Abstract
In coeliac disease, gluten-containing diet challenges over many years are sometimes required for diagnosis, especially if the initial diagnosis was equivocal. The rectal gluten challenge has been proposed to simplify coeliac disease diagnosis. We were interested in studying whether the oral mucosa could be used for local challenge with gliadin as an aid in finalizing the diagnosis of coeliac disease. The study groups consisted of 37 treated coeliac disease patients and 10 controls. The challenges on the oral mucosa were performed either supramucosally with gliadin powder (coeliac disease patients) or by submucosal injection of dissolved gliadin (10 μg/ml) (coeliac disease patients and controls). A control challenge with submucosal gliadin solvent was made in the coeliac disease patients. B and T cells, mast cells and T cell subsets were counted and HLA-DR expression was determined. Biopsies were taken from each provoked area 24 h post-challenge. A significant increase in the number of CD4+ lymphocytes in the lamina propria (observed in 27/37 patients), but a decrease in the number of mast cells was observed in treated coeliac disease patients after submucosal challenge with gliadin. Following supramucosal challenge with gliadin the counts of intraepithelial CD4+ (in 25/37 patients) and CD8+ T cells (in 27/37 patients) increased significantly and the number of CD4+ T cells in the lamina propria was also significantly increased. Control subjects were tested by submucosal gliadin challenge and no significant changes in the number of cells were observed. HLA-DR expression did not show increased positivity in coeliac disease patients on submucosal challenge. For the first time the oral mucosa has been used for immunological testing and shown to react to gliadin challenge in coeliac disease patients. Recruitment of T cells upon submucosal gliadin challenge occurred towards the lamina propria, whereas it occurred towards the epithelium in supramucosal gliadin challenge. The numbers of T cells increased in the lamina propria after submucosal challenge. The results suggest that local oral challenge with gliadin may be used as a diagnostic method in coeliac disease; however, further studies in untreated coeliac disease patients are needed to evaluate the usefulness of this method.
Keywords: coeliac disease, oral mucosa, gluten challenge, T cells, T cell subsets, HLA-DR expression, late immune response
INTRODUCTION
Patients with coeliac disease are permanently sensitized to cereal gluten, resulting in jejunal villous atrophy and cryptal hyperplasia, histological changes that are typical for coeliac disease [1–3]. Several studies suggest that activated intestinal intraepithelial lymphocytes (IELs) and lymphocytes in the lamina propria, mostly bearing the T cell receptor αβ (TCRαβ), may play a role in the development of villous atrophy. One characteristic feature of coeliac disease is the prominent increase in IELs, especially IELs bearing a γδ T cell receptor (TCRγδ). There is also a considerable expansion of CD4+ and CD8+ TCRαβ+ IELs in untreated coeliac disease [4–6]. In addition, the expression of mucosal HLA-DR is enhanced in the gut mucosa in untreated coeliac disease [7]. After the eviction of gluten, the number of TCRγδ+ IELs remains increased, but the number of CD8+ TCRαβ+ IELs returns to normal. The only gliadin-specific T cell clones derived from the intestine of patients with coeliac disease, however, are of the CD4+ TCRαβ+ phenotype [8,9].
As most research has concentrated on assessing the effects of gluten on the jejunal mucosa, the idea that gluten or other prolamins may also damage other mucosal surfaces within the gut-associated lymphoid tissue (GALT) in gluten-sensitized persons has been largely neglected [6,10]. Two sites are of particular interest: the mouth as the first part of the gastrointestinal tract, and the rectum at its end. In fact, rectal gluten challenges have previously been performed [11–15], but the mouth has never been used as an area of immunological testing. In their extensive studies on the rectal mucosa, Loft and co-workers showed a primary response to local gluten challenge with a rapid increase in lamina propria volume and a dramatic decline in the number of granulated mast cells. Lymphocytes emigrated into the lamina propria and the number of IEL increased progressively. Loft et al. showed that a 10% increase in epithelial lymphocyte populations could be used to predict gluten sensitivity with 90% sensitivity and 91% specificity [13]. In a recent study on rectal gluten challenge with gliadin enema in a paediatric setting a substantial infiltration of IEL was noted, showing a recruitment of CD3 and TCRγδ+ lymphocytes [15].
In the present study, the oral mucosa was used as a site for immunological testing for the first time to our knowledge in the diagnosis of coeliac disease. We hypothesized that in coeliac disease there is an immunopathological response of the oral mucosa to local gliadin challenge, which might offer the easiest access for testing the gastrointestinal system, and thus be helpful in the diagnosis of coeliac disease.
MATERIALS and METHODS
Patients
Thirty-seven patients with gluten-free diet (GFD)-treated coeliac disease (29 women and eight men, mean age 46.6 years, range 24–65 years) were enrolled in this study from among the members of the Coeliac Association in Turku, Finland. Coeliac disease had been confirmed by small intestine biopsies. All but one of the 37 treated patients were negative for serum endomysial antibodies (EmA) [16]. Ten previously healthy volunteers (seven women and three men, mean age 56.1 years, range 42–75 years) formed the control group for the coeliac disease patients.
