Abstract
Immune response to retinal autoantigens plays a central role in the pathogenesis of uveitis. A synthetic peptide (B27PD) from a common sequence of various HLA-B molecules associated with uveitis, such as HLA-B27 and 51, which shares amino acid homologies with a retinal-S antigen (S-Ag)-derived peptide (PDSAg), was shown to be immunogenic in human and experimental uveitis in the rat. In this study we investigated T cell responses to B27PD and PDSAg in patients with Behçet’s disease and posterior uveitis (BD-posterior uveitis; n = 33) in comparison with non-Behçet anterior uveitis (AU, n = 14), Behçet’s patients without uveitis (BD, n = 15) and healthy controls (HC, n = 32) in a 6-day proliferation assay. Patients with BD and posterior uveitis had significantly higher responses (stimulation index (SI) 2·8 ± 1·3) than those with AU (SI 1·5 ± 0·4), BD without uveitis (SI 1·1 ± 0·4) and HC (SI 1·1 ± 0·6) for B27PD (P < 0·0001). Responses to PDSAg were also higher in BD with posterior uveitis patients (SI 3·3 ± 1·6) than AU (SI 1·5 ± 0·4), BD without uveitis (SI 1·2 ± 0·3) and HC (SI 1·1 ± 0·6) (P < 0·0001). A significant correlation between the responses to PDSAg and B27PD (r = 0·56, P < 0·001) was observed. Elevated levels of IL-2 and tumour necrosis factor-alpha were also observed in culture supernatants obtained from peripheral blood mononuclear cells after stimulation with the peptides, but no correlation was found between the proliferative responses and cytokine levels. These results suggest that cellular immunity to cross-reactive HLA-B and S-Ag-derived peptides might play a role in the pathogenesis of posterior uveitis in BD.
Keywords: Behçet’s disease, uveitis, retinal autoimmunity, HLA-B27, B51
INTRODUCTION
Behçet’s disease (BD) is a systemic vasculitis of unknown aetiology [1]. The clinical spectrum may vary from mild mucocutaneous involvement with recurrent oro-genital ulcerations and joint involvement to severe neurological or ocular manifestations which may lead to blindness in 10–20% of patients. Histopathological studies of BD lesions demonstrated either polymorphonuclear and mononuclear or T cell-dominated infiltrates in pathergy reactions, erythema nodosum-like lesions and in enucleated eyes [2–5]. Furthermore, changes in peripheral TCR Vβ repertoire [6], decreased CD45RA+‘naive’ and increased CD45RO+‘memory’ cells [7–9] suggest the role of cellular immunity in the pathogenesis of BD.
Uveitis is a T cell-mediated disease and leads to decreased vision and sometimes retinal destruction, resulting in blindness [10]. Retinal soluble antigen (S-Ag) is one of the best characterized retinal antigens which is capable of inducing experimental autoimmune uveitis (EAU) in rats and thus assumed to be the retinal target of autoreactive cells [11]. Cellular immune response to S-Ag has been shown in both anterior uveitis patients and in BD patients with uveitis [12,13]. Recurrent posterior uveitis is one of the leading causes of disability due to blindness in BD. In some autoimmune uveitis entities, associations with certain HLA antigens have been described, such as anterior uveitis with HLA-B27 [14] and Birdshot chorioretinopathy with HLA-A29 [15]. For ocular involvement in BD, an association with HLA-B51 was described [16,17]. However, the relationship between a CD4+ T cell-mediated, HLA-class II-restricted immune response and disease-associated HLA class I antigens is unclear. Recently, peptides derived from degraded class I antigens were shown to be presented by HLA class II molecules [18]. In inflammatory conditions, aberrant assembly or enhanced expression of MHC antigens may probably increase the presentation of class I peptides on class II antigens. Wildner et al. have described a peptide from a polymorphic region of HLA-B molecules associated with uveitis, such as HLA-B27 and 51 (B27PD), which shares amino acid homologies with a retinal-S Ag-derived peptide (PDSAg) and probably also with interphotoreceptor binding protein (IRBP)-derived peptides [19,20]. This peptide could not only induce uveitis in animals, but also reduced the severity of S-Ag-induced EAU when given by the oral route (oral tolerance) [19]. A first successful therapeutic trial using oral tolerance induced with peptide B27PD for patients with uveitis was reported [21]. Another HLA-B peptide which was suggested as a possible trigger for pathogenesis has been recently described for ankylosing spondylitis, a disease highly associated with HLA-B27 [22].
