Abstract
Advanced glycation end-products (AGE) play a role in diabetes complications and in RA. An autoantibody to IgG-AGE has been shown to correlate with RA disease activity. Thus we sought to analyse serum immune complexes (IC) and AGE-modified proteins in Caucasians and North American Indians to see if the presence of anti-IgG-AGE influenced their composition. Polyethylene glycol precipitation of IC from the serum of anti-IgG-AGE-positive or -negative RA patients, and healthy and diabetic controls were examined. Concentrations of circulating IC were highest in anti-IgG-AGE+ RA patients, followed by anti-IgG-AGE− RA patients, which were greater than healthy controls. IC amounts in the Ojibwe were consistently higher than in Caucasians. Affinity purification of AGE-modified proteins from IC and immunoblotting with antibodies against Ig γ and μheavy chains, κ and λ light chains, and AGE Nε(carboxymethyl)lysine and imidazolone yielded similar results: anti-AGE+ RA patients had elevated levels relative to those without the autoantibody. Levels in both RA groups were higher than in controls. Glycated albumin amounts followed a similar distribution, but were not influenced by the presence of anti-AGE antibodies. A heavily glycated κ-chain was present primarily in IC from anti-IgG-AGE+ patients. These studies indicate that anti-AGE antibodies have a direct impact on the accumulation of IgG-AGE but not glycated albumin, and may block the normal clearance of IgG-AGE through AGE receptors.
Keywords: advanced glycation end-products, autoantibody, diabetes, rheumatoid arthritis, rheumatoid factor
INTRODUCTION
RA is a common, chronic autoimmune inflammatory disease with debilitating articular and systemic manifestations. The aetiology and pathogenesis are still unclear, but appear to be multifactorial, and current treatment is not curative. Long-term outcome markers such as rheumatoid factor (RF) [1–3] and certain susceptibility alleles of the HLA-DR4β chain [4–6] are only partially indicative of disease course and treatment, and better prognostic tools are needed.
Recently, levels of certain circulating advanced glycation end-products (AGE) have been identified as a novel factor which correlates with RA disease activity [7,8]. Initially studied for their direct pathogenic role in diabetes [9,10], AGE are increasingly recognized for their contribution to a number of diseases, including RA. AGE formation is the non-enzymatic glycation of proteins on exposed amino groups such as on lysine and arginine residues, and is accelerated in the pathophysiological conditions of hyperglycaemia and/or oxidative stress.
While haemoglobin (glycated form HbA1c), albumin and collagen are common targets of AGE modification in diabetes [9], glycated IgG (IgG-AGE) is associated with inflammation in the context of arthritis (El-Gabawaly, manuscript in preparation). Both the heavy chain and the light chain of IgG can be AGE-modified and it is likely the 60 amino acid hinge region unique to IgG3, which has several lysine and arginine residues, is also glycated. Interestingly, a subset of patients with RA also has novel autoantibodies directed against IgG-AGE [11–13], which are linked to the presence of RF but represent a different population of antibodies. These antibodies to IgG-AGE but not RF were found to correlate with RA disease activity and have led us to question the impact of anti-IgG-AGE antibodies on the accumulation of IgG-AGE-containing immune complexes (IC). This theoretically could occur if the antibodies blocked the normal uptake and clearance mediated by receptors for AGE (RAGE) of the reticuloendothelial system [9,10].
Several tribes of North American Indians such as the Pima and Ojibwe exhibit RA and type 2 diabetes at frequencies exceeding that of any other ethnic group [14,15]. The coexistence of these diseases in the population and the exaggerated anti-AGE response in North American Indians [12] suggest a possible link in disease pathogenesis, perhaps mediated by non-enzymatic glycation.
We therefore undertook a study of native American and Caucasian RA and diabetic patients to identify the range and relative abundance of glycated proteins in these different patient groups, and to assess the impact of anti-IgG-AGE antibodies on IC composition and the accumulation of glycated proteins.
