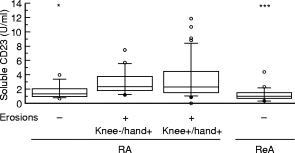
Synovial fluid soluble CD23 levels in RA and reactive arthritis (ReA), a control inflammatory non-erosive arthropathy. Boxes represent the interquartile range, i.e. the middle 50% of the data, between 25th and 75th quartile. Whiskers represent the 10th and 90th quartiles, and circles represent values out of this range. Asterisks indicate a significant difference versus erosive knee−/hand+ RA: *P < 0·05; ***P < 0·001 (Mann–Whitney test).
Table 2.
Synovial fluid parameters
| Rheumatoid arthritis | Reactive arthritis | |||
|---|---|---|---|---|
| Non-erosive | Erosive knee−/hand+ | Erosive knee+/hand+ | Non-erosive | |
| sCD23 (U/ml) | 1·5 (1–2·2)* | 2·6 (2–4) | 2·5 (1·7–4·7) | 1·25 (0·8–1·8)*** |
| IL-10 (pg/ml) | 9·5 (1·1–26·7)* | 40·7 (17·5–65) | 30·3 (18·5–62) | 10 (5·9–17·8)** |
| IL-12 (pg/ml) | 219 (156–239)* | 806 (249–1040) | 269 (143–355)* | 516 (197–984) |
| sCD25 (pmol/l) | 98 (77·5–116·7)** | 150 (116–195) | 185 (112–242) | 138 (89–205) |
| IL-1β (pg/ml) | 9·3 (4·6–46) | 6 (3–14·1) | 14 (5·5–52)* | 7·5 (4–14) |
| IL-3 (pg/ml) | 52 (22·5–95·5) | 68 (30–112) | 117 (49–358)* | 39 (18–57) |
| IL-4 (pg/ml) | 4 (0–19·5) | 4 (2·2–15·5) | 22 (6–57·5)** | 0 (0–7) |
| IL-8 (ng/ml) | 176 (128–391) | 232 (100–759) | 1415 (588–4219)*** | 127 (39–193) |
| IL-6 (ng/ml) | 9 (3·5–24) | 7·6 (3·8–12·2) | 19 (8–33·8)* | 25 (7–48)* |
P < 0·05
P < 0·01
P < 0·001 in comparison to erosive knee−/hand+ RA (Mann–Whitney).
Values in bold highlight specific characteristics of erosive RA.
Comparison of the two erosive RA groups which matched for RF but not for disease duration demonstrated significantly increased SF levels of IL-1β, IL-3, IL-4 and IL-8 in the k+/h+ RA group of longer disease duration (Table 2). The increased SF levels of these cytokines seem specific for a longer duration of disease, since levels were comparable in non-erosive and erosive k−/h+ groups which matched for shorter disease duration. SF IL-6 levels were increased in the longer disease duration RA group compared with the erosive k−/h+ RA group, but were also increased in ReA.
Correlations were sought between sCD23 and the other parameters in the three RA populations. Statistically significant correlations between sCD23 and IL-12 (Fig. 2) and between sCD23 and sCD25 (Fig. 3) were found in the two erosive RA groups whether studied together or separately (Table 3). These correlations were not found in any of the non-erosive diseases, RA or ReA. These correlations remained however statistically significant when all patient groups were studied together (r = 0·41, P = 0·0001 for IL‐12; r = 0·38; P = 0·0001 for sCD25).
Fig. 2.
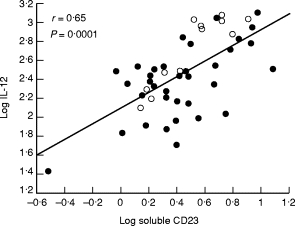
Positive linear correlation between log soluble CD23 and log IL-12 in erosive knee−/hand+ RA (○) and erosive knee+/hand+ RA (•). r and P values are those obtained for the two groups studied together.
Fig. 3.
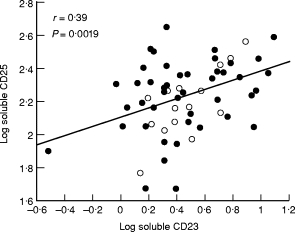
Positive linear correlation between log soluble CD23 and log soluble CD25 in erosive knee−/hand+ RA (○) and erosive knee+/hand+ RA (•). r and P values are those obtained for the two groups studied together.