In this study we investigated whether peripheral blood lymphocytes (PBL) from BD patients with uveitis (BD-posterior uveitis) responded to B27PD and PDSAg and compared our results with those from patients with HLA-B27-associated anterior uveitis (AU), HLA-B51+ BD patients without uveitis (BD) and healthy controls (HC).
PATIENTS and METHODS
Patients and controls
This study was approved by the University of Marmara Institutional review board. Thirty-three uveitis patients with BD who fulfilled The International Study Group Criteria for BD (23 males and 10 females, age 26 ± 7 years) and were followed in Cerrahpasa and Marmara Medical School, Departments of Ophthalmology and Rheumatology in Istanbul, were included in this study [23]. Their disease duration was between 3 months and 8 years (mean 39 ± 51 months). As current therapies for uveitis they received combined therapy with cyclosporin and azathioprine (n = 15), azathioprine only (n = 7), cyclosporin only (n = 4), cyclophosphamide only (n = 4) and oral corticosteroids (60 mg/day) in three out of 33 patients. All patients had active posterior uveitis with or without retinal vasculitis at the time of the study. We also evaluated PBL samples from 15 BD patients without uveitis. None of them had uveitis either in their previous history or in current ophthalmic examination (six males, nine females, mean age 35 ± 3 years). They were under treatment with colchicine, non-steroidal anti-inflammatory drugs or topical corticosteroids. Two patients were using azathioprine for vascular involvement and one cyclophosphamide for neuro-BD. All BD patients were HLA-B51+, which was determined by standard microcytotoxicity assays.
Fourteen patients with anterior uveitis (AU, 12 with ankylosing spondylitis, two with idiopathic uveitis) who were HLA-B27+ were studied as diseased controls (10 males, four females, mean age 30 ± 9 years). Thirty-two healthy individuals (HC, 24 males, eight females, mean age 30 ± 8 years) were also studied. None of them had a personal or family history of BD or evidence of uveitis.
Antigens
Retinal S-Ag was prepared from bovine eyes as described [24]. Synthetic peptides PDSAg and B27PD were purchased from Biotrend (Cologne, Germany). Peptide B7PD (Biotrend) representing the same epitope as B27PD but differing by a single amino acid and shown to be non-uveitogenic previously, was used as a control [19]. Amino acid sequences of the peptides are shown in Table 1. All peptides were dissolved in RPMI 1640. Phytohaemagglutinin (PHA-p; Sigma, St Louis, MO) was used at a concentration of 10 μg/ml as a positive control for T cells.
Table 1.
Amino acid sequences of retinal-S-Ag and HLA peptides
| Source of peptides | Amino acid position | Designation | Amino acid sequence |
|---|---|---|---|
| S-Ag | aa 342–355 | PDSAg | FLGELTSS EVATEV |
| HLA-B (e.g. B27,B51) | aa 125–138 | B27PD | ALNEDLSSWTAADT |
| HLA-B7 | aa 125–138 | B7PD | ALNEDLRSWTAADT |
Sequence similarities (in bold letters) between PDSAg and B27PD are shown. B7PD differs from B27PD in only amino acid and shows no antigenic mimicry with S-Ag peptide.
Separation of PBL and proliferation assay
PBL from patients and controls were isolated by centrifugation on a Ficoll–Hypaque gradient for 20 min, washed twice and resuspended in culture medium RPMI 1640 supplemented with 2 mm l-glutamine (Sigma), 100 U penicillin G (Sigma), 100 μg/ml streptomycin sulphate and 10% heat-inactivated autologous human serum. Cells (1 × 105/well) were incubated in a total volume of 200 μl with the peptides or PHA in triplicates in 96-well round-bottomed culture plates (Nunc, Roskilde, Denmark) at 37°C in a humidified atmosphere with 5% CO2. Culture supernatant (100 μl) was collected after 48 h and 96 h of culture and fresh medium was added. Cultures were incubated for 6 days and then 0·3 μCi 3H-thymidine was added to each well for the last 16 h. Cells were harvested and incorporated radioactivity was determined by a liquid scintillation counter. The results are given as a stimulation index (SI: (mean ct/min with antigen)/(mean ct/min without antigen)).