The study was authorized by the Ethical Committee of the Faculty of Medicine, University of Turku.
Gluten challenge
The gluten challenge was performed at supramucosal and submucosal sites as follows. In the supramucosal challenge, 0·020 g gliadin powder (Sigma-Gliadin G 3375; Sigma Chemical Co., St Louis, MO) was applied on the upper labial mucosa in the region of the second upper incisors with the aid of an oral adhesive bandage (SQUIBB ConvaTec ORAHESIVE; Princeton, NJ). It was intended that the oral bandage should stay in place overnight. A stock solution (2 mg/ml in 50% ethanol) was prepared for the submucosal gliadin challenge. For the experiment 0·3 ml of the solution with a final concentration of 10 μg gliadin/ml (further dilution in 0·9% NaCl; Orion, Helsinki, Finland) was injected into the buccal submucosa (beneath the occlusal line) of patients with treated coeliac disease and the controls. In addition, as a control to the injected gliadin solution, 0·3 ml of the dilution solution without gliadin was injected into the contralateral buccal mucosa of 12/37 patients with coeliac disease. The biopsies were taken from each provoked area 24 h post-challenge. A biopsy specimen of buccal mucosa taken 1 month earlier from a different area than that used in the provocation served as a control.
Clinical examination of oral mucosa
The oral mucosal clinical changes were registered after challenges, as were the oral symptoms reported by the study subjects.
Biopsy specimen processing and immunohistochemical staining
The oral mucosal biopsies were taken under local anaesthesia (Xylocain adrenalin; Astra, Södertälje, Sweden). Each specimen was divided into two parts. One part was processed using routine histological methods, fixed in formalin and embedded in paraffin. Mast cells were stained with toluidine blue. The presence of B and T lymphocytes was detected using MoAb CD20+ and MoAb CD45RO+, respectively, in sequential sections. The MoAbs were provided by Dako (Glostrup, Denmark). The sections were placed on organosilane-coated slides, deparaffinized in xylene and rehydrated with graded alcohol. The sections were then treated with pepsin at 37°C for 10 min. Endogenous peroxidase activity was blocked using 5% H2O2 for 5 min. The sections were first incubated with 1·5% normal goat serum (Vector Labs, Burlingame, CA) for 15 min, followed by incubation with primary antibody (in blocking serum) overnight at 4°C. Then the sections were incubated with secondary biotinylated antibody (anti-mouse IgG; Vector Labs) for 30 min, followed by incubation with avidin-biotin-peroxidase complex (Vectastain Elite ABC Kit; Vector Labs) for 30 min. The immunoperoxidase reaction was developed using 3,3-diaminobenzidine (DAB; Sigma) for 5 min. Finally, the sections were counterstained with Meyer’s haematoxylin, dehydrated and mounted with permount. Positive and negative control sections were included in all stainings. Sections treated with blocking serum without primary antibodies served as negative controls. Formalin-fixed, paraffin-embedded sections from the tonsils and minor salivary glands of patients with Sjögren’s syndrome were used as positive controls.
The other part of the specimen was embedded in optimal cutting temperature (OCT) compound (Miles Labs, Elkhart, IN), snap-frozen in liquid nitrogen, and stored at −70°C until analysis. Sections of 5 μm were fixed in acetone for 10 min and in chloroform for 17 min at 4°C. Thereafter, the specimens were washed three times in PBS pH 7·4. To stain MoAbs, we used the Vectastain Elite ABC kit (Vectastain PK-6102; Vector Labs) according to the manufacturer’s instructions. The sections were covered with diluted MoAb in PBS/0·1% bovine serum albumin (BSA) for 1 h. Endogenous peroxidase was blocked by incubation in 0·3% peroxide in methanol for 30 min. 3-amino-9-ethylcarbazole (Sigma) was diluted in 10 ml N,N-demethylformamide and used as substrate. The slides were counterstained with Harris haematoxylin (Diagnostica Merck, Darmstadt, Germany).
CD3+ intraepithelial T cells were examined using MoAb Leu-4 (Becton Dickinson, San Jose, CA), αβ TCR molecules using MoAb β F1 (Endogen, Woburn, MA) and γδ+ T cells using MoAb panTcRgd (Endogen). To detect CD4+ T cells, MoAb Leu-3a + 3b (Becton Dickinson) was used. For CD8+ cells, MoAb Leu-2a (Becton Dickinson) was used. MoAb Leu-4 was used at a dilution of 1:15, β F1 1:40, panTcRgd 1:140, Leu-3a + 3b 1:20, and Leu-2a 1:30.
