Abstract
SLE is associated with the production of autoantibodies to self-constituents. In particular, certain ribonucleoprotein particles are targeted. Despite the multitude of autoantibodies produced and the remarkable concentrations of these antibodies in the sera of SLE patients, there have been little data that the autoantibodies found in SLE are involved in the pathogenesis of disease or its manifestations. The present work demonstrates that anti-Ro (or SSA) is associated with granulocytopenia, binds the surface of granulocytes and fixes complement to this membrane surface. Binding is a property of anti-Ro Fab fragments and can be inhibited by 60-kD Ro. However, the antigen bound on the surface of granulocytes is a 64 000 mol. wt protein that is a novel autoantigen in SLE. As suggested by inhibition studies, sequence identity between 60-kD Ro and eight tandem repeats in the 64-kD antigen may be responsible for the observed serologic cross-reactivity. These data imply that anti-Ro antibodies that also bind the 64-kD protein mediate neutropenia in patients with SLE.
Keywords: neutropenia, systemic lupus erythematosus, anti-Ro/SS-A, D1 antigen, mass spectrometry
Introduction
Among the various manifestations of SLE haematological abnormalities are common. Included are haemolytic anaemia, thrombocytopenia, lymphopenia and neutropenia [1]. Neutropenia was first described in SLE over 70 years ago [2] and mild neutropenia is found in about one-half of patients with the disease [1,3,4]. The absolute fall of neutrophils can be large, usually larger than the absolute fall of lymphocytes [3], but only rarely does severe neutropenia occur as a result of SLE [1,3,4]. Data have implicated binding of antibody to the surface of neutrophils, followed by fixation of complement, as mediating the fall of circulating neutrophils found in SLE [5,6].
Anti-Ro (or SSA) is found in the sera of up to 40% of patients with SLE as well as most patients with Sjögren’s syndrome (SS), subacute cutaneous lupus and mothers of infants with neonatal lupus [7]. All sera with a Ro precipitin bind a 60-kD protein (60-kD Ro), whose function is not completely known [8,9]. The 60-kD Ro binds one of the several hY RNAs [10]. The La (or SSB) protein transiently associates with the Ro ribonucleoprotein particle [11] and antibodies to La are found in a subset of sera with anti-Ro [12]. Meanwhile, the relationship of the hY RNAs and the Ro particle to calreticulin or 52-kD Ro is controversial [13–16]. Antibodies binding 52-kD Ro are found in sera that also have anti-60-kD Ro [13, 17, 18]. Anti-Ro has been associated with several clinical features of illness in either SLE or SS [7]. Lymphopenia is a common feature of SLE, and is found more commonly in patients with anti-Ro [19]. Other studies have found anti-Ro related to low total leucocyte count in either SS [20], SLE [21], or rheumatoid arthritis (RA) [22].
We undertook the present study to test the hypothesis that anti-Ro is associated with granulocytopenia in SLE and to determine whether anti-Ro can bind the surface of neutrophils in an antigen-specific manner. SLE patients with anti-Ro had significantly lower neutrophil counts than patients without anti-Ro. Anti-Ro from the sera of SLE patients or from animals immunized with 60-kD Ro bound neutrophils. In addition, binding was inhibited by 60-kD Ro, was present when only the Fab portion of immunoglobulin was used, and fixed complement. Neutrophil membrane contained a protein of approximately 60 000 mol. wt that was bound by anti-Ro sera. However, this protein was identified as a previously sequenced cell surface molecule. The data demonstrate that anti-Ro antibodies are cross-reactive towards this protein and that these antibodies may mediate granulocytopenia in SLE.
PATIENTS and METHODS
Patients and sera
Consecutive SLE patients attending the Rheumatology Clinic in the University Hospital at the University of Oklahoma Health Science Center were studied. Each patient met the 1982 revised criteria for the classification of SLE [23]. Complete blood counts were obtained as part of their routine care. Patients were studied for the presence of rheumatic disease-related autoantibodies by double immunodiffusion using standard methods [24,25]. Categorical data were compared with a χ2 analysis. Numerical data were compared with Student’s t-test. Sera were drawn from patients under protocols approved by the Institutional Review Board and stored at −80°C until use. Sera from 20 of these patients were studied further for binding to neutrophils. Normal control sera were gathered from 14 individuals, none of whom had received a blood transfusion or had been pregnant. The patient and control sera were tested for antibodies to class I HLA molecules using an ELISA (Quickscreen, QS3G Solid Phase HLA Class I Screening Kit; Genetic Testing Institute, Brookfield, WI). No control had these antibodies, while four of the patients did so. Three patients with a Ro precipitin had anti-HLA antibodies, two of whom had a neutrophil count <3000/mm3 and one of whom had a neutrophil count above this level.
The rabbits immunized with 60-kD Ro have been previously described [24]. Neutrophils of either rabbits or humans were isolated from peripheral blood cells by centrifugation over a continuous density gradient Lymphocyte Separation Medium from Mediatech (Herndon, VA). The neutrophils were then separated from the erythrocytes by centrifugation after layering over a discontinuous Percoll density gradient (Pharmacia, Uppsala, Sweden). Fab was produced and purified using reagents and instructions provided by the supplier (Immunopure Fab; Pierce, Rockford, IL).
Anti-Ro purification
Anti-Ro IgG was purified from sera by affinity chromatography. Purified bovine 60-kD Ro (10 mg) [26,27] was coupled to cyanogen bromide (CNBr)-preactivated Sepharose CL 4B (Sigma Chemical Co., St Louis, MO) according to instructions from the manufacturer. Sera were passed over the column at 4°C and bound antibody was eluted with 3 m sodium thiocyanate. The eluate was concentrated with centrifugation using a Centricon 10 and diluted to the original volume of sera with PBS.