Determination of cytokines
IL-2 and tumour necrosis factor-alpha (TNF-α) levels were assayed in culture supernatants collected at 48 h and 96 h of culture. Only BD patients with posterior uveitis and healthy controls were used in these experiments. Cytokine levels were determined by standard ELISA procedures according to the manufacturer’s instructions (Duo-Set; Genzyme, Cambridge, MA). The higher cytokine value is given and levels exceeding 15 pg/ml were defined as positive responses.
Statistical analysis
Statistical evaluation of proliferative responses was performed by Mann–Whitney U-test, using SPSS 5.0. Results are given as mean ±s.d. The correlations between the T cell responses were evaluated by Spearman’s rank correlation analysis. P <0·05 was regarded as significant.
RESULTS
Proliferative responses of PBL to S-Ag, PDSAg and B27PD
In a prior dose–response study, optimal concentrations of the peptides were determined at 20 μg/ml and S-Ag at 10 μg/ml. S-Ag induced positive PBL proliferation (SI > 2) in 23 of the 33 BD-posterior uveitis patients (69·6%) (SI 3·1 ± 1·7). This was significantly higher compared with patients with AU (SI 1·1 ± 0·4), BD without uveitis (1·1 ± 0·5) and HC (1·4 ± 0·7) (P < 0·0001 BD-posterior uveitis versus AU, BD and HC). Responses to PDSAg were also higher in BD-posterior uveitis patients (SI 3·3 ± 1·6) (22/33, 67% positive) than in patients with AU (1·5 ± 0·4), BD without uveitis (1·2 ± 0·3) and HC (1·1± 0·6). The differences were statistically significant (P < 0·0001 BD-posterior uveitis versus AU, BD and HC) (Fig. 1).
Fig. 1.
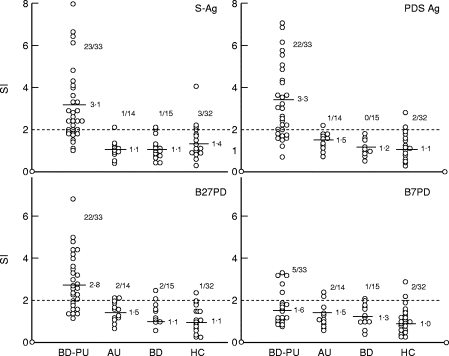
Responses of peripheral blood lymphocytes (PBL) to S-Ag, PDSAg, B27PD and B7PD in a standard proliferation assay. All results are given as stimulation index (SI). Number of patients with positive results (SI > 2) with mean values for each group is given. The mean ct/min of Behçet’s disease (BD)-posterior uveitis patients are 2550 for S-Ag, 3441 for PDSAg, 4149 for B27PD, 1664 for B7PD and 7597 for phytohaemagglutinin (PHA). Statistically significant responses to the peptides were obtained from BD patients with posterior uveitis (BD-PU) in comparison with anterior uveitis (AU), BD and healthy blood donors (HC), except B7PD.
BD-posterior uveitis patients had also higher responses to B27PD (SI 2·8 ± 1·3) (22/33, 67% positive) than patients with AU (SI 1·5 ± 0·4), BD without uveitis (SI 1·1 ± 0·4) and HC (SI 1·1±0·6) (P < 0·0001 BD-posterior uveitis versus AU, BD and HC).
Fifteen patients with BD-posterior uveitis (46%) responded to all three antigens, nine (27%) patients only to PDSAg and S-Ag, six (18%) to only B27PD and PDSAg, and three of 33 (9%) responded only to S-Ag. Responses to control peptide B7PD were not statistically different between the four groups of patients and controls (BD-posterior uveitis, 1·6 ± 1·0 (5/33, 15% positive); AU, 1·5 ± 1·3; BD, 1·3 ± 0·9; HC, 1·0 ± 0·6) (Fig. 1).