Assessment of the immunopositivity
The total counts of the positively stained mast cells, B cells, T cells and CD3+, CD4+, CD8+, TCRαβ+ and TCRγδ+ T cells were quantified in the epithelium and in the lamina propria under a light microscope through a graticule at ×400 flat field objective. Ten fields (0·10 × 0·10 mm) were counted in the buccal epithelium and lamina propria immediately below the basement membrane. The results are given as cells/mm2.
MoAb HLA-DR (Becton Dickinson) at a dilution of 1:1000 was used to stain the epithelium for HLA class II expression. Again 10 fields were counted in the epithelium and lamina propria. The expression of HLA-DR was interpreted as not present or slight, slightly enhanced or enhanced, and the results are given as percentages of the distribution according to the staining intensity.
Blood samples
Serum EmA titres were analysed from the patients with treated coeliac disease at the time of, and 4 weeks after, the challenges. Blood samples for EmA determination were also collected from controls at the time of the challenge.
Serum antibody determinations
Serum EmA (IgA class) was measured by an indirect immunofluorescence method using commercial monkey oesophagus as the substrate (BioSystems, Barcelona, Spain) [3,17].
Statistical analysis
The statistical significance of differences in cell types between the numbers before the challenge and upon supramucosal and submucosal challenge and solvent provocation was tested using a paired t-test with every response, except that for TCRγδ+ cells, which were tested using Wilcoxon’s signed rank test. A Bonferroni correction was done on all comparisons when needed. Differences between patients and controls were tested using a two-sample t-test, with the exception of TCRγδ+ cells, which were tested using Wilcoxon’s rank sum test. P <0·05 was interpreted as statistically significant. Statistical computing was performed with the SAS system for Windows, Release 6.12/1996 and SPSS for Windows, Release 8.01/1998.
RESULTS
We show that the epithelium and lamina propria of oral mucosa both react to local gliadin challenge. The challenge did not have any influence on the coeliac disease-associated serum EmA in patients with coeliac disease 4 weeks after challenge. One patient had an elevated titre for EmA (titre 1:640) at the time of, and 4 weeks after the challenge. At the site of the submucosal gliadin injection 28 out of 37 patients with treated coeliac disease reported some complaint (mild pain or burning sensation), nine patients had mucosal redness and one patient had small blister formation. In the area of supramucosal gliadin challenge, four out of 37 patients with coeliac disease had a burning sensation and two patients had mild blister formation. Two patients reported mild swelling in the area of the supramucosal provocation, and two others had gastrointestinal symptoms. Six patients had mild pain in the contralateral mucosa after the injection of the pure solvent without gliadin. The controls had no oral symptoms or changes after the submucosal injection of gliadin. All control subjects were negative for EmA.
The results of immunohistochemical stainings of mast cells and T cell subsets are given in Tables 1,2 and 3. The data concerning expression of the HLA-DR staining are not shown.
Table 1.
Local submucosal challenge of oral mucosa with gliadin. The presence of mast cells in oral mucosal lamina propria of patients with treated coeliac disease and controls
| Lamina propria | Cells/mm2; mean ±s.d. |
|---|---|
| Coeliac disease patients, n = 37 | |
| Before challenge | 120·8 ± 77·0* |
| Supramucosal challenge | 138·8 ± 80·5 |
| Submucosal challenge with gliadin solution | 74·2 ± 60·4 |
| Solvent provocation, n = 12 | 128·7 ± 55·8 |
| Controls, n = 10 | |
| Before challenge | 114·5 ± 88·1 |
| Submucosal challenge with gliadin solution | 103·4 ± 59·7 |
Before versus submucosal challenge, P = 0·002.
Table 2.
Local challenge of oral mucosa with gliadin. The presence of T cell subsets in the oral mucosal epithelium of patients with treated coeliac disease and healthy controls. The results of solvent provocation without gliadin in patients with treated coeliac disease are also shown
| Cells/mm2; mean ±s.d. | |||||
|---|---|---|---|---|---|
| Epithelium | CD3+ | CD4+ | CD8+ | αβ+ | γδ+ |
| Coeliac disease patients, n = 37 | |||||
| Before challenge | 193·0 ± 76·1* | 76·1 ± 56·2 | 120·9 ± 93·7 | 114·5 ± 81·0**** | 3·7 ± 12·4 |
| Supramucosal challenge | 230·1 ± 162·0 | 162·9 ± 126·6** | 204·1 ± 127·7*** | 125·9 ± 117·3 | 43·7 ± 12·4 |
| with gliadin powder | |||||
| Submucosal challenge | 148·9 ± 85·8 | 90·0 ± 58·3 | 93·3 ± 69·2 | 81·4 ± 53·5 | 1·4 ± 4·2 |
| with gliadin solution | |||||
| Solvent provocation†, n = 12 | 88·0 ± 60·0 | 81·1 ± 71·4 | 95·3 ± 72·6 | 60·1 ± 50·7 | 0 |
| Healthy controls, n = 10 | |||||
| Before challenge | 86·3 ± 86·5 | 46·3 ± 40·3 | 83·8 ± 85·3 | 67·5 ± 61·4 | 1·3 ± 3·5 |
| Submucosal challenge | 80·0 ± 50·0 | 58·9 ± 39·1 | 57·8 ± 38·3 | 84·4 ± 50·8 | 4·0 ± 9·7 |
| with gliadin solution | |||||
Comparison of values in epithelium After a Bonferroni correction
CD patients:
versus submucosal P = 0·034
versus before P = 0·002
versus before P = 0·002
versus submucosal P = 0·052
Controls: Non-significant differences
The counts of cell types before not shown.