Table 3.
Significant correlations for sCD23 and IL-10 in erosive rheumatoid arthritis
| Knee−/hand+ erosive RA | Knee+/hand+ erosive RA | |||
|---|---|---|---|---|
| r | P | r | P | |
| sCD23 and | ||||
| IL-12 | 0·91 | 0·0001 | 0·61 | 0·0001 |
| sCD25 | 0·65 | 0·0043 | 0·34 | 0·0249 |
| IL-10 and | ||||
| IL-6 | 0·66 | 0·014 | 0·34 | 0·0327 |
| IL-12 | 0·21 | NS | 0·37 | 0·0301 |
| sCD25 | 0·44 | NS | 0·41 | 0·0088 |
Correlations were sought by linear regression, after logarithmic transformation.
Because IL-10 and sCD23 levels displayed a similar profile, we searched for correlations between IL-10 and IL-12 and IL-10 and sCD25. Positive correlations were indeed observed but only in the k+/h+ RA group (Table 3). We also correlated IL-10 levels with those of the other immune parameters and found a positive linear correlation between IL-10 and IL-6 in the two erosive RA groups (Table 3). While sCD23 and IL-10 displayed a similar profile and similar correlations with the other parameters, we did not find a significant correlation between them in the RA groups. Further, no correlations were found between SF levels of sCD23 and IL-1β, IL-3, IL-6, IL-10, or between SF sCD23 and serum CRP or ESR.
Discussion
These data demonstrate that higher levels of sCD23 are found in the knee SF of RA patients presenting a high positivity for serum RF and x-ray-diagnosed erosions in hands, whether or not they present erosions in knees. In contrast, lower sCD23 levels are found in the SF of non-erosive diseases whether rheumatoid or ReA. To the best of our knowledge, this is the first study evaluating the SF status of sCD23 in RA with respect to the degree of joint destruction. sCD23 has been previously detected in RA SF [18,22] but no SF controls were available, nor was the radiological stage of the disease mentioned. It has been proposed that sCD23 might contribute to the up-regulation of the inflammatory reaction [30], since it induces the production of proinflammatory cytokines [23,24] and since its inhibition improves collagen-induced arthritis (CIA) in mice [26]. Furthermore, CD23-deficient mice exhibit a marked reduction of CIA [31]. Histological studies have also shown an increased expression of CD23 in inflammatory tissues, whether rheumatoid or not [21]. Our data suggest that SF sCD23 is not simply a parameter reflecting local inflammation. Indeed, an inflammatory arthropathy such as ReA displays SF sCD23 levels similar to those of non-erosive RA while having a more inflammatory profile, as shown by higher SF IL-6 and serum CRP levels. Furthermore, SF sCD23 levels in these two inflammatory but non-erosive arthropathies are similar to those detected in degenerative arthropathies such as osteoarthritis or meniscus pathology.
Erosive RA patients usually exhibit a 70–80% positivity for serum RF [32]. Albeit higher sCD23 levels are found in the serum of RF+ RA patients compared with RF− patients, no difference is found between erosive and non-erosive RA [20]. In the SF, sCD23 levels are also related to the presence of serum RF in the sense that the two patient groups with 70% RF positivity had higher SF sCD23 levels than the group with 30% RF positivity. While production of RF and sCD23 might be directly related in the joint, this cannot be ascertained as SF RF levels were not determined in our study. The measurement of sCD23 in the SF might be of greater interest than in the serum because SF sCD23 levels were significantly higher in erosive than in non-erosive RA patients. Furthermore, increased levels were found in erosive RA, even when x-ray erosions were not yet detected in the knee with an effusion (erosive k−/h+ RA). This suggests that sCD23 may be of predictive value for joint destruction in RA. However, an alternative explanation is that the appearance of knee erosions is related to disease duration and that sCD23 is an epiphenomenon associated with the immunopathology of the established phase of RA.
The correlations found between sCD23 and IL-12 on one hand and sCD25 on the other are of particular interest since they were only found in the two erosive RA groups. These three parameters are therefore probably involved in the mechanisms leading to joint erosions. The pathological relevance of the positive correlation between IL-12 and sCD23 is emphasized by the fact that the incidence and severity of CIA is reduced in CD23-deficient mice [31] and in IL-12-deficient mice [33]. Furthermore, administration of IL-12 causes more severe joint destruction in DBA/1 mice when given in combination with type II collagen [34].