Flow cytometry
Sera containing 500 μg of IgG were incubated with about 0·7 × 106 rabbit or human neutrophils for 20 min on ice at 4°C. Species-appropriate FITC conjugates were added after washing the cells with cold PBS and incubated for 20 min on ice. The cells were then washed with PBS and underwent FACS (Coulter, Hialeah, FL). This same procedure was repeated for the anti-Ro Fab fragments except that binding was detected with a Fab-specific FITC conjugate. For inhibition studies the sera were incubated with 20 μg/ml of the 60-kD Ro or an unrelated protein for 20 min before incubation with the cells. For inhibition study using MAPs, the same procedure was followed.
Complement fixation
This assay was carried out according to an earlier method [6] with some modifications. The isolated neutrophils were washed with Alsever’s medium (8·0 g trisodium citrate, 0·55 g citric acid, 4·2 g NaCl, 20·5 gd-glucose in 1 l water, pH 6·1) to minimize agglutination, resuspended and treated with one volume of 2% paraformaldehyde in PBS for 10 min at room temperature. The paraformaldehyde-treated cells were washed twice and resuspended in Alsever’s at about 20 million cells/ml and stored at −80°C. Just before use, the cells were thawed and washed with PBS. The test sera, sera that served as complement source, heat-inactivated sera and C3-depleted sera were diluted 1:5 with PBS. Fixed neutrophils (50 μl) were added to 50 μl of each of the diluted sera for 45 min at 37°C. After washing with PBS, anti-C3 antibody was added to the cells. This was followed by anti-C3 FITC conjugate and read on FACS.
Nitrogen cavitation
Surface biotinylation
The cells were washed and suspended in 6 ml PBS containing 1 mm MgCl2, and 0·1 mm CaCl2. Sulfo-NHS-biotin made up in DMSO was added to the cells at a final concentration of 0·5 mg/ml and incubated for 40 min at 4°C with gentle shaking. After washing with PBS the cells were lysed with buffer containing 1% Triton X-100, 10 mm Tris, 150 mm NaCl and 1 mm EDTA in the presence of the protease inhibitors pepstatin, leupeptin and PMSF for 30 min at 4°C. After centrifugation, 50 μl of streptavidin beads (Pierce) per ml of sample were added to the supernatant and rotated end-over-end overnight at 4°C. The streptavidin beads were washed six times with Tris-buffered saline containing 0·05% Tween. SDS-loading buffer was added to the beads and the mixture boiled for 5 min with 5% β-mercaptoethanol (β-ME). β-ME (5%) was added two more times with boiling and the samples were analysed on SDS–PAGE and immunoblot.
Immunoblot and anti-Ro ELISA
Affinity column for membrane ligand
Polyclonal rabbit anti-60-kD Ro was coupled to CNBr-preactivated Sepharose 4B according to instructions supplied by the manufacturer. Granulocyte membranes (200 μl), purified as described above, were homogenized with 5 ml of prechilled lysis buffer (0·5% Nonidet, 10 mm HEPES, 0·15 m NaCl, 0·08% sodium azide, 0·10 mm CaCl2, 0·01 mm MnCl2, 0·20 mm PMSF, and 0·20 U/ml aprotinin), pH 7·5. The homogenization was carried out on ice. The homogenate was centrifuged at 100 000 g for 1 h and the supernatant passed through the anti-Ro column after equilibration with PBS. Then, bound antigen was eluted with 300 μl of NaSCN and the column washed with 10 ml of PBS. The eluate was concentrated using a Centricon-30 to 500 μl, then diluted with cold PBS and concentrated to 500 μl again in order to remove NaSCN. This step was repeated once more. Next, the sample was diluted with cold deionized water and concentrated to 100 μl using N2 gas. The sample was analysed after 10% SDS–PAGE and non-electrophoretic transfer to nitrocellulose [31] by probing with anti-Ro sera. A single non-electrophoretic transfer left the majority of protein in the gel [31] and the gel was stained with coomassie blue and stored at 4°C for further use.
Tryptic digestion of purified membrane protein
The portion of the gel containing the purified protein, as identified in both immunostaining after transfer to nitrocellulose and coomassie blue staining of the gel, was carefully cut out and placed in a 1·5-ml conical tube. One millilitre of 50% acetonitrile in 200 mm ammonium bicarbonate buffer pH 8·9 was added to the tube. The mixture was shaken for 15 min. After removal of the acetonitrile solution, this procedure was repeated twice. The gel pieces were then removed and let dry for 10 min on clear plastic wrap. Then, 0·5 μl of TPCK-treated trypsin (Sigma) was added to each side of the gel. This was absorbed readily by the dried gel. The gel slices were rinsed with 50 μl of 200 mm ammonium bicarbonate buffer and then placed in 1·5-ml conical tubes once again. To these tubes were added 150 μl of 200 mm ammonium bicarbonate buffer. This was incubated at 30°C for 24 h. After this time, the samples were stored at −20°C until use in mass spectrometry.
Mass spectrometry
To the sample described above, 150 μl of 60% acetonitrile with 0·1% trifluoroacetic acid (TFA) were added and the mixture was shaken for 15 min at room temperature. The supernatant was removed and the procedure repeated twice. The supernatants were combined and dehydrated using a Speed-Vac Concentrator (Savant Instruments Inc., Farmingdale, NY). Resulting pellets were suspended in 20 μl of 70% acetonitrile containing 5% TFA. After mixing with 0·5 μl of ferrulic acid, a 0·5-μl aliquot was subjected to collision-induced dissociation (CID) using a API-III triple quadruple mass spectrometer (Perkin-Elmer Sciex, Thornhill, Ontario, Canada) with an electrospray atmosphere pressure ionization source. Polyethylene glycol was used for tuning and calibration. CID was conducted at 75 eV energy with 10% N2 and Ar gas. The product ions were scanned in a positive ion mode in the third quadruple Q3 from m/z = 10–600 with a mass step of 0·2 D.
If no spectrum was obtained, then further purification was performed. Five micolitres of the 70% acetonitrile sample were spotted on PVDF membrane and dried for 30 min. The dried spot was cut out and placed in 1 ml of deionized water and shaken for 15 min and the supernatant discarded. This procedure was done four times, the first two with shaking and the second two with vortexing for 1 min. The PVDF membrane was then extracted as was done with the gel as above and then subjected to mass spectroscopy as described above.