Table 3.
Local challenge of oral mucosa with gliadin. The presence of T cell subsets in the oral mucosal lamina propria of patients with treated coeliac disease and healthy controls. The results of solvent provocation without gliadin in patients with treated coeliac disease are also shown
| Cells/mm2; mean ±s.d. | |||||
|---|---|---|---|---|---|
| Lamina propria | CD3+ | CD4+ | CD8+ | αβ+ | γδ+ |
| Coeliac disease patients, n = 37 | |||||
| Before challenge | 498·2 ± 403·1 | 406·1 ± 321·8 | 207·6 ± 152·8 | 276·4 ± 278·3 | 9·1 ± 29·2 |
| Supramucosal challenge | 623·5 ± 551·4 | 557·6 ± 316·5* | 237·2 ± 151·5 | 392·9 ± 314·9 | 2·9 ± 9·7 |
| with gliadin powder | |||||
| Submucosal challenge | 1022·6 ± 1561·8 | 1092·5 ± 1701·6** | 293·3 ± 389·7 | 490·3 ± 603·9 | 7·5 ± 20·5 |
| with gliadin solution | |||||
| Solvent provocation†, n = 12 | 347·8 ± 168·2 | 400·3 ± 323·2 | 208·3 ± 142·8 | 163·3 ± 163·1 | 2·5 ± 8·7 |
| Healthy controls, n = 10 | |||||
| Before challenge | 196·3 ± 150·9 | 222·5 ± 218·5 | 180·0 ± 165·5 | 278·6 ± 258·7 | 0 |
| Submucosal challenge | 258·6 ± 115·1 | 281·1 ± 121·1 | 173·3 ± 85·1 | 241·1 ± 137·8 | 3·0 ± 6·7 |
| with gliadin solution | |||||
Comparison of values in lamina propria after a Bonferroni correction
CD patients
versus before P = 0·038
versus before P = 0·04
Controls: Non-significant.
The counts of cell types before not shown.
Before gliadin challenge
Epithelium
Before gliadin challenge, the intraepithelial T cells were elevated in coeliac disease patients (versus controls, P < 0·001). B cells were seen only occasionally in the coeliac disease patients and they were not detected in the controls (data not shown). The counts of CD3+ cells were significantly higher in the coeliac disease patients than in the controls (P = 0·046, not shown in Table 2). HLA-DR expression was similar in both study groups.
Lamina propria
In the oral mucosa of coeliac disease patients there were more CD3+ lymphocytes in the lamina propria than in that of the controls (P = 0·045, not shown in Table 3). In general, in both study groups the infiltrates of lymphocytes were much more intense immediately adjacent to the basement membrane than deeper in the lamina propria. Only sporadic B cells were detectable in the oral mucosa of the coeliac disease patients. There was no statistically significant difference in the counts of the mast cells between coeliac disease patients and controls. The specimens of patients with coeliac disease and the controls before challenge showed no difference in HLA-DR staining.
Supramucosal gliadin challenge of oral mucosa in coeliac disease patients
The oral adhesive bandages with gliadin remained in the same place as follows: for 6 h in two, 10 h in eight, 20 h in three and 24 h in 24 out of 37 seven patients with coeliac disease.
Epithelium
The counts of CD4+ (P = 0·002) and CD8+ (P = 0·002) T cells increased when compared with those before supramucosal challenge. Supramucosal challenge induced an increase in the expression of HLA-DR positivity which was not however statistically significant (NS).
Lamina propria
There was a significant increase in the number of CD4+ cells upon supramucosal challenge (P = 0·038). The numbers of mast cells in the lamina propria were similar before and after supramucosal challenge. Nor did the expression of HLA-DR show any change.
Submucosal gliadin challenge of oral mucosa
Epithelium
The challenge resulted in a sharp decline in the numbers of T cells (P = 0·014, data not shown), CD3+ (P = 0·034) and TCRαβ+ cells (P = 0·052) in coeliac disease patients, but not in the controls. The expression of HLA-DR remained unchanged in both groups studied.
Lamina propria
In coeliac disease patients there was a highly significant increase in the numbers of total T cells (determined on paraffin-embedded sections, P = 0·005, data not shown) and CD4+ (P = 0·04) T cells after challenge. Importantly, the challenge induced no statistically significant difference in the cell densities of CD3+, CD4+ and TCRαβ+ T cells in healthy control persons. Interestingly, a significant decline in the total numbers of mast cells (P = 0·002) was found in the coeliac disease patients but not in the controls after challenge, indicating the degranulation of mast cells.