Glucocorticoids are known to decrease CD23 expression and sCD23 release in vitro and in vivo[35]. However, in our study, non-erosive RA patients presenting lower SF sCD23 levels did not take more corticosteroids than erosive RA patients. Furthermore, no differences in sCD23 SF levels were observed in RA patients treated with MTX or other disease-modifying anti-rheumatic drugs (DMARDs) compared with levels in untreated patients.
The SF profile of IL-10 was similar to that of sCD23 inasmuch as increased SF levels were exclusively found in both RA groups exhibiting an erosive status. IL-10 and sCD23 are however probably regulated by different mechanisms since they are not correlated with each other in RA patients, nor is IL-10 correlated with SF IL-12 and sCD25 in erosive k−/h+ RA patients in contrast to sCD23. Our data confirm earlier studies showing that increased SF IL-10 levels are a dominant feature of established RA [36–39]. Moreover, they indicate that elevated SF IL-10 levels are restricted to an erosive behaviour of the disease, since patients with a non-erosive disease have lower SF levels. This association between IL-10 and joint erosions seems paradoxical in view of the immunoregulatory and anti-inflammatory properties generally assigned to this cytokine [40]. Two not mutually exclusive hypotheses can be proposed. First, IL-10 is a potent growth and differentiation factor for activated human B lymphocytes [41]. Interestingly, we found a significant positive correlation between IL-10 and IL-6, another B cell growth and differentiation factor. Furthermore, IL-10 may serve as a major cofactor facilitating T cell-dependent B cell differentiation and immunoglobulin production—including RF—in rheumatoid synovium [37]. These observations are supported by the sustained B lymphocyte hyperactivity classically observed in RA [36], which may be incriminated in the development of erosions. Second, elevated SF IL-10 levels in erosive disease may reflect an attempt to counteract the inflammatory cascade operating in the rheumatoid joint [38]. Recent data indicate that while serum and SF IL-10 levels are higher in RA, a relative IL-10 deficiency exists in RA patients [42]. Supporting this concept is the beneficial result obtained in RA patients treated with human recombinant IL-10 in a phase I study [43]. Furthermore, IL-10 decreases surface CD23 expression and CD23 mRNA levels in human monocytes in vitro[44]. Therefore, the finding that both increased SF levels of sCD23 and enhanced numbers of CD23+ monocytes are found in RA [45] supports the concept of a relative deficit of available IL-10 in RA.
The predictive value of SF analysis to monitor knee joint destruction has been previously proposed. Longitudinal studies in RA patients presenting progressive deterioration of Larsen’s grade have identified SF biochemical characteristics such as lower C3, higher acid phosphatase levels [46] and higher levels of cross-linked carboxyterminal telopeptide of type I collagen [47]. However, few immune parameters have been studied in relationship to the erosive course of RA. A cross-sectional study found higher SF TNF-α and IL-8 levels in erosive RA compared with non-erosive disease [48]. In our study however, higher SF IL-8 levels were only found in erosive RA of longer disease duration and did not discriminate between non-erosive and erosive RA groups matched for disease duration, in contrast to sCD23.
In conclusion, we have found elevated SF levels of sCD23 to be specific of an erosive behaviour of RA but also to be present before erosions are x-ray-diagnosed. Our study therefore demonstrates that SF sCD23 may be a parameter of predictive value for joint destruction. Longitudinal studies are however needed to confirm the potential clinical interest of SF sCD23.
Acknowledgments
The authors wish to thank A. Desoroux, R. Meulemans, Y. Pirard and Y. Vrindts-Gevaert for technical assistance. C.R., N.F. and Y.B. are, respectively, Research Fellow, Post-doctoral Researcher and Research Director of the Belgian National Fund for Scientific Research (FNRS). This work was supported in part by grants from the Lejeune-Lechien Foundation and from the ‘Fond d’Investissement pour la Recherche Scientifique’ (CHU Sart-Tilman).