PATIENTS and METHODS
Patients and controls
Members of the Ojibwe tribe of Treaty First Nations Indians from the insular reserve Wikwemikong in Ontario were recruited for this study following informed consent. The study was approved by the University of Toronto ethics review board. RA was diagnosed according to the American College of Rheumatology criteria [16]. Serological studies, including random blood glucose measurements using the glucose 100 Trinder kit (Sigma, St Louis, MO), AGE measurements (both albumin-AGE and IgG-AGE) and autoantibody status (RF and anti-IgG-AGE) were completed on the RA patients and healthy controls, as previously reported [12]. In the present study 15 normal Ojibwe (of whom six had anti-IgG-AGE antibodies), 20 Ojibwe with RA (10 with anti-IgG-AGE antibodies), none of whom had diagnosed diabetes, and 12 Ojibwe with type 2 diabetes (two with anti-IgG-AGE antibodies) were studied. Unfortunately the HbA1c levels were not measured. Of the Ojibwe with RA, 10 of the 20 were receiving prednisone (divided roughly equally between the anti-IgG-AGE+ and anti-IgG-AGE− groups).
Sera were drawn from randomly selected Caucasian patients with RA from the Montreal General Hospital [13]. Diabetic patients (n = 10), selected for elevated haemoglobin A1c, were kindly provided by Dr R. Gardiner (Montreal General Hospital). Healthy controls were selected as previously described [12]. Information on clinical parameters, blood glucose levels and haemoglobin A1c (HbA1c; values >6·1 were considered elevated) were obtained where available from the charts. RF and anti-IgG-AGE antibodies were detected as previously described [11]. In the present study 10 normal Caucasians, and 36 patients with RA (10 were anti-IgG-AGE+) were studied. No abnormal HbA1c levels were detected in the Caucasian RA patients studied. Of the Caucasian RA patients studied, 13 of the 36 (35%) were receiving corticosteroids, but blood glucose levels were within the normal range.
Isolation of IC and purification of AGE modified proteins
Polyethylene glycol (PEG) was used to precipitate the IC [17]. In brief, delipidated sera were made to a final concentration of 2·5% PEG 8000 (American Chemicals, Montreal, Canada), and incubated overnight at 4°C. The samples were centrifuged at 13 000 g for 15 min to obtain a pellet (IgG-rich IC) and supernatant (lower molecular weight proteins). The pellet was resuspended in PBS. The AGE-modified proteins in IC were isolated via affinity chromatography, using aminophenylboronic acid (APB)–Sepharose (Sigma), according to the manufacturer’s instructions. The sorbitol-eluted fractions containing the AGE-modified proteins were analysed by 10% SDS–PAGE in the presence of β-mercaptoethanol [18]. The proteins resolved on the gel were silver-stained following the method of Wray et al. [19].
Immunoblot analyses
AGE modifications of proteins in the PEG supernatant, pellet, or APB-purified IC were characterized by immunoblotting. In brief, 2·5 μg or 5 μg of protein were resolved on 10% SDS–PAGE under reducing conditions. Proteins were transferred to nitrocellulose membranes, which were then blocked with 0·1% Tween 20 or 5% milk in PBS for 1 h at room temperature. AGE determinants were detected by incubating membranes at 37°C for 2 h with the MoAbs 6D12 (Wako Bioproducts, Richmond, VA) specific for Nε(carboxymethyl)lysine (CML) diluted 1:1000 in PBS–Tween and 2% goat serum, or AG1 specific for imidazolone (kindly provided by Professor T. Niwa, Nagoya, Japan) diluted 1:500. After washing three times with PBS–Tween, the blots were incubated with biotin-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch, West Grove, PA) diluted 1:20 000 in PBS–Tween for 2 h at 37°C. This was followed by washing and a 20-min room temperature incubation with horseradish peroxidase (HRP)-conjugated avidin (Vector Labs, Burlingame, CA) diluted 1:20 000. The bound antibodies were detected by enhanced chemiluminescence (ECL; Amersham, Oakville ON) and visualized on Biomax film (Kodak, Rochester, NY). The amounts of protein in the bands were quantified by laser densitometry.