No significant enhancement in the expression of HLA-DR in patients with coeliac disease was observed after submucosal gliadin challenge.
Submucosal provocation of oral mucosa with solvent solution without gliadin in 12 coeliac disease patients
Epithelium
No significant differences were detected in the intraepithelial cell counts after the solvent provocation.
Lamina propria
Solvent provocation did not change cell counts in the lamina propria. Enhanced HLA-DR staining was less frequent after solvent provocation (7·9%) than before (15·8%) (data not shown).
Figure 1 illustrates an example of oral mucosal biopsy specimens of a treated coeliac disease patient stained for CD3+, CD4+, CD8+ and TCRαβ+. This patient participated in supramucosal and submucosal gliadin challenge, and in solvent provocation without gliadin. The oral mucosa reacted strongly, with an increase of CD3+ and CD4+ T cells to submucosal challenge with gliadin solution. HLA-DR was also expressed strongly after submucosal gliadin challenge.
Fig. 1.
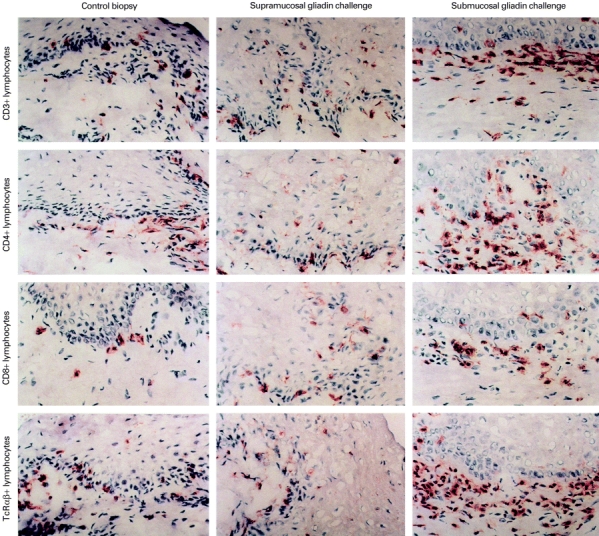
(See next page) Immunohistochemical staining of CD3+, CD4+, CD8+ and TCRαβ+ cells in the oral mucosa. A panel of examples of oral mucosal slides in patients with treated coeliac disease before (left panel) and after local oral challenge with gliadin made supramucosally with gliadin powder (middle panel), and submucosally with dissolved gliadin (right panel).
Summary of the results
Both supramucosal and submucosal gliadin challenge distinguished coeliac disease patients from the controls. After supramucosal gliadin challenge, the number of intraepithelial CD4+ and CD8+ T cells was increased in 25 out of 37 and in 27 out of 37 coeliac disease patients, respectively. In this study, controls were not tested for supramucosal gliadin. After submucosal gliadin challenge, CD4+ T cells increased in number in the lamina propria in 27 out of 37 coeliac disease patients but in only two out of 10 controls. Based on these counts the sensitivity of submucosal gliadin challenge was 73% (27/37) and the specificity 80% (8/10).
Since the controls were not tested for supramucosal gliadin, the sensitivity and specificity cannot be counted. The positive predictive value of submucosal gliadin challenge was 93% (27/29) and the negative predictive value 44% (8/18).
DISCUSSION
For the first time we have used the oral mucosa as an immunological testing site in the diagnosis of coeliac disease. The ultimate aim of our study was to investigate whether a local oral challenge with gliadin could provoke oral mucosal changes typical only for coeliac disease, and thus be helpful for diagnosis and serve as a challenge method when needed [18]. Our results support the hypothesis that the oral mucosa responds to gliadin when locally provoked in coeliac disease patients. This does not happen in healthy individuals.
Normal mucosa of the lips and cheeks is quite different from the gut mucosa and consists of a non-keratinized stratified squamous epithelium, basal cell layer with basement membrane, lamina propria, submucosal connective tissue and fat. A single line of epithelial, mostly cylindrical, cells covers the inner surface of the gut. The mouth is the first part of the gastrointestinal system and a part of the GALT [6]. The extravasation of primed memory or effector cells is particularly effective in the gut mucosa, but dissemination takes place from GALT to more distant effector sites and also to the oral mucosa and salivary glands [6]. Therefore, although the structure of the oral mucosa differs from that of the gut, we expected the oral mucosa to react when challenged with gliadin.
In an earlier study on small intestinal gliadin challenge the initial damage to the gut mucosa was observed at 1 h after the infusion and it was most marked at 4–6 h, after which the mucosa gradually improved. On the other hand, the inflammatory response in rectal gluten challenge reached its first peak at 24 h [13]. On the basis of that study, we took the biopsies 24 h post-challenge. No earlier studies exist for oral gluten challenge.