In BD-posterior uveitis, a significant correlation was observed between the responses to PDSAg and B27PD (r = 0·56, P < 0·001), but no correlations were observed between S-Ag and PDSAg (r = −0·06) and S-Ag and B27PD (r = 0·09) (Fig. 2). There were also no correlations between the proliferative responses to the peptides and sex, age, treatment or duration of uveitis.
Fig. 2.
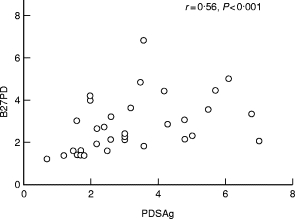
The correlation between the proliferative responses to B27PD and PDSAg in Behçet’s disease (BD) patients with posterior uveitis
Secretion of IL-2 and TNF-α
Increased IL-2 levels in supernatants of unstimulated wells from five of nine (56%) patients with BD-posterior uveitis were found. In three of these patients, further increase in IL-2 levels could not be induced by antigenic stimulation (Table 2). However, PBL from five patients responded to B27PD, three to S-Ag and two to PDSAg with elevated IL-2 levels compared with unstimulated cultures. One healthy control had a high spontaneous release of IL-2, two had an increased secretion in response to PDSAg and one each to S-Ag and B27PD.
Table 2.
IL-2, tumour necrosis factor-alpha (TNF-α) and proliferative responses to S-Ag, PDSAg and B27PD in Behçet’s disease (BD) patients with uveitis (UV) and healthy controls (HC)
| SI | IL-2 (pg/ml) | TNF-α (pg/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S-Ag | PDSAg | B27PD | Without antigen | S-Ag | PDSAg | B27PD | Without antigen | S‐Ag | PDSAg | B27PD | |
| UV | 1·7 | 7 | 2 | * | * | * | 37 | 51 | 15 | * | 48 |
| UV | 2 | 1·2 | 1·4 | * | * | * | 116 | 15 | 36 | * | 21 |
| UV | 1·8 | 0·7 | 1·2 | * | 37 | 154 | 89 | * | * | 558 | 181 |
| UV | 1·9 | 1·6 | 1·4 | 186 | 197 | 318 | 37 | * | * | 302 | 205 |
| UV | 6·1 | 3·5 | 4·8 | 173 | 154 | * | * | * | * | * | 16 |
| UV | 2·4 | 1·6 | 3 | * | * | * | 67 | * | * | * | * |
| UV | 1·1 | 2·2 | 2·6 | 167 | 279 | 75 | 296 | * | 17 | 179 | 307 |
| UV | 1·5 | 3 | 2·4 | 746 | 64 | 29 | 24 | * | * | * | * |
| UV | 3·6 | 3·6 | 1·8 | 38 | * | * | * | * | 43 | * | * |
| HC | 2 | 0·9 | 1·1 | * | * | 26 | * | * | 85 | 43 | 25 |
| HC | 1·4 | 1·2 | 2 | * | 69 | 46 | * | * | * | * | * |
| HC | 1·4 | 0·9 | 1·5 | * | * | * | 116 | * | * | * | * |
| HC | 1 | 1·1 | 0·7 | 17 | * | * | * | * | * | * | * |
| HC | 0·9 | 1·1 | 0·8 | * | * | * | * | * | * | * | * |
< 15 pg/ml. Culture supernatants collected at 48 h and 96 h were used for analysis. The higher cytokine value is given.
SI, Stimulation index; UV, uveitis; HC, healthy controls.
Only two BD patients had high TNF-α levels in unstimulated cultures (Table 2). PBL from five of nine BD-posterior uveitis patients showed increased secretion of TNF-α after stimulation with B27PD. Stimulation with PDSAg or S-Ag led to enhanced TNF-α production in three patients each. Only one healthy donor showed increased TNF-α secretion in response to S-Ag, PDSAg and B27PD. Interestingly, a correlation between peptide-specific T cell proliferation and production of cytokines such as IL-2 and TNF-α could not be observed.