In order to identify the AGE-modified proteins in the IC, blots were also probed with primary antibodies of known specificity for target proteins. Immunoglobulin components were detected with HRP-conjugated antibodies specific for the γ or μheavy chains (Jackson) diluted 1:20 000 in PBS–Tween and 2% goat serum, κ light chain (Protos, San Francisco, CA) diluted 1:1000, or λ light chain (Tago, Burlingame, CA) diluted 1:15 000. Albumin was detected with HRP-conjugated goat anti-human albumin antibody (EY Labs, San Mateo, CA) diluted 1:20 000. Bound antibodies were detected using chemiluminescence and quantified as above.
RESULTS
Circulating IC
The protein concentrations of circulating IC isolated from the serum were not different in the patient or control groups when examined statistically (n = 10–26, data not shown). There was however a trend for the Ojibwe First Nations People to have elevated levels of circulating IC relative to the Caucasian group. As can be seen in Table 1, levels of serum IC were more than four-fold higher in the randomly selected normal Ojibwe individual relative to the Caucasian normal individual examined. Within each ethnic group there was a trend for the RA patients to have increased levels of circulating IC compared with the healthy controls. In the patients selected for further study, serum IC levels were 40% higher in the anti-IgG-AGE+ Ojibwe RA patient than the anti-AGE− individual, and 15% higher in the Caucasian RA patient who was anti-IgG-AGE+ compared with the patient who lacked the autoantibodies.
Table 1.
Concentration of advanced glycation end-product (AGE)-modified proteins in the circulating immune complexes (IC) of RA patients and normal controls
| Patient group | Total IC (mg/ml) | Total AGE-protein in IC (mg/ml) | IC AGE-protein as % total |
|---|---|---|---|
| Ojibwe normal | 11·10 | 2·38 | 21·4 |
| Ojibwe RA anti-AGE− | 14·32 | 2·14 | 14·9 |
| Ojibwe RA anti-AGE+ | 20·46 | 4·10 | 20·0 |
| Caucasian normal | 2·54 | 0·06 | 2·4 |
| Caucasian RA anti-AGE− | 13·42 | 2·20 | 16·4 |
| Caucasian RA anti-AGE+ | 15·70 | 1·12 | 8·2 |
| Caucasian Type 1 diabetic | 6·94 | 0·52 | 7·5 |
AGE-modified proteins in IC
Elevated amounts of AGE-modified proteins in the IC were detected in the Ojibwe when compared with their Caucasian counterparts (Table 1). Interestingly, the level of IC glycation was greater in the Ojibwe normal individual even when compared with the RA anti-IgG-AGE− patient. Consistent with the trends seen in the IC levels, the Caucasian normal exhibited much lower levels of glycated IC proteins (0·06 mg/ml) than the RA patients. The presence of anti-IgG-AGE antibodies was associated with higher levels of AGE-modified IC in the Ojibwe RA patients.
Characterization of the IC AGE-modified proteins
A number of different proteins were captured by the APB column from the IC, as can be seen in Fig. 1. From the apparent molecular weights it was likely that immunoglobulin and albumin were among the proteins that bore AGE determinants. This was confirmed by immunoblot analysis using specific antisera (for a representative immunoblot see Fig. 2). Interestingly, when the AGE-modified proteins were probed with the anti-κ antisera an additional band of 28–30 kD was identified along with the typical κ band of 24 kD, but only in some of the lanes. Notably, the IC from anti-IgG-AGE+ patients were enriched with this heavily glycated κ-chain (lanes 4 and 7, Fig. 2).
Fig. 1.
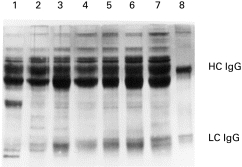
SDS–PAGE resolution of aminophenylboronic acid (APB)-purified proteins (5 μg/lane) from the immune complexes of patient and control representatives of North American Indian (NAI) or Caucasian (C) descent. Proteins were silver stained. Lane 1, NAI normal; lane 2, NAI RA anti-advanced glycation end-product (AGE)-negative; lane 3, NAI RA anti-AGE+ lane 4, C normal; lane 5, C RA anti-AGE−; lane 6, C RA, anti-AGE+ lane 7, C diabetic; lane 8, in vitro glycated IgG heavy chain (HC) and light chain (LC) purified by APB chromatography.
Fig. 2.