Computer comparisons
Comparison of the tryptic fragments obtained was performed using the EMBL Protein and Peptide Group databases and programs. For sequence comparison of 60-kD Ro and the 64-kD membrane antigen, the Compare program of the Genetics Computer Group Package was used with a word size of 4 and an exact amino acid match required (A = 1·5 as a command-line command).
Results
Seventy-two patients with SLE were evaluated. There were 69 women and three men. The average age was 32 years. One patient had an SLE/systemic sclerosis overlap. Thirteen patients had anti-Ro/SSA, five of whom also had anti-La/SSB. Twenty-two patients had anti-RNP, with six also having anti-Sm. Seven had anti-P while 19 had no precipitating antibodies. Anti-native DNA was found in 17. Six of the 13 anti-Ro/SSA patients had total neutrophil counts <3000/mm3. Only eight of the remaining 59 anti-Ro/SSA− SLE patients had neutrophils <3000/mm3 (P = 0·03 by Fisher’s exact test, odds ratio (OR) = 7·6). The average neutrophil count of the anti-Ro/SSA+ patients was significantly lower than that of the anti-Ro/SSA− patients (Table 1). There was no relationship among the other SLE-associated antibodies and neutropenia. Patients of African-American descent, who may have benign ethnic neutropenia [32], were not over-represented among the anti-Ro+ patients.
Table 1.
Relationship of anti-Ro/SSA and neutrophil count (per mm3) in 72 patients with SLE
| Anti-Ro/SSA | ||
|---|---|---|
| Positive n= 13 | Negative n = 59 | |
| Mean neutrophil count | 3602* | 6012 |
| s.d. | 3357 | 2631 |
Significantly lower than the neutrophil counts of anti-Ro/SSA − patients (t = 2·85, P < 0·0001).
Binding of anti-Ro to neutrophils was studied with flow cytometry. Sera from anti-Ro+ SLE patients bound neutrophils more than either sera from SLE patients without anti-Ro (Fig. 1a) or sera from normal individuals (Fig. 1b). Patients were categorized as to the presence of anti-Ro and granulocytopenia, and then studied for binding to granulocytes (Table 2). Patients with low granulocytes and anti-Ro tended to have more intense fluorescence in flow cytometry than any other group (P = 0·0001 by Wilcoxon rank order test) and more than normal controls (P = 0·02 by Student’s t-test). We also found that sera from rabbits immunized with 60-kD Ro bound neutrophils, while pre-immune sera or sera from animals immunized with only Freund’s adjuvant did not (Fig. 2).
Fig. 1.

Flow cytometry analysis of sera from normal persons as well as SLE patients with and without anti-Ro binding to human neutrophils. Human sera with equal amounts of protein based on A280 were added to the granulocytes and assayed for binding to the neutrophil surface as given in Patients and Methods. (a) Two anti-Ro SLE sera and two anti-Ro− SLE sera binding to neutrophils. (b) A normal serum and four neutropenic anti-Ro sera analysed for binding to neutrophils.
Table 2.
Sera from patients with SLE were divided according to total neutrophil count of <3000 and the presence of anti-Ro, then each serum was assayed for neutrophil binding IgG in FACS
| Anti-Ro+ | Anti-Ro− | |
|---|---|---|
| < 3000 neutrophils | 264 | 184 |
| 365 | 220 | |
| 305 | 237 | |
| 305 | ||
| > 3000 neutrophils | 212 | 175 |
| 273 | 245 | |
| 143 |
Data are given for the median fluorescence of individual sera with 14 normal sera averaging a value of 165 and FITC conjugate alone giving a value of 22. The anti-Ro/neutrophil <3000 group was significantly different from the other three groups by a Wilcoxon rank analysis (P < 0·0001).
Fig. 2.
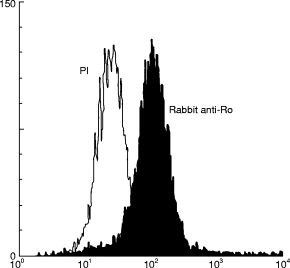
Flow cytometry analysis of granulocyte binding by 60-kD Ro-immunized rabbit sera and pre-immune rabbit sera. FACS analysis of cells incubated with the pre-immune sera is shown by the open graph and fluorescence by cells incubated with 60-kD Ro-immunized rabbit sera is shown by the shaded graph.
In order to demonstrate that anti-Ro was specifically responsible for the binding seen, we purified anti-Ro from several human sera. Purified antibody bound the 60-kD Ro antigen in high titre in an ELISA and did not bind any other protein species in immunoblot of a human tissue extract (not shown). The purified anti-Ro did bind neutrophils as assessed by flow cytometry, compared with Cohn fraction II at four times the concentration of immunoglobulin (Fig. 3).
Fig. 3.
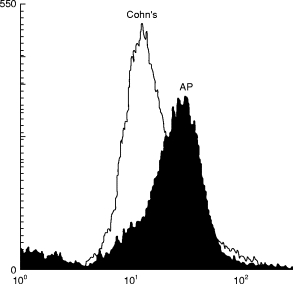
Flow cytometry analysis of affinity-purified human anti-Ro (AP) binding to neutrophils. Cohn Fraction II (Cohn’s) at four times the concentration of the anti-Ro or purified anti-Ro was incubated with neutrophils and bound fluorescence was measured.