REFERENCES
- 1.Delespesse G, Suter U, Mossalayi D, et al. Expression, structure and function of the CD23 antigen. Adv Immunol. 1991;49:149–91. doi: 10.1016/s0065-2776(08)60776-2. [DOI] [PubMed] [Google Scholar]
- 2.Delespesse G, Sarfati M, Wu CY, Fournier S, Letellier M. The low-affinity receptor for IgE. Immunol Rev. 1992;125:77–97. doi: 10.1111/j.1600-065x.1992.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 3.Sarfati M, Fournier S, Wu CY, Delespesse G. Expression, regulation and function of human Fc epsilon RII (CD23) antigen. Immunol Res. 1992;11:260–72. doi: 10.1007/BF02919132. [DOI] [PubMed] [Google Scholar]
- 4.Liu YJ, Cairns JA, Holder MJ, Abbot SD, Jansen KU, Bonnefoy JY, Gordon J, MacLennan ICM. Recombinant 25-kDa CD23 and interleukin 1α promote the survival of germinal center B cells: evidence for bifurcation in the development of centrocytes rescued from apoptosis. Eur J Immunol. 1991;21:1107–14. doi: 10.1002/eji.1830210504. [DOI] [PubMed] [Google Scholar]
- 5.Matsumo T, Miike T, Yamaguchi K, Murakami M, Kawabe T, Yodoi J. Serum levels of soluble IL-2 receptor, IL-4 and IgE-binding factors in childhood allergic diseases. Clin Exp Immunol. 1991;85:288–92. doi: 10.1111/j.1365-2249.1991.tb05720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarfati M, Bron D, Lagneaux L, Fonteyn C, Frost H, Delespesse G. Elevation of IgE-binding factors in serum patients with B-cell-derived chronic lymphocytic leukemia. Blood. 1988;71:94–98. [PubMed] [Google Scholar]
- 7.Beguin Y, Lampertz S, De Groote D, Igot D, Malaise M, Fillet G. Soluble CD23 and other receptors (CD4, CD8, CD25, CD71) in serum of patients with chronic lymphocytic leukemia. Leukemia. 1993;7:2019–25. [PubMed] [Google Scholar]
- 8.Bansal A, Roberts T, Hay EM, Kay R, Pumphrey RSH, Wilson PB. Soluble CD23 levels are elevated in the serum of patients with primary Sjögren’s syndrome and systemic lupus erythematosus. Clin Exp Immunol. 1992;89:452–5. doi: 10.1111/j.1365-2249.1992.tb06979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal AS, Ollier W, Marsh MN, Pumphrey RSH, Wilson PB. Variations in serum sCD23 in conditions with either enhanced humoral or cell-mediated immunity. Immunology. 1993;79:285–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Takei M, Azuhata T, Yoshimatu T, Shigihara S, Hashimoto S, Horie T, Horikoshi A, Sawada S. Increased soluble CD23 molecules in serum/saliva and correlation with the stage of sialoectasis in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol. 1995;13:711–5. [PubMed] [Google Scholar]
- 11.Sato S, Fujimoto M, Kikuchi K, Ihn H, Tamaki K, Takehara K. Elevated soluble CD23 levels in the sera from patients with localized scleroderma. Arch Dermatol Res. 1996;288:74–78. doi: 10.1007/BF02505047. [DOI] [PubMed] [Google Scholar]
- 12.Roblot P, Morel F, Lelievre E, Biais-Sauvetre MH, De Groote D, Preud’Homme JL, Lecron JC. Serum soluble CD23 levels in giant cell arteritis. Immunol Letters. 1996;53:41–44. doi: 10.1016/0165-2478(96)02612-0. [DOI] [PubMed] [Google Scholar]
- 13.Kutukculer N, Caglayan S. Plasma and synovial fluid soluble CD23 concentrations in children with juvenile chronic arthritis. Autoimmunity. 1998;27:155–8. doi: 10.3109/08916939809003863. [DOI] [PubMed] [Google Scholar]
- 14.Massa M, Pignatti P, Oliveri M, De Amici M, De Benedetti F, Martini A. Serum soluble CD23 levels and CD23 expression on peripheral blood mononuclear cells in juvenile chronic arthritis. Clin Exp Rheumatol. 1998;16:611–6. [PubMed] [Google Scholar]
- 15.Chomarat P, Briolay J, Banchereau J, Miossec P. Increased production of soluble CD23 in rheumatoid arthritis, and its regulation by interleukin-4. Arthritis Rheum. 1993;36:234–42. doi: 10.1002/art.1780360215. [DOI] [PubMed] [Google Scholar]