DISCUSSION
Antigenic mimicry between a peptide from retinal S-Ag and a peptide from a polymorphic region of HLA-B was postulated as a mechanism for the induction of organ-specific autoaggressive immune response in endogenous human uveitis [19]. PBL from patients with different entities of uveitis (anterior, intermediate, posterior uveitis with or without underlying systemic diseases) can proliferate in response to S-Ag peptide PDSAg as well as to the HLA-peptide B27PD. Peptide B27PD represents a region not only specific for B27 but for several other HLA-B antigens, including B51. Therefore we investigated responses of PBL from B51+ BD patients with or without ocular involvement to these peptides. Although all patients with posterior uveitis were under immunosuppressive therapy, including selective T lymphocyte inhibitor cyclosporin, we obtained peptide-specific responses from PBL of these patients.
In BD, with its severe uveitis and also multiple inflammatory manifestations, an enhanced general autoreactivity with elevated cytokine productions such as TNF-α, IL-2, IL-6, IL-8 and interferon-gamma (IFN-γ) [25–29] might cause a highly increased expression of MHC class I and class II antigens, probably resulting in an enhanced presentation of an epitope equivalent to class I peptide B27PD on class II antigens. This could cause the activation of peripheral B27PD-specific T lymphocytes, and consequently PDS-Ag cross-reactive T cells. An autoimmune disease with high peripheral immunological activity (increased proinflammatory cytokines, activation of innate immunity, etc.) such as BD could therefore increase the probability of detecting autoreactive T cells. In case of Behçet’s uveitis, vasculitis of retinal vessels might have destroyed the blood–retina barrier and therefore facilitated immigration of pathogenic effector cells. These can cause severe retinal damage, which enhances stimulation of retinal autoantigen-specific T cells. This could result in a perpetuation of cross-reactive, autoaggressive T cell responses to B27PD and PDSAg. Alternatively, enhanced MHC expression without activation of costimulatory molecules might lead to immune tolerance, but observation of increased T cell responses only in active uveitis patients suggests that T cell activity is possibly pathogenic in our group.
We found only low responses to S-Ag, PDSAg and B27PD in non-Behçet’s patients with anterior uveitis. It has been shown that PBL responses to retinal autoantigens are not detectable at all time points, even when patients with uveitis (not related to BD) suffer from acute or chronic intra-ocular inflammation [21], and therefore reactivity in the periphery may not always represent the autoimmune response within the target organ. We showed that PBL of patients with ocular BD could secrete TNF-α after incubation with B27PD. TNF-α, a proinflammatory cytokine, is produced by many cell types, including macrophages, T cells and natural killer cells. TNF-α is postulated to play a central role in experimental and clinical autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and uveitis [30]. Regarding TNF-α production in BD, variable responses were observed with blood monocytes in vitro[25]. An increase in spontaneous secretion of TNF-α is reported from patients with active disease [27], which might be linked to genetic polymorphisms of TNF-α or its promoter [17,31]. Animal models of uveitis suggest that the ability to produce TNF-α contributes to susceptibility to EAU [30]. Proinflammatory effects of TNF-α include induction and/or up-regulation of the expression of MHC class I and class II molecules as well as IL-2 receptors. We observed spontaneous production of the Th1 cytokine IL-2 in five of nine patients with ocular BD, as was also shown by Sugi-Ikai et al. with intracellular cytokine staining of PBL from BD patients [29]. With regard to the pathogenesis of uveitis in BD, we speculate that increased secretion of Th1-type cytokines and TNF-α leads to over-expression of MHC antigens, which then might activate B27PD-specific T cells that cause uveitis.
Our results suggest that uveitis in BD might have similar pathogenic features as proposed for other uveitis entities, although posterior uveitis in BD is usually more destructive than other forms of uveitis. Studies of the characteristics of the T cells responding to HLA-peptide B27PD are underway. With respect to animal studies, where peptide B27PD was used as an effective oral tolerogen [19], and a successful first therapeutic trial for patients with various uveitis entities (anterior, intermediate, posterior uveitis, ankylosing spondylitis, sarcoidosis) [21], we believe that oral tolerance induction with the HLA peptide B27PD might also be a useful, non-toxic therapeutic approach for patients with uveitis in Behçet’s disease.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (project Wi 1382/1-1) and the Turkish Scientific and Technical Research Council (TUBITAK; project no. SBAG 1849).