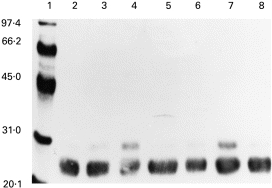
Immunoblot of aminophenylboronic acid (APB)-purified proteins (5 μg/lane) from the immune complexes of patient and control representatives of North American Indian (NAI) or Caucasian (C) descent probed with anti-κ antibodies. Lane 1, Molecular weight markers; lane 2, NAI normal; lane 3, NAI RA anti-advanced glycation end-product (AGE)-negative; lane 4, NAI RA anti-AGE+ lane 5, C normal; lane 6, C RA anti-AGE−; lane 7, C RA, anti-AGE+ lane 8, C diabetic.
A summary of the relative quantities of glycated proteins in serum IC from the selected patients and controls, as determined by densitometry of the immunoblots, is shown in Table 2. Within each ethnic group, greater amounts of glycated protein were present in IC from RA patients compared with healthy controls.
Table 2.
Relative amounts of advanced glycation end-product (AGE)-modified protein affinity purified from serum immune complexes of different patient and control individuals as determined by densitometry of immunoblots
| AGE-modified protein | Ojibwe normal | Ojibwe RA anti-AGE− | Ojibwe RA anti-AGE+ | Caucasian normal | Caucasian RA anti-AGE− | Caucasian RA anti-AGE+ | Caucasian IDDM anti-AGE− |
|---|---|---|---|---|---|---|---|
| γ heavy chain | 43·1* | 67·7 | 100 | 5·0 | 22·5 | 26·0 | 11·1 |
| μheavy chain | 7·4 | 100 | 84·3 | 3·3 | 7·1 | 20·3 | 8·0 |
| κ light chain | 35·9 | 59·5 | 100 | 4·6 | 21·3 | 31·7 | 17·6 |
| κ light chain† | 27·3 | 53·0 | 100 | 1·5 | 9·1 | 62·6 | 7·3 |
| λ light chain† | 21·1 | 76·7 | 100 | 4·6 | 10·6 | 24·4 | 8·4 |
| Albumin | 55·3 | 100 | 83·4 | 4·1 | 25·3 | 26·0 | 20·7 |
Values for a given protein are shown as a percentage of the highest relative density assigned as 100.
Heavily glycated form of approx. 28–30 kD.
Glycated IgG is selectively elevated in the IC from anti-IgG-AGE+ individuals
For the immunoglobulin components (heavy and light chains), the Ojibwe had up to 8–10-fold increased levels of such proteins with AGE modification relative to the Caucasian group. Interestingly, whereas IgG was the predominant glycated immunoglobulin in the complexes of Caucasians, both IgG and IgM were heavily glycated in the complexes of the North American Indians. The presence of anti-IgG-AGE antibodies in the RA patients was linked to an increase in the amount of glycated γ heavy chain present in the IC in both ethnic groups examined.
Glycated albumin was also present in the IC in all groups examined (Table 2), with elevated amounts detected in the Ojibwe. In contrast to what was observed with immunoglobulin, albumin-AGE levels were not elevated in the RA patients with anti-IgG-AGE antibodies in comparison with the RA patients lacking the anti-IgG-AGE antibodies. This suggests that the specificity of the anti-IgG-AGE antibodies is to a determinant on IgG-AGE which is not present on albumin-AGE.
As can be seen in Fig. 3, the immunoblot of the APB-purified IC probed with the anti-CML MoAb revealed a range of proteins with apparent molecular weights consistent with those seen in the SDS–PAGE (Fig. 1). Similar results, although with minor differences in the proteins and amounts recognized, were obtained with the anti-imidazolone MoAb (data not shown). CML-modified proteins of 58 kD were detected principally in the Ojibwe patients with RA. The Ojibwe also had CML-modified proteins of 42 kD and 48 kD which were not present in the Caucasians. The 42-kD CML-modified protein which was detected in the IC from one Ojibwe individual who was classified as normal was not detected in the IC from either his daughter (apparently normal) or another randomly chosen Ojibwe normal individual (data not shown). The identities of the 42-, 48- and 58-kD proteins are as yet unknown.
Fig. 3.