The present study clearly shows that supramucosal gliadin challenge significantly increases the recruitment of CD4+ and CD8+ T cells intraepithelially in the oral mucosa of coeliac disease patients, suggesting an increased traffic of the cells across the basement membrane towards the epithelium. Furthermore, the submucosal gliadin challenge induced a significant increase in the number of CD4+ T cells in the oral mucosal lamina propria of coeliac disease patients, but not in the controls. In coeliac disease patients, in contrast to the controls, the TCRαβ+ T cells in the lamina propria were much lower in number than the predominating CD4+ T cell subset of CD3+ T cells. Low numbers of both αβ+ and γδ+ T cells, simultaneously with high numbers of CD3+ cells, indicate that many T cells bore no TCR. Since almost all T cells detected bearing a TCR were αβ+, it appears that there was no tendency to recruit TCRγδ+ T cells. The reason for the high numbers of CD3+ cells is unclear. It is possible that there were differences in the affinities of the antibodies used, which could affect the sensitivity of the staining. Alternatively, other cells bearing the CD3 marker, some of which could be natural killer cells (NK), may have been detected.
The small intestinal mucosal immunopathological changes seem to be dependent on gluten-activated CD4+ lymphocytes within the mucosal lamina propria. The whole spectrum of mucosal changes observed in coeliac disease has been interpreted in terms of T cell activation [8,9,19,20]. The role of CD8+ TCRαβ+ IEL in the pathogenesis of villous atrophy in the small intestine is unclear [4,21]. In the lamina propria, CD8+ TCRαβ+ T cells might be activated by gliadin and contribute to the jejunal epithelial lesions [5]. We now show that in the oral mucosa of coeliac disease patients, the intraepithelial CD4+ and CD8+ T cell counts increased significantly upon supramucosal challenge, as did the CD4+ lymphocyte counts within the lamina propria upon submucosal gliadin challenge. The roles of CD4+ and CD8+ T cells might have similar effects on the mucosal integrity in both the jejunal and oral mucosa in coeliac disease patients.
According to our earlier results, patients with untreated coeliac disease do not have increased oral mucosal inflammation despite the partial or total villous atrophy seen in the small intestinal mucosa [16]. The present study shows that in the oral mucosa of coeliac disease patients the number of TCRγδ+ cells is very small, a finding which is in agreement with a recent report on the oral mucosal findings of patients with dermatitis herpetiformis [22]. Nor did gliadin increase the numbers of TCRγδ+ cells in the oral epithelium. This is in contrast to that which has been found in the jejunal mucosa of coeliac disease patients. Untreated coeliac disease patients have an increased density of γδ+ T cells intraepithelially in the jejunal mucosa [23–26]. Savilahti et al. have shown that the ingestion of gluten seems to trigger intraepithelial γδ+ T cell recruitment in the gut, as the density of γδ+ T cells was higher in patients whose initial coeliac disease diagnosis was made at an older age [27]. Recently, this was shown to be the case [28]. Our results show again that the determination of TCRγδ+ T cell numbers in the oral mucosa in coeliac disease does not aid the diagnosis of the disease. This study also indicates that the oral mucosal pathology differs from that of the jejunal mucosa in this respect. The role of intraepithelial TCRγδ+ lymphocytes in the gluten-induced oral and jejunal lesion in coeliac disease is not known. In mice, a protective role for these cells has been proposed [29].
One of the first characteristic features of in vitro gliadin challenge of the small intestinal mucosa in coeliac disease is the up-regulation of HLA-DR expression, consistent with the T cell activation observed [30]. In our study of the oral mucosa of patients with coeliac disease, the submucosal gliadin challenge did not produce a significant induction of this activation marker.
When performing supramucosal oral challenge, the most difficult thing is to keep the material used for the challenge in place. When the challenge substance is injected under the epithelium it remains at the injection site for a longer period, but the injection procedure itself might irritate the oral mucosa, because crude gliadin is soluble only in ethanol. We decided to use this procedure however to obtain maximal antigenic stimulation. The effects of the ethanol in the gliadin challenge were excluded by making a separate solution challenge. Using submucosal gliadin challenge for detection of coeliac disease enabled us to identify 10 out of 37 patients with disease that would otherwise have been missed. The sensitivity of the submucosal gliadin challenge in these study subjects was 73% and the specificity 80%.
Exposure of the small intestinal mucosa to ethanol causes histamine release from intestinal mast cells through degranulation, which can provoke intestinal damage [31,32]. In the rectal gluten challenge the count of mast cells has been shown to decrease quickly following their degranulation process [13]. In the present study the mast cells did not decrease in number when using ethanol-containing solvent in the provocation alone. Nor did the control individuals react to ethanol upon submucosal gliadin challenge. Thus, we infer that ethanol was not the cause of the T cell recruitment seen in the present study in the oral mucosa. Rather, we believe that gliadin injected into the oral submucosa results in degranulation of mast cells in the lamina propria of coeliac disease patients.