- 16.Izikawa K, Yanagihara Y, Kajiwara K, Koshio T, Shida T, Yamada A. Possible role of CD5+ B cells expressing CD23 in mediating elevation of serum-soluble CD23 in patients with rheumatoid arthritis. Int Arch Allergy Immunol. 1993;101:416–24. doi: 10.1159/000236485. [DOI] [PubMed] [Google Scholar]
- 17.Rezonzew R, Newkirk MM. Impaired release of sCD23 by activated B-cells from RA patients. Clin Immunol Immunopathol. 1994;71:156–63. doi: 10.1006/clin.1994.1066. [DOI] [PubMed] [Google Scholar]
- 18.Loza E, Tinturé T, Sanchez-Ibarrola A. CD5 and CD23 expression on B cells in peripheral blood and synovial fluid of rheumatoid arthritis: relationship with interleukin-4, soluble CD23 and tumor necrosis factor alpha levels. Rheumatol. 1999;38:325–8. doi: 10.1093/rheumatology/38.4.325. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Gutierrez B, Hernandez-Garcia C, Banares AA, Jover JA. CD23 hyperexpression in rheumatoid arthritis: evidence for a B cell hyperresponsiveness to cognate and noncognate T-cell signals. Clin Immunol Immunopathol. 1994;72:321–7. doi: 10.1006/clin.1994.1148. [DOI] [PubMed] [Google Scholar]
- 20.Bansal AS, MacGregor AJ, Pumphrey RSH, Silman AJ, Ollier WER, Wilson PN. Increased levels of sCD23 in rheumatoid arthritis are related to disease status. Clin Exp Rheumatol. 1994;12:281–5. [PubMed] [Google Scholar]
- 21.Hellen EA, Rowlands DC, Hansel TT, Kitas GD, Crocker J. Immunohistochemical demonstration of CD23 expression on lymphocytes in rheumatoid synovitis. J Clin Pathol. 1991;44:293–6. doi: 10.1136/jcp.44.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roussou E, Gordon J, Sykes C, Farr M, Luqmani R, Sheeran T, Bacon PA, Emery P. Soluble CD23 expression in patients with rheumatoid arthritis. Br J Rheumatol. 1991;30(Suppl. 2):110. (Abstr.) [Google Scholar]
- 23.Armant M, Ishihara H, Rubio M, Delespesse G, Sarfati M. Regulation of cytokine production by soluble CD23: costimulation of interferon γ secretion and triggering of tumor necrosis factor α release. J Exp Med. 1994;180:1005–11. doi: 10.1084/jem.180.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecoanet-Henchoz S, Gauchat JF, Aubry JP, et al. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Immunity. 1995;3:119–25. doi: 10.1016/1074-7613(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 25.Partsch G, Steiner G, Leeb BF, Dunky A, Bröll H, Smolen JS. Highly increased levels of tumor necrosis factor-α and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol. 1997;24:518–23. [PubMed] [Google Scholar]
- 26.Plater-Zyberk C, Bonnefoy JY. Marked amelioration of established collagen-induced arthritis by treatment with antibodies to CD23 in vivo. Nature Med. 1995;1:781–5. doi: 10.1038/nm0895-781. [DOI] [PubMed] [Google Scholar]
- 27.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 28.Steiner G, Studnicka-Benke A, Witzmann G, Höfler E, Smolen J. Soluble receptors for tumor necrosis factor and interleukin-2 in serum and synovial fluid of patients with rheumatoid arthritis, reactive arthritis and osteoarthritis. J Rheumatol. 1995;22:406–12. [PubMed] [Google Scholar]
- 29.Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn. 1977;18:481–9. doi: 10.1177/028418517701800415. [DOI] [PubMed] [Google Scholar]
- 30.Bonnefoy JY, Plater-Zyberk C, Lecoanet-Henchoz S, Gauchat JF, Aubry JP, Graber P. A new role for CD23 in inflammation. Immunol Today. 1996;17:418–20. doi: 10.1016/0167-5699(96)10054-2. [DOI] [PubMed] [Google Scholar]
- 31.Kleinau S, Martinsson P, Gustavsson S, Heyman B. Importance of CD23 for collagen-induced arthritis: delayed onset and reduced severity in CD23-deficient mice. J Immunol. 1999;162:4266–70. [PubMed] [Google Scholar]
- 32.Walker DJ, Pound JD, Griffiths ID, Powell RJ. Rheumatoid factor tests in the diagnosis and prediction of rheumatoid arthritis. Ann Rheum Dis. 1986;45:684–90. doi: 10.1136/ard.45.8.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIntyre KW, Shuster DJ, Gillooly KM, et al. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur J Immunol. 1996;26:2933–8. doi: 10.1002/eji.1830261219. [DOI] [PubMed] [Google Scholar]