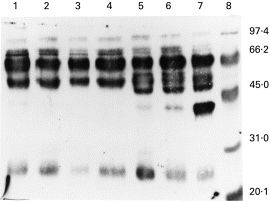
Immunoblot of aminophenylboronic acid (APB)-purified proteins (5 μg/lane) from the immune complexes of patient and control representatives of Caucasian (C) or North American Indian (NAI) descent probed with anti-Nε(carboxymethyl)lysine (CML) MoAbs. Lane 1, C diabetic; lane 2, C RA, anti-advanced glycation end-product (AGE)-positive; lane 3, C RA anti-AGE−; lane 4, C normal; lane 5, NAI RA anti-AGE+ lane 6, NAI RA anti-AGE−; lane 7, NAI normal; lane 8, molecular weight markers.
There was a significant linear correlation between the relative amounts of κ light chains, as recognized by the anti-κ antisera in the immunoblots, and the amounts of CML or imidazolone as recognized by the respective MoAbs in the 24-kD light chain band when all patient and control groups were analysed (r = 0·98, P = 0·0001 and r = 0·97, P = 0·0003, respectively).
DISCUSSION
RA engenders two conditions which accelerate AGE formation: hyperglycaemia, due to insulin resistance [20] most notably in some tribes of North American Indians [12,21], and oxidative stress, due to the release of free oxygen radicals which are a result of chronic inflammation [22,23]. However, only a very small percentage of patients with RA has concomitant diabetes, and the percent varies with the population studied. We have found that there are increased levels of glycated proteins in the IC of RA patients, relative to controls. The amounts of glycated proteins were found to be higher in the Ojibwe than in the Caucasians. Type II diabetes and RA are prevalent in North American Indian populations, with some tribes exhibiting the highest disease rates of any ethnic group. The Pima Indians were found to have an overall prevalence of RA of 5·3% (7% in females, 3% in males) [14] with familial aggregation of the disease [24], suggesting underlying genetic factors play an important role. In contrast, the prevalence in Caucasians in the USA is 1% (1·6% in females and 0·7% in males) [25]. RA disease onset is also earlier by approximately a decade in North American Indians when compared with other ethnic groups [26]. Thus, finding an over-representation of AGE-modified proteins in this ethnic group that has an earlier, more frequent and severe disease than in Caucasians has important implications in RA pathogenesis.
While ethnicity appeared to influence the levels of circulating AGE-modified proteins, so did the presence of a novel autoantibody directed against glycated IgG [11–13]. Anti-IgG-AGE antibodies could theoretically prevent the clearance of IgG-AGE by forming IC and thus blocking the recognition of AGE by specific receptors of the reticuloendothelial system [10]. This is the likely explanation for the elevated levels of glycated γ heavy chains in RA patients positive for anti-IgG-AGE antibodies, relative to those RA patients lacking the anti-AGE immune response. Furthermore, neither the levels of glycated μheavy chain nor glycated albumin were increased in the presence of anti-IgG-AGE, which is compatible with the hypothesis that the autoantibody is specific for glycated IgG. The anti-IgG-AGE immune response was also associated with the elevated levels of κ and λ light chains which are an integral part of IgG. Interestingly, elevated levels of a heavily glycated κ, in particular, were associated with the presence of the anti-IgG-AGE antibodies. We did not detect a similarly heavily glycated λ light chain, and this may reflect both the fact that 75% of all light chain genes used are κ and that there are more free amino groups that could be potentially glycated in κ chains than in λ (8–10 lysines and arginines/Vκ region, depending on the subgroup, compared with 4–8/Vλ with seven in the Cκ compared with six in the Cλ[27]). The precise locations of the AGE modifications on these molecules are as yet unknown.
From our previous studies, the anti-IgG-AGE immune response has been linked to the presence of RF [11,13], which suggests that RF-expressing B cells may mediate the induction of anti-AGE autoimmunity. RF B cells could endocytose IgG-AGE via membrane immunoglobulin (mIg), and process and present an AGE-containing IgG epitope in association with class II MHC (perhaps disease-conferring DR4) to autoreactive T cells. Indeed, B cells have been shown to be efficient antigen-presenting cells for self-antigen by virtue of the mIg, which can initiate processing of exogenously provided self-peptide into forms recognized by self-T cells [28]. The specificity of the anti-AGE response for IgG heavy and/or light chains rather than IgM is probably due to the longer half-life of IgG, and the specificity of the linked RF for IgG. Interestingly, we found a 90% frequency of RF, and a 52% frequency of anti-AGE seropositivity in a study of Ojibwe RA patients [12], both of which exceed rates in other ethnic groups.