Several experiments were performed to demonstrate that binding to the surface of neutrophils was via antibody–antigen interaction. The binding of either rabbit or human anti-Ro could be inhibited by incubation of the anti-Ro with 60-kD Ro with median fluorescence falling from 245·6 to 68·4 for a representative serum preincubated with 1 μg of 60-kD Ro. Incubation with purified RNP autoantigen did not result in inhibition of binding with median fluorescence of 264·2. Use of an anti-Ro− serum in these experiments gave a mean fluorescence of just 77·7. In another test of antibody–antigen interaction, IgG from an anti-Ro+ patient was used to produce anti-Ro Fab fragments. The anti-Ro Fab fragments bound neutrophils while Fab antibody fragments produced from IgG of normals or anti-Ro− SLE patients did not bind the cells (Table 3). Previous data have indicated that neutropenia in SLE patients is quantitatively related to fixation of complement by antibody that binds the surface of neutrophils. Anti-Ro sera from SLE patients with low neutrophil counts were studied for their ability to fix complement on the surface of neutrophils. Antibody from each serum bound neutrophils, and when a complement source was added flow cytometry indicated that complement was on the surface of the cells (Table 4).
Table 3.
FACS analysis of anti-Ro and control Fab fragments binding to granulocytes
| Sample | Median fluorescence |
|---|---|
| Unstained | 4·7 |
| FITC anti-Fab conjugate alone | 28 |
| Normal control Fab | 340 |
| Anti-Ro− SLE Fab | 470 |
| Anti-Ro+ SLE Fab | 866 |
Neutrophils were incubated with FITC conjugate alone, or Fab from a normal individual, or Fab from an SLE patient with anti-Ro, or Fab from an SLE patient without anti-Ro. No sera used in these studies contained anti-HLA class I antibodies.
Table 4.
Fixation of complement by anti-Ro antibody binding the surface of neutrophils
| Serum complement source | |||
|---|---|---|---|
| Whole | C3-depleted | Heat-inactive | |
| Anti-Ro+ | 460 | 246 | 255 |
| Anti-Ro+ | 462 | 379 | 316 |
| Normal | 284 | 264 | ND |
| Anti-Ro− | 328 | 178 | 284 |
Data are given for the median fluorescence of each experiment as assessed by FACS analysis using an anti-C3 FITC-labelled antibody to determine deposition of complement on the neutrophil cell surface. Heat-inactivated and C3-depleted sera were used as control sources of complement.
The above data show that anti-Ro interacts with intact neutrophils in a manner that is dependent on the binding of an antigen on the surface of neutrophils. Thus, the next set of experiments was designed to identify this antigen. Enriched neutrophil cell membranes were isolated after nitrogen cavitation of purified neutrophils and analysed by immunoblot. Immunized rabbit serum was used in these analyses because its ability to bind 60-kD Ro in immunoblot far exceeds that of most naturally arising anti-Ro in human SLE patients. When subjected to SDS–PAGE and transferred to nitrocellulose, the product obtained from nitrogen cavitation contained a prominent band that was bound by anti-Ro and migrated at an approximate mol. wt of 60 000 (Fig. 4a). To confirm these results, we next biotinylated the surface of intact neutrophils. Biotinylated surface proteins were then isolated by using streptavidin. Using SDS–PAGE and immunoblotting, we found no contaminating nuclear, cytoplasmic or mitochondrial proteins when assessed by probing with specific antisera. There was however binding of a protein that migrated at 60 kD by anti-Ro sera (Fig. 4b).
Fig. 4.
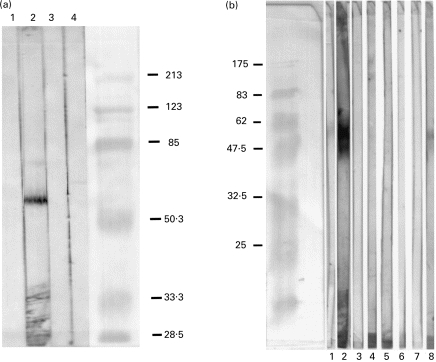
(a) Western immunoblot of granulocyte membrane prepared via nitrogen cavitation and probed with control or 60-kD Ro-immunized rabbit sera. Lane 1, Conjugate control; lane 2, 60-kD immunized rabbit; lane 3, rabbit pre-immune sera; lane 4, Freund’s adjuvant-immunized rabbit sera. (b) Western immunoblot of biotinylated granulocyte cell membrane. Granulocyte membrane-bound proteins were biotinylated and analysed on SDS–PAGE, followed by immunoblot. Lane 1, Pre-immune rabbit sera; lane 2, 60-kD Ro-immunized rabbit sera; lane 3, conjugate control; lane 4, normal human sera; lane 5, SLE sera with anti-Sm; lane 6, SLE sera without anti-Ro; lane 7, anti-P protein sera; lane 8, anti-Ro human sera.
In order to identify the antigen bound by anti-Ro sera, affinity chromatography was used. Immunoglobulin from a rabbit immunized with 60-kD Ro was coupled to Sephadex 4B and purified granulocyte membrane exposed to the column. The eluted product was concentrated and then subjected to SDS–PAGE. A single protein band was identified by coomassie staining of the gel, or probing with anti-Ro sera after transfer to nitrocellulose. The band of interest was cut from the gel and subjected to trypsin digestion. The products of the digestion were analysed using quadruple electrospray ionization and mass spectroscopy. The trypsin fragments were compared with predicted trypsin digestion fragments of known proteins using the EMBL Protein and Peptide programs and database. The trypsin fragments produced from the neutrophil membrane antigen bound by anti-Ro matched those predicted from a 64-kD protein referred to as D1 [33–36] (Table 5).
Table 5.