In conclusion, the results of the present study confirm our hypothesis: the oral mucosa is suitable for immunological provocation tests and changes after gliadin challenge can be seen in the mucosa. In coeliac disease, the oral mucosa responds to a local gliadin challenge by T cell recruitment and mast cell degranulation. In fact, the oral mucosa offers the easiest access to a gluten challenge test when compared with the classical challenge lasting even years [33], or to a rectal gluten challenge [13,30]. After receiving the invitation to participate in this study, the willingness was great among the members of The Coeliac Association, as they found our proposal to search for easier methods than the small intestinal biopsy procedure to diagnose coeliac disease very important. Further studies in untreated and GFD‐treated coeliac disease patients are needed, however, to evaluate the usefulness of this method and to establish the optimal modes for a local oral gliadin challenge on oral mucosa.
Acknowledgments
We thank Ms Sari Mäki, Marja Uola, Irene Toivonen and Marja-Leena Koskinen for skilful technical assistance and Mr Hans Helenius for assistance in the statistical calculations. The Emil Aaltonen Foundation, The Research Foundation of Raisio Yhtymä, The Research Foundation of Orion Corporation, The Yrjö Jahnsson Foundation, The Finnish Dental Society and The Medical Fund of Tampere University Hospital supported the study.
REFERENCES
- 1.Trier JS. Celiac sprue. N Engl J Med. 1991;325:1709–19. doi: 10.1056/NEJM199112123252406. [DOI] [PubMed] [Google Scholar]
- 2.Mäki M, Collin P. Coeliac disease. Lancet. 1997;349:1755–9. doi: 10.1016/S0140-6736(96)70237-4. [DOI] [PubMed] [Google Scholar]
- 3.Trier J. Diagnosis of celiac sprue. Gastroenterology. 1998;115:211–6. doi: 10.1016/s0016-5085(98)70383-x. [DOI] [PubMed] [Google Scholar]
- 4.Kutlu T, Brousse N, Rambaud C, Le Deist F, Schmitz J, Cerf-Bensussan N. Numbers of T cell receptor (TCR) α/β+ but not of TCR γ/δ+ intraepithelial lymphocytes correlate with the grade of villous atrophy in coeliac patients on a long term normal diet. Gut. 1993;34:208–14. doi: 10.1136/gut.34.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerf-Bensussan N, Cuenod-Jabri B, Guy-Grand D. Subsets of intraepithelial lymphocytes in normal intestine and in coeliac disease. In: Mäki M, Collin P, Visakorpi JK, editors. Coeliac disease. Tampere: University of Tampere; 1997. pp. 291–309. [Google Scholar]
- 6.Brandtzaeg P, Farstad IN, Helgelund L. Phenotypes of T cells in the gut. In: MacDonald TT, editor. Mucosal T cells. Vol. 71. Basel: Chem Immunol, Karger; 1998. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 7.Maiuri L, Picarelli A, De Marco G, Auricchio S, Londei M. In vitro gluten challenge. In: Mäki M, Collin P, Visakorpi JK, editors. Coeliac disease. Tampere: University of Tampere; 1997. pp. 291–309. [Google Scholar]
- 8.Nielsen EM, Lundin KEA, Krajci P, Soli LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with TH1 or TH0 profile dominated by interferon γ. Gut. 1995;37:766–76. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundin KEA, Scott H, Hansen T, Paulsen G, Halstensen TS, Fausa O, Thorsby E, Sollid LM. Gliadin-specific, HLA-DQ (α1*0501, β1*0201) restricted T cells isolated from small intestinal mucosa of celiac patients. J Exp Med. 1993;178:187–96. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh MN. The gut associated lymphoid tissue and immune system. In: Whitehead P, editor. Gastrointestinal and oesophageal pathology. Edinburgh: Churchill Livingstone; 1989. pp. 161–86. [Google Scholar]
- 11.Dobbins WO, Rubin CE. Studies of rectal mucosa in coeliac sprue. Gastroenterology. 1962;42:691–705. [PubMed] [Google Scholar]
- 12.Austin L, Dobbins WO. Studies of the rectal mucosa in celiac sprue: the intraepithelial lymphocyte. Gut. 1988;29:200–5. doi: 10.1136/gut.29.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loft DE, Marsh MN, Sandle GI, Crowe PT, Garner V, Gordon D, Baker R. Studies of intestinal lymphoid tissue. XII. Epithelial lymphocyte and mucosal responses to rectal gluten challenge in celiac sprue. Gastroenterology. 1989;97:29–37. doi: 10.1016/0016-5085(89)91411-x. [DOI] [PubMed] [Google Scholar]
- 14.Ensari A, Marsh MN, Loft DE, Morgan S, Moriarty K. Morphometric analysis of intestinal mucosa. V. Quantitative histological and immunocytochemical studies of rectal mucosae in gluten sensitivity. Gut. 1993;34:1225–9. doi: 10.1136/gut.34.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troncone R, Greco L, Meyer M, et al. In siblings of coeliac children rectal gluten challenge reveals sensitisation to gluten not restricted to coeliac HLA. Gastroenterelogy. 1996;111:318–24. doi: 10.1053/gast.1996.v111.pm8690196. [DOI] [PubMed] [Google Scholar]