- 34.Germann T, Szeliga J, Hess H, Storkel S, Podalski FJ, Gately MK, Schmitt E, Rude E. Administration of interleukin 12 in combination with type II collagen induces severe arthritis in DBA/1 mice. Proc Natl Acad Sci USA. 1995;92:4823–7. doi: 10.1073/pnas.92.11.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer A, König W. Regulation of CD23 expression, soluble CD23 release and immunoglobulin synthesis of peripheral blood lymphocytes by glucocorticoids. Immunology. 1990;71:473–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Llorente L, Richaud-Patin Y, Fior R, Alcocer-Varela J, Wijdenes J, Morel Fourrier B, Galanaud P, Emilie D. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjögren’s syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum. 1994;37:1647–55. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 37.Cush JJ, Splawski JB, Thomas R, McFarlin JE, Schulze-Koops H, Davis LS, Fujita K, Lipsky PE. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:96–104. doi: 10.1002/art.1780380115. [DOI] [PubMed] [Google Scholar]
- 38.Bucht A, Larsson P, Weisbrot L, Thorne C, Pisa P, Smedegard G, Keystone EC, Gronberg A. Expression of interferon-gamma (IFN-gamma), IL-10, IL-12 and transforming growth factor-beta (TGF-beta) mRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis (RA) Clin Exp Immunol. 1996;103:357–67. doi: 10.1111/j.1365-2249.1996.tb08288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isomäki P, Luukkainen R, Saario R, Toivanen P, Punnonen J. Interleukin-10 functions as an antiinflammatory cytokine in rheumatoid synovium. Arthritis Rheum. 1996;39:386–95. doi: 10.1002/art.1780390306. [DOI] [PubMed] [Google Scholar]
- 40.Howard M, O’Garra A, Ishida H, de Waal Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–47. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- 41.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keystone E, Wherry J, Grint P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 1998;24:629–39. doi: 10.1016/s0889-857x(05)70030-2. [DOI] [PubMed] [Google Scholar]
- 43.Maini RN, Paulus H, Breedveld PC, et al. rHuIL-10 in subjects with active rheumatoid arthritis (RA): a phase I and cytokine response study. Arthritis Rheum. 1997;40(Suppl.):S224. (Abstr.) [Google Scholar]
- 44.Morinobu A, Kumagai S, Yanagida H, Ota H, Ishida H, Matsui M, Yodoi J, Nakao K. IL-10 suppresses cell surface CD23/Fc epsilon RII expression, not by enhancing soluble CD23 release, but by reducing CD23 mRNA expression in human monocytes. J Clin Immunol. 1996;16:326–33. doi: 10.1007/BF01541668. [DOI] [PubMed] [Google Scholar]
- 45.Becker H, Potyka P, Weber C, Federlin K. Detection of circulating FcεR2/CD23+ monocytes in patients with rheumatic diseases. Clin Exp Immunol. 1991;85:61–65. doi: 10.1111/j.1365-2249.1991.tb05682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luukkainen R, Alanaatu A, Kaarela K, Huhtala H. Predictive value of synovial fluid analysis in rheumatoid arthritis. A 7.5-year follow-up study. Eur J Med. 1993;2:284–6. [PubMed] [Google Scholar]
- 47.Aman S, Risteli J, Luukkainen R, Risteli L, Kauppi M, Nieminen P, Hakal M. The value of synovial fluid analysis in the assessment of knee joint destruction in arthritis in a three year follow up study. Ann Rheum Dis. 1999;58:559–62. doi: 10.1136/ard.58.9.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neidel J, Schulze M, Lindschau J. Association between degree of bone-erosion and synovial fluid-levels of tumor necrosis factor α in the knee-joints of patients with rheumatoid arthritis. Inflamm Res. 1995;44:217–21. doi: 10.1007/BF01782262. [DOI] [PubMed] [Google Scholar]