Trypsin fragments identified in the 64-kD granulocyte membrane protein bound by anti-Ro sera
| Fragment mol. wt (measured) | D1 match mol. wt (predicted) | Sequence |
|---|---|---|
| 474·357 | 474·256 | 262-TPEK |
| 502·364 | 502·335 | 100-GGLKK |
| 506·295 | 506·21 | 239-DDEK |
| 507·396 | 507·224 | 442-NMDK |
| 520·37 | 520·226 | 223-EDEK |
| 532·42 | 532·31 | 142-KEAGK |
| 524·434 | 534·241 | 160-EEEK |
| 548·432 | 548·304 | 556-SSNLK |
| 634·316 | 634·305 | 238-KDDEK |
| 635·323 | 635·2521 | 190-EDDEK |
| 715·474 | 715·433 | 446-QRQKR |
| 758·524 | 758·453 | 135-VRAAVDK |
| 784·506 | 784·457 | 538-VLPAQEK |
| 804·36 | 804·447 | 415-ENTSLLK |
| 860·489 | 860·459 | 142-KEAGKDGR |
| 862·524 | 862·416 | 190-EDDEKVK |
The molecular weight determined by mass spectroscopy is given along with the calculated molecular weight of the predicted fragment from the D1 protein, all of which were the best match among sequenced proteins to the fragments obtained. The residue position is given for the amino terminal amino acid residue in each sequence from D1.
The amino acid sequences of 60-kD Ro and the identified 64-kD D1 protein were compared for identity. There was no evidence of evolutionary homology between the two proteins. However, 60-kD Ro contains a tetrapeptide that is represented as part of a series of eight tandem repeats in the 64-kD antigen. In three of the tandem repeats the 60-kD Ro tetrapeptide sequence is matched exactly and in the five other repeats there is partial identity (Table 6).
Table 6.
Sequence comparison of 60-kD Ro and the 64-kD D1 antigen
| Antigen | Residues | Sequence |
|---|---|---|
| 60-kD Ro | 233–236 | E K V K |
| 64-kD D1 | 143–146 | – A G – |
| 162–165 | – E K – | |
| 176–179 | D –– K | |
| 193–196 | –––– | |
| 209–212 | – − M – | |
| 225–228 | –––– | |
| 241–244 | –––– | |
| 251–254 | –– E A | |
| 264–267 | –– Q T |
Based on these sequence similarities, we hypothesized that the autoantibody cross-reactivity between 60-kD Ro and the 64-kD antigen as well as the binding of granulocytes by anti-Ro might be mediated by antibodies binding the region of 60-kD Ro mimicked eight times in the 64-kD antigen. To test this hypothesis inhibition studies of granulocyte binding were carried out using a MAP peptide representing the 60-kD Ro sequence of residues 230–243 (amino acid sequence EAVEKVKRTKDELE). We found that this peptide, which contains the shared sequence, inhibited the binding of anti-Ro sera, either from 60-kD Ro-immunized animals or human SLE patients, to intact, living granulocytes (Fig. 5). There was a dose-dependent effect of increasing amounts of this Ro-MAP, with 5 μg of the Ro-MAP giving 67% inhibition and 15 μg giving 80% inhibition. Increasing amounts of control MAPs did not result in increasing inhibition. Control MAPs, including SM-MAP-115 with the sequence PSQQVMTP and Ro-MAP-331 with the sequence YKTGHGLRKLKWRP, did not produce inhibition of binding.
Fig. 5.
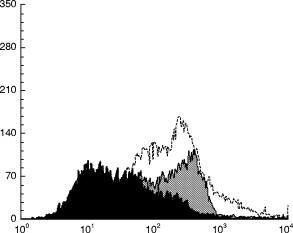
Inhibition of anti-Ro binding intact, living neutrophils with 60-kD Ro MAP-230. Binding of anti-Ro to neutrophils is shown without inhibition (shaded grey), after incubation of the antibody with 10 μg of 60-kD Ro MAP-230 (shaded black), and after incubation with 10 μg of Ro-MAP-331 (unshaded), representing another sequence from 60-kD Ro. Sm-MAP-115 with residues 115–122 from Sm-B/B′ antigen served as another control and produced no inhibition (not shown).
Discussion
Neutropenia that is mild in nature is common in SLE, while severe neutropenia is uncommon. The mechanisms of severe, life-threatening neutropenia are diverse and include both peripheral and bone marrow suppression [5]. Both the humoral and cellular arms of the immune system may be involved [5]. In contrast, previous data suggest the mild neutropenia found in about one-half of SLE patients is caused by binding of antibody to neutrophils with subsequent fixation of complement and peripheral destruction of the cells [5,6].
Because the presence of anti-Ro/SSA has been associated with lymphopenia and leukopenia, we undertook a clinical study to determine whether anti-Ro was related to neutropenia in SLE patients. These data show that neutropenia in SLE is associated with the presence of anti-Ro/SSA. This autoantibody is related to a number of the clinical features of SLE or SS. Included among these are lymphopenia, photosensitive and subacute cutaneous rash, pneumonitis, hereditary complement deficiencies, and neonatal lupus syndromes (reviewed in [7]). A decreased total leucocyte count is associated with anti-Ro in SS [20], SLE [21], or RA [22]. The present work adds to the list of features of SLE and SS that are associated with anti-Ro/SSA.
We have shown that anti-Ro antibodies from SLE patients or Ro-immunized animals bind to neutrophils via an interaction with a cell surface antigen. Inhibition of binding by 60-kD Ro and the ability of Fab fragments to bind are both convincing evidence that the interaction is one of specific antibody binding antigen. Anti-Ro antibody can fix complement on the surface of neutrophils. Taken together, these data in conjunction with the clinical data suggest that anti-Ro has the capacity to mediate neutropenia in patients with SLE.
However, the antigen to which these antibodies bind is not 60-kD Ro, but instead a 64-kD membrane protein that has been described as an antigen in autoimmune thyroid disease [34–38]. A partial sequence of this protein, called D1, was obtained by Dong and colleagues by screening of a thyroid expression library with sera from patients with Hashimoto’s thyroiditis [33]. These same investigators later sequenced and cloned a full length cDNA encoding a 572 amino acid protein.
Initial studies of tissue distribution were thought to demonstrate that D1 was a candidate antigen for Graves’ ophthalmopathy [37]; however, subsequent studies have shown mRNA for D1 is present in a wide range of tissues. In fact, every tissue type studied has had D1 mRNA, including lymphocytes [33,35]. Granulocytes have not been studied to our knowledge. Furthermore, antibodies to D1 are found in the sera of Graves’ disease patients either with or without ophthalmopathy as well as the sera of those with Hashimoto’s thyroiditis [33, 35, 36, 38] and some normal controls [35]. Sera from patients with other autoimmune diseases have not been studied. The wide distribution of this antigen is similar to others found in SLE. Also similar to other SLE antigens, the tissue distribution of the antigen does not explain the clinical manifestations.