REFERENCES
- 1.Yazici H, Yurdakul S, Hamuryudan V. Behçet’s syndrome. Curr Opin Rheumatol. 1999;11:53–57. doi: 10.1097/00002281-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ergun T, Gürbüz O, Harvell J, Jorizzo J, White W. The histopathology of pathergy: a chronological study of skin hyperreactivity in Behçet’s disease. Int J Dermatol. 1998;37:929–33. doi: 10.1046/j.1365-4362.1998.00474.x. [DOI] [PubMed] [Google Scholar]
- 3.Gül A, Esin S, Dilsen N, Koniçe M, Wigzell H, Biberfeld P. Immunohistology of skin pathergy reaction in Behçet’s disease. Br J Dermatol. 1995;132:901–7. doi: 10.1111/j.1365-2133.1995.tb16946.x. [DOI] [PubMed] [Google Scholar]
- 4.Poulter LW, Lehner T. Immunohistology of oral lesions from patients with recurrent oral ulcers and Behçet’s syndrome. Clin Exp Immunol. 1989;78:189–95. [PMC free article] [PubMed] [Google Scholar]
- 5.Charteris DG, Barton K, McCartney ACE, et al. CD4+ lymphocyte involvement in ocular Behçet’s disease. Autoimmunity. 1992;12:201–6. doi: 10.3109/08916939209148460. [DOI] [PubMed] [Google Scholar]
- 6.Direskeneli H, Eksioglu-Demiralp E, Kibaroglu A, et al. Oligoclonal T cell expansions in patients with Behçet’s disease. Clin Exp Immunol. 1999;117:166–70. doi: 10.1046/j.1365-2249.1999.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahan A, Hamzaoui K, Ayed K. Abnormalities of T lymphocyte subsets in Behçet’s disease demonstrated with anti-CD45RA and anti-CD29 monoclonal antibodies. J Rheumatol. 1992;19:742–6. [PubMed] [Google Scholar]
- 8.Fortune F, Walker J, Lehner T. The expression of γδ T cell receptor and the prevalence of primed, activated and IgA-bound T cells in Behçet’s syndrome. Clin Exp Immunol. 1990;82:326–32. doi: 10.1111/j.1365-2249.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eksioglu-Demiralp E, Kibaroglu A, Direskeneli H, et al. Phenotypic characteristics of B cells in Behçet’s disease: increased activity in B cell subsets. J Rheumatol. 1999;26:526–32. [PubMed] [Google Scholar]
- 10.Forrester JV. Uveitis: pathogenesis. Lancet. 1991;338:1498–501. doi: 10.1016/0140-6736(91)92309-p. [DOI] [PubMed] [Google Scholar]
- 11.De Kozak Y, Sakai J, Faure JP. S-Antigen induced experimental autoimmune uveo-retinitis in rats. Curr Eye Res. 1981;6:327–37. doi: 10.3109/02713688108998359. [DOI] [PubMed] [Google Scholar]
- 12.De Smet MD, Yamamoto JH, Mochizuki M, et al. Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am J Ophthalmol. 1990;110:135–42. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto JH, Minami M, Inaba G, et al. Cellular autoimmunity to retinal specific antigens in patients with Behçet’s disease. Br J Ophthalmol. 1993;77:584–9. doi: 10.1136/bjo.77.9.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewerton DA, Caffrey M, Nicholls A, Walters D, James DC. Acute-anterior uveitis and HLA-B27. Lancet. 1973;2:994–6. doi: 10.1016/s0140-6736(73)91090-8. [DOI] [PubMed] [Google Scholar]
- 15.Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal-S antigen. Am J Ophthalmol. 1982;94:147–58. doi: 10.1016/0002-9394(82)90069-1. [DOI] [PubMed] [Google Scholar]
- 16.Sakamato M, Akazawa K, Nishioka Y, et al. Prognostic factors of vision in patients with Behçet’s disease. Ophthalmology. 1995;102:317–21. doi: 10.1016/s0161-6420(95)31022-6. [DOI] [PubMed] [Google Scholar]