- 16.Lähteenoja H, Toivanen A, Viander M, Mäki M, Irjala K, Räihä I, Syrjänen S. Oral mucosal changes in coeliac patients on a gluten-free diet. Eur J Oral Sci. 1998;106:899–906. doi: 10.1046/j.0909-8836.1998.eos106501.x. [DOI] [PubMed] [Google Scholar]
- 17.Hällström O. Comparison of IgA-class reticulin and endomysium antibodies in coeliac disease and dermatitis herpetiformis. Gut. 1989;30:1225–32. doi: 10.1136/gut.30.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker-Smith JA, Guandalini S, Schmitz J, Shmerling DH, Visakorpi JK. Working Group of European Society of Pediatric Gastroenterology and Nutrition: revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson A. Models of immunologically-driven small intestinal damage. In: Marsh MN, editor. Immunology of the small intestine. Chichester: Wiley; 1987. pp. 225–52. [Google Scholar]
- 20.MacDonald TT. T cell-mediated intestinal injury. In: Marsh MN, editor. Coeliac disease. Oxford: Blackwell; 1992. pp. 283–304. [Google Scholar]
- 21.Halstensen TS, Brantzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4(+) αβ cells in lamina propria but proliferation (Ki-67) of αβ and γδ cells in the epithelium. Eur J Immunol. 1993;23:505–10. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- 22.Patinen P, Savilahti E, Hietanen J, Malmström M, Mäki M, Reunala T. Intraepithelial lymphocytes bearing the gamma/delta receptor in the oral and jejunal mucosa in patients with dermatitis herpetiformis. Eur J Oral Sci. 1997;105:130–5. doi: 10.1111/j.1600-0722.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 23.Trejdosiewicz LK, Calabrese A, Smart CJ, et al. γδ T cell receptor-positive cells of the human gastrointestinal mucosa: occurrence and V region gene expression in Helicobacter pylori-associated gastritis, coeliac disease and inflammatory bowel disease. Clin Exp Immunol. 1991;84:440–4. [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer J, Isaacson PG, Diss TC, MacDonald TT. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor gamma/delta heterodimer in human intestinal intraepithelial lymphocytes. Eur J Immunol. 1989;19:1335–8. doi: 10.1002/eji.1830190728. [DOI] [PubMed] [Google Scholar]
- 25.Halstensen TS, Scott H, Brantzaeg P. Intraepithelial T cells of the TcR gamma/delta+CD8− and V delta 1/J delta 1+ phenotypes are increased in celiac disease. Scand J Immunol. 1989;30:665–72. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 26.Holm K, Mäki M, Savilahti E, Lipsanen V, Laippala P, Koskimies S. Intraepithelial gamma/delta T-cell-receptor lymphocytes and genetic susceptibility to celiac disease. Lancet. 1992;39:1500–3. doi: 10.1016/0140-6736(92)91262-7. [DOI] [PubMed] [Google Scholar]
- 27.Savilahti E, Örmälä T, Arato A, Hacsek G, Holm K, Klemola T, Nemeth A, Mäki M. Density of γδ+ T cells in the jejunal epithelium of patients with coeliac disease and dermatitis herpetiformis is increased with age. Clin Exp Immunol. 1997;109:464–7. doi: 10.1046/j.1365-2249.1997.4811377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iltanen S, Holm K, Ashorn M, Ruusku T, Laippala P, Mäki M. Changing jejunal γδTcR-bearing intraepithelial lymphocyte density in coeliac disease. Clin Exp Immunol. 1999 doi: 10.1046/j.1365-2249.1999.00948.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts SC, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes towards natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiuri L, Picarelli A, Boirivant M, Coletta S, Mazzilli MC, De Vincenzi M, Londei M, Auricchio S. Definition of the initial immunological modifications upon in vitro challenge in the small intestine of celiac patients. Gastroenterology. 1996;110:1368–78. doi: 10.1053/gast.1996.v110.pm8613040. [DOI] [PubMed] [Google Scholar]
- 31.Dotto P, Vianello F, Laino G, et al. Alcohol-dependent mast cell activation in ulcer. Minerva Gastroenterol Dietol. 1993;39:7–10. [PubMed] [Google Scholar]
- 32.Dinda PK, Wasan S, Beck IT, Kosev P. Adaptive cytoprotection against ethanol-induced small intestinal mucosal injury. Can J Physiol Pharmacol. 1996;74:598–602. [PubMed] [Google Scholar]
- 33.Mäki M, Lähdeaho M-L, Hällström O, Viander M, Visakorpi JK. Postpubertal gluten challenge in coeliac disease. Arch Dis Child. 1989;64:1604–7. doi: 10.1136/adc.64.11.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]