Thus, D1 is an antigen in autoimmune thyroid disease and has serologic cross-reactivity with anti-Ro, which is found in SLE and SS. A possible association of either SLE or SS has been examined in several, but rarely controlled, studies [38]. The evidence is strongest that cohorts with SS have an excess of thyroid disease [39]. While SLE patients have a high risk of acquiring thyroid autoimmunity and disease [40], two well controlled studies have examined cohorts with SLE for autoimmune thyroid and neither found an excess [41,42]. However, one report found increased thyroid disease in SLE patients with secondary SS compared with SLE patients without SS [43,44]. Seven of eight with SLE and secondary SS had autoimmune thyroid disease. The majority of patients with either primary or secondary SS will have anti-Ro in their sera.
Our data demonstrate that 60-kD Ro and D1 are immunologically cross-reactive. Anti-Ro has been found to bind immunoglobulin, and thus 60-kD Ro is cross-reactive for at least one other protein with which it shares no evolutionary homology [25,45]. The D1 protein contains eight tandem repeats within which there is a sequence shared with 60-kD Ro (Table 6). Inhibition experiments using a peptide from 60-kD Ro that contains the shared sequence demonstrate that binding to the granulocyte surface can be partially blocked by this peptide. Other control peptides from Ro or from another lupus autoantigen do not inhibit binding of anti-Ro to granulocytes. Thus, these data suggest that the serologic cross-reactivity between 60-kD Ro and D1 is mediated by these shared sequences. Furthermore, the data imply that these cross-reactive antibodies mediate granulocytopenia in some SLE patients with neutropenia and anti-Ro.
There are only a few other examples of autoantibodies in SLE involved in the pathogenesis of disease. Hahn and co-workers have described several anti-dsDNA MoAbs that are pathogenic [46]. When injected into naive mice these antibodies produce kidney disease. Recent work has shown that these pathogenic antibodies enter cells, some localizing to the nucleus while others remain cytoplasmic [47]. Other data have implicated anti-Ro in the pathogenesis of neonatal SLE [48–51] or subacute cutaneous lupus [52,53]. For example, anti-Ro was concentrated in the heart of an infant dying of congenital complete heart block [51]. Also, in studies of isolated heart cells and intact hearts, anti-52-kD Ro produces a direct effect on conduction [49,50]. In mice bearing a human skin graft, injected purified human anti-Ro binds the human skin in a pattern identical to that found in subacute cutaneous lupus [52].
In conclusion, mild neutropenia is a common occurrence in humans with SLE. Past evidence suggests that this manifestation is, at least in part, mediated by autoantibodies that bind the surface of neutrophils. The present study demonstrates that anti-Ro is associated with neutropenia, can bind neutrophils and potentially induce injury by activation of the complement cascade. However, the antigen bound on the surface of these blood cells is not 60-kD Ro. Instead it is a 64-kD protein that shares a repeated sequence with 60-kD Ro. Antibodies to the shared sequences may account for serologic cross-reactivity between these two proteins, and the data are consistent with this subset of anti-60-kD Ro autoantibodies mediating granulocytopenia in SLE.
REFERENCES
- 1.Nossent JC, Swaak AJG. Prevalence and significance of haematological abnormalities in patients with systemic lupus erythematosus. Quart J Med. 1991;80:605–12. [PubMed] [Google Scholar]
- 2.Goeckerman WH. Lupus erythematosus as a systemic disease. JAMA. 1923;80:542–7. [Google Scholar]
- 3.Michael SR, Vural IL, Bassen FA, Schaefer L. The hematological aspects of disseminated (systemic) lupus erythematosus. Blood. 1951;6:1059–72. [PubMed] [Google Scholar]
- 4.Harvey AM, Shulman LE, Tumulty PA, Conley CL, Schoenrich EH. Systemic lupus erythematosus: review of the literature and clinical analysis of 138 cases. Medicine (Baltimore) 1954;33:291–437. [PubMed] [Google Scholar]
- 5.Starkebaum G, Arend WP. Neutrophil-binding immunoglobulin G in systemic lupus erythematosus. Evidence for the presence of both soluble immune complexes and immunoglobulin G antibodies to neutrophils. J Clin Invest. 1979;64:902–12. doi: 10.1172/JCI109556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rustagi PK, Currie MS, Logue GL. Complement-activating antineutrophil antibody in systemic lupus erythematosus. Am J Med. 1985;78:971–7. doi: 10.1016/0002-9343(85)90220-7. [DOI] [PubMed] [Google Scholar]
- 7.Harley JB, Scofield RH, Reichlin M. Anti-Ro in Sjögren’s syndrome and systemic lupus erythematosus. Rheum Dis Clin N Am. 1992;18:337–58. [PubMed] [Google Scholar]
- 8.O’Brien CA, Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, O’Brien CA, Van Horn DJ, Wolin SL. A misfolded form of 5S rRNA is complexed with the Ro and La autoantigens. RNA. 1996;2:769–84. [PMC free article] [PubMed] [Google Scholar]
- 10.Farris AD, Gross JK, Hanas JS, Harley JB. Genes for murine Y1 and Y3 Ro RNAs have class 3 RNA polymerase III promoter structures and are unlinked on mouse chromosome 6. Gene. 1996;174:35–42. doi: 10.1016/0378-1119(96)00279-x. [DOI] [PubMed] [Google Scholar]