- 17.Verity DH, Wallace GR, Vaughan RW, et al. HLA and tumour necrosis factor (TNF) polymorphisms in ocular Behçet’s disease. Tissue Antigens. 1999;54:264–72. doi: 10.1034/j.1399-0039.1999.540307.x. [DOI] [PubMed] [Google Scholar]
- 18.Chicz RM, Urban RG, Gorga JC, et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildner G, Thurau SR. Cross-reactivity between an HLA-B27-derived peptide and a retinal autoantigen peptide: a clue to major histocompatibility complex association with autoimmune disease. Eur J Immunol. 1994;24:2579–85. doi: 10.1002/eji.1830241103. [DOI] [PubMed] [Google Scholar]
- 20.Wildner G, Thurau SR. Database screening for molecular mimicry. Immunol Today. 1997;18:252. doi: 10.1016/s0167-5699(97)90086-4. [DOI] [PubMed] [Google Scholar]
- 21.Thurau SR, Diedrichs-Möhring M, Fricke H, Arbogast S, Wildner G. Molecular mimicry as a therapeutic approach for an autoimmune disease: oral treatment of uveitis patients with an MHC-peptide crossreactive with autoantigen—first results. Immunol Letters. 1997;57:193–201. doi: 10.1016/s0165-2478(97)00058-8. [DOI] [PubMed] [Google Scholar]
- 22.Marker-Hermann E, Meyer zum Buschenfelde KH, Wildner G. HLA B-27-derived peptides as autoantigens for T lymphocytes in ankylosing spondylitis. Arthritis Rheum. 1997;11:2047–54. doi: 10.1002/art.1780401118. [DOI] [PubMed] [Google Scholar]
- 23.International Study Group for Behçet’s Disease. Criteria for diagnosis of Behçet’s disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 24.Dorey C, Cozette J, Faura JP. A simple and rapid method for isolation of retinal-S antigen. Ophthalmic Res. 1982;14:249–55. doi: 10.1159/000265199. [DOI] [PubMed] [Google Scholar]
- 25.Verity DH, Holland-Gladwish J, Kanawati C, et al. TNF production by peripheral blood monocytes in Arabic patients with Behçet’s disease from Israel and Jordan (Abstract) Rev Rheum. 1996;7–8:529, A03. [Google Scholar]
- 26.Akoglu TF, Direskeneli H, Yazici H, et al. TNF-α, soluble-IL-2 and soluble CD-8 in Behçet’s disease. J Rheumatol. 1990;17:1107–8. [PubMed] [Google Scholar]
- 27.Mege JL, Dilsen N, Sanguedolce V, et al. Overproduction of monocyte derived TNF-α, IL-6, IL-8 and increased neutrophil superoxide generation in Behçet’s disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol. 1993;20:1544–9. [PubMed] [Google Scholar]
- 28.BenEzra D, Maftzir G, Kalichman I, et al. Serum levels of IL-2R in ocular Behçet’s disease. Am J Ophthalmol. 1993;115:26–30. doi: 10.1016/s0002-9394(14)73520-2. [DOI] [PubMed] [Google Scholar]
- 29.Sugi-Ikai N, Nakazawa M, Nakamura S, et al. Increased frequencies of interleukin-2 and interferon γ producing T cells in patients with active Behçet’s disease. Invest Ophthalmol Vis Sci. 1998;39:996–1004. [PubMed] [Google Scholar]
- 30.Nakamura S, Yamakawa T, Sugita M, et al. The role of tumor necrosis factor-alpha in the induction of experimental autoimmune uveoretinitis in mice. Invest Ophthalmol Vis Sci. 1994;35:3884–9. [PubMed] [Google Scholar]
- 31.Mizuki N, Inoko H, Sugimura K, et al. RFLP analysis in the TNF-β gene and the susceptibility to alloreactive NK cells in Behçet’s disease. Invest Ophthalmol Vis Sci. 1992;33:3084–90. [PubMed] [Google Scholar]