- 11.Hendrick JP, Wolin SL, Rinke J, Lerner MR, Steitz JA. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981;1:1138–49. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harley JB, Alexander EL, Bias WB, Fox OF, Provost TT, Reichlin M, Yamagata H, Arnett FC. Anti-Ro/SSA and anti-La/SSB in patients with Sjögren’s syndrome. Arthritis Rheum. 1986;29:196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Chetrit E, Chan EK, Sullivan KF, Tan EM. A 52-kD protein is a novel component of the SSA/Ro antigenic particle. J Exp Med. 1988;167:1560–71. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boire G, Gendron M, Monast N, Bastin B, Ménard HA. Purification of antigenically intact Ro ribonucleoproteins; biochemical and immunological evidence that the 52-kD protein is not a Ro protein. Clin Exp Immunol. 1995;100:489–98. doi: 10.1111/j.1365-2249.1995.tb03728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peek R, Pruijn GJ, van der Kamp AJ, van Venrooij WJ. Subcellular distribution of Ro ribonucleoprotein complexes and their constituents. J Cell Sci. 1993;106:929–35. doi: 10.1242/jcs.106.3.929. [DOI] [PubMed] [Google Scholar]
- 16.Kelekar A, Saitta MR, Keene JD. Molecular composition of Ro small ribonucleoprotein complexes in human cells. Intracellular localization of the 60- and 52-kD proteins. J Clin Invest. 1994;93:1637–44. doi: 10.1172/JCI117145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh Y, Reichlin M. Autoantibodies to the Ro/SSA antigen are conformation dependent. I: anti-60 kD antibodies are mainly directed to the native protein; anti-52kD antibodies are mainly directed to the denatured protein. Autoimmunity. 1992;14:57–65. doi: 10.3109/08916939309077357. [DOI] [PubMed] [Google Scholar]
- 18.Itoh Y, Itoh K, Frank MB, Reichlin M. Autoantibodies to the Ro/SSA autoantigen are conformation dependent. II: antibodies to the denatured form of 52 kD Ro/SSA are a cross reacting subset of antibodies to the native 60 kD Ro/SSA molecule. Autoimmunity. 1992;14:85–95. doi: 10.3109/08916939209083125. [DOI] [PubMed] [Google Scholar]
- 19.Harley JB, Sestak AL, Willis LG, Fu SM, Hansen JA, Reichlin M. A model for disease heterogeneity in systemic lupus erythematosus. Relationships between histocompatibility antigens, autoantibodies and lymphopenia or renal disease. Arthritis Rheum. 1989;32:826–36. [PubMed] [Google Scholar]
- 20.Alexander EL, Arnett FC, Provost TT, Stevens MB. Sjögren’s syndrome: association of anti-Ro (SS-A) antibodies with vasculitis, hematologic abnormalities, and serologic hyperreactivity. Ann Intern Med. 1983;98:155–9. doi: 10.7326/0003-4819-98-2-155. [DOI] [PubMed] [Google Scholar]
- 21.de Rooij DJ, van de Putte LB, Habets WJ, Verbeek AL, van Venrooij WJ. The use of immunoblotting to detect antibodies to nuclear and cytoplasmic antigens. Clinical and serologic associations in rheumatic diseases. Scand J Rheumatol. 1988;17:353–64. doi: 10.3109/03009748809105271. [DOI] [PubMed] [Google Scholar]
- 22.Boire G, Menard HA, Gendron M, Lussier A, Myhal D. Rheumatoid arthritis: anti-Ro antibodies define a non-HLA-DR4 associated clinicoserological cluster. J Rheumatol. 1993;20:1654–60. [PubMed] [Google Scholar]
- 23.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 24.Scofield RH, Henry WE, Kurien BT, James JA, Harley JB. Immunization with short peptides from the sequence of the systemic lupus erythematosus-associated 60 kDa Ro autoantigen results in anti-Ro ribonucleoprotein autoimmunity. J Immunol. 1996;156:4059–66. [PubMed] [Google Scholar]
- 25.Mamula MJ, Fox OF, Yamagata H, Harley JB. The Ro/SSA autoantigen as an immunogen: some anti-Ro/SSA antibody binds immunoglobulin G. J Exp Med. 1986;164:1889–901. doi: 10.1084/jem.164.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamagata H, Harley JB, Reichlin M. Molecular properties of the Ro(SSA) antigen and enzyme-linked immunosorbant assay for quantitation of antibody. J Clin Invest. 1984;74:625–33. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickey WD, van Egmond JE, Hardgrave KL, Harley JB, Scofield RH. Presence of anti-La (SS-B) is associated with binding to the 13 kD carboxyl terminus of 60 kD Ro(SS-A) in systemic lupus erythematosus. J Invest Dermatol. 1993;100:412–6. doi: 10.1111/1523-1747.ep12472055. [DOI] [PubMed] [Google Scholar]
- 28.Eklund EA, Gabig TG. Purification and characterization of a lipid thiobis ester from human neutrophil cytosol that reversibly deactivates the O2-generating NADPH oxidase. J Biol Chem. 1990;265:8426–30. [PubMed] [Google Scholar]
- 29.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, McEver RP. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992;118:445–56. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh Y, Rader MD, Reichlin M. Heterogeneity of the Ro/SSA antigen and autoanti-Ro/SSA response: evidence of the four antigenically distinct forms. Clin Exp Immunol. 1990;81:45–51. doi: 10.1111/j.1365-2249.1990.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurien BT, Scofield RH. Multiple immunoblots after non-electrophoretic bidirectional transfer of a single SDS–PAGE gel with multiple antigens. J Immunol Methods. 1997;205:91–94. doi: 10.1016/s0022-1759(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 32.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. 1999;133:15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]
- 33.Dong Q, Ludgate M, Vassart G. Cloning and sequencing of a novel 64-kDa autoantigen recognized by patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1991;72:1375–81. doi: 10.1210/jcem-72-6-1375. [DOI] [PubMed] [Google Scholar]
- 34.Dong Q, Ludgate M, Vassart G. Towards an antigenic map of thyroglobulin: identification of ten epitope-bearing sequences within the primary structure of thyroglobulin. J Endocrinol. 1989;122:169–76. doi: 10.1677/joe.0.1220169. [DOI] [PubMed] [Google Scholar]
- 35.Ross PV, Koenig RJ, Arscott P, Ludgate M, Waier M, Nelson CC, Kaplan MM, Baker Jr., Jr Tissue specificity and serologic reactivity of an autoantigen associated with autoimmune thyroid disease. J Clin Endocrinol Metab. 1993;77:433–8. doi: 10.1210/jcem.77.2.8345048. [DOI] [PubMed] [Google Scholar]
- 36.Bernard NF, Nygen TN, Tyutyunikov A, et al. Antibodies against 1D, a recombinant 64-kDa membrane protein, are associated with ophthalmopathy in patients with thyroid autoimmunity. Clin Immunol Immunopathol. 1994;70:225–33. doi: 10.1006/clin.1994.1033. [DOI] [PubMed] [Google Scholar]
- 37.Zhang ZG, Dong Q, Rodien P, et al. Antibodies in the serum of patients with autoimmune thyroid disorders react with a recombinant 98 amino acid fragment of a full length 64 kDa eye muscle membrane protein which is also expressed in the thyroid. Autoimmunity. 1992;13:151–7. doi: 10.3109/08916939209001916. [DOI] [PubMed] [Google Scholar]
- 38.Scofield RH. Autoimmune thyroid disease in systemic lupus erythematosus and Sjögren’s syndrome. Clin Exp Rheumatol. 1996;14:321–30. [PubMed] [Google Scholar]
- 39.Foster H, Fay A, Kelly C, Charles P, Walker D, Griffiths I. Thyroid disease and other autoimmune phenomena in a family study of primary Sjögren’s syndrome. Br J Rheumatol. 1993;32:36–40. doi: 10.1093/rheumatology/32.1.36. [DOI] [PubMed] [Google Scholar]
- 40.Kausman D, Isenberg DA. Thyroid autoimmunity in systemic lupus erythematosus: the clinical significance of a fluctuating course. Br J Rheumatol. 1995;34:361–4. doi: 10.1093/rheumatology/34.4.361. [DOI] [PubMed] [Google Scholar]
- 41.Vianna JL, Haga HJ, Asherson RA, Swana G, Hughes GRV. A prospective evaluation of antithyroid antibody prevalence in 100 patients with systemic lupus erythematosus. J Rheumatol. 1991;18:1193–5. [PubMed] [Google Scholar]
- 42.Weetman AP, Walport MJ. The association of autoimmune thyroiditis with systemic lupus erythematosus. Br J Rheumatol. 1987;26:359–61. doi: 10.1093/rheumatology/26.5.359. [DOI] [PubMed] [Google Scholar]
- 43.Jonsson H, Nived O, Sturfelt G. Thyroid disorders are related to secondary Sjögren’s syndrome in unselected systemic lupus erythematosus patients (letter) Arthritis Rheum. 1988;31:1079–80. doi: 10.1002/art.1780310826. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson H, Nived O, Sturfelt G. Thyroid disorders in systemic lupus erythematosus are associated with secondary Sjögren’s syndrome (letter) Ann Rheum Dis. 1987;46:349. doi: 10.1136/ard.46.4.349-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mamula MJ, Harley JB. Anti-Ro autoantibody with cross-reactive binding to the heavy chain of immunoglobulin G. Yale J Biol Med. 1992;65:277–87. [PMC free article] [PubMed] [Google Scholar]
- 46.Ohnishi K, Ebling FM, Mitchell B, Singh RR, Hahn BH, Tsao BP. Comparison of pathogenic and non-pathogenic murine antibodies to DNA: antigen binding and structural characteristics. Int Immunol. 1994;6:817–30. doi: 10.1093/intimm/6.6.817. [DOI] [PubMed] [Google Scholar]
- 47.Koren E, Koscec M, Wolfson-Reichlin M, Ebling FM, Tsao B, Hahn BH, Reichlin M. Murine and human antibodies to native DNA that cross-react with the A and D SnRNP polypeptides cause direct injury of cultured kidney cells. J Immunol. 1995;154:4857–64. [PubMed] [Google Scholar]
- 48.Harley JB, Kaine JL, Fox OF, Reichlin M, Gruber B. Ro(SSA) antibody and antigen in a patient with congenital complete heart block. Arthritis Rheum. 1985;28:1321–5. doi: 10.1002/art.1780281202. [DOI] [PubMed] [Google Scholar]
- 49.Garcia S, Nascimento JHM, Bonfa E, Levy R, Olivera SF, Tavares AV, de Carvalho AC. Cellular mechanism of the conduction abnormalities induced by serum from anti-Ro/SSA-positive patients in rabbit hearts. J Clin Invest. 1994;93:718–24. doi: 10.1172/JCI117025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boutjdir M, Chen L, Zhang ZH, et al. Arrhythmogenicity of IgG and anti-52-kD SSA/Ro affinity purified antibodies from mothers of children with congenital heart block. Circ Res. 1997;80:354–62. doi: 10.1161/01.res.80.3.354. [DOI] [PubMed] [Google Scholar]
- 51.Reichlin M, Brucato A, Frank MB, Maddison PJ, McCubbin VR, Wolfson-Reichlin M, Lee LA. Concentration of autoantibodies to native 60-kd Ro/SSA and denatured 52-kd Ro/SSA in eluates from the heart of a child who died with congenital complete heart block. Arthritis Rheum. 1994;37:1698–703. doi: 10.1002/art.1780371120. [DOI] [PubMed] [Google Scholar]
- 52.Lee LA, Gaither KK, Coulter SN, Norris DA, Harley JB. Pattern of cutaneous immunoglobulin G deposition in subacute cutaneous lupus erythematosus is reproduced by infusing purified anti-Ro(SSA) autoantibodies into human skin-grafted mice. J Clin Invest. 1989;83:1556–62. doi: 10.1172/JCI114052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sontheimer RD, Maddison PJ, Reichlin M, Jordan RE, Stasny P, Gilliam JN. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664–71. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]