From the SDS–PAGE and immunoblots of the APB-purified IC proteins, although there were many commonly AGE-modified proteins present in the IC of all of the groups examined, there appeared to be unique proteins that were AGE-modified, primarily in the Ojibwe when compared with the Caucasians. Although we can only speculate on their identity, it is possible that the 58-kD protein may be the glycated heavy chain of IgG3, but further studies are clearly warranted to characterize these proteins and determine how representative these data are among these ethnic groups.
The correlation observed between AGE determinants, namely CML and imidazolone, and κ light chain indicates that the variation between patient and control groups was due to accumulation of different amounts of similarly glycated proteins, rather than differing degrees of glycation per protein. The levels of IC were highest in RA patients, particularly the anti-IgG-AGE+ individuals.
The presence of an enrichment of glycated proteins in IC, especially when anti-IgG-AGE antibodies are present, has important clinical implications. Deposition of IgG-AGE-containing IC in the synovial space and elsewhere could contribute to the localized and systemic complement activation and inflammation in RA. The capacity of AGE to cross-link proteins could further facilitate IC deposition, e.g. by cross-linking IgG-AGE with AGE-modified proteins in the vascular wall or synovial space. Indeed, AGE-modified proteins have been detected co-localizing with IgG and IgM in the perineurion and blood vessels of an anti-IgG-AGE+ patient with RA vasculitis and peripheral neuropathy, as well as in synovial fluid from patients with RA (Newkirk, unpublished data).
Whether AGE accumulation and the anti-AGE autoimmune response are critical steps in disease pathogenesis or merely concomitant with its progression, their contribution to disease activity and severity is very likely. In further studies, development of an appropriate animal model to assess directly the role of AGE and anti-AGE antibodies in RA will be required. AGE-modified proteins, along with the anti-AGE immune response, could act as important diagnostic and prognostic markers of disease, and therapies which prevent or control their progression could potentially result in an improved quality of life for RA patients.
Acknowledgments
The authors thank Dr Laurence Rubin (The Toronto Hospitals), Dr Paul Fortin (The Montreal General Hospital) and Michelle Lucey for making available the clinical specimens. We thank Professor T. Niwa for making available the monoclonal antibodies specific for imidazolone. M.M.N. is a Senior Scholar of the Arthritis Society of Canada. This research was supported by grants from the Arthritis Society of Canada and the Alpha Omicron Pi Arthritis Fund.
REFERENCES
- 1.Robbins DL, Feigal Dw, Jr, Leek JC. Relationship of serum IgG rheumatoid factor and disease activity in rheumatoid arthritis. J Rheumatol. 1986;13:259–62. [PubMed] [Google Scholar]
- 2.Eberhardt KB, Truedsson L, Petersson H, et al. Disease activity and joint damage progression in early rheumatoid arthritis: relationship to IgG, IgA and IgM rheumatoid factors. Ann Rheum Dis. 1990;49:906–9. doi: 10.1136/ard.49.11.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Schaardenburg D, Hazes JMW, de Boer A, et al. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J Rheumatol. 1993;20:45–52. [PubMed] [Google Scholar]
- 4.Wagner U, Kaltenhauser S, Sauer H, et al. HLA markers and prediction of clinical course and outcome in rheumatoid arthritis. Arthritis Rheum. 1997;40:341–51. doi: 10.1002/art.1780400219. [DOI] [PubMed] [Google Scholar]
- 5.Weyand CM, Hicok KC, Conn DL, Goronzy JJ. The influence of HLA-DRβ1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992;117:801–6. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- 6.Hammer J, Gallazzi F, Bono E, et al. Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J Exp Med. 1995;181:1847–55. doi: 10.1084/jem.181.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Garcia J, Requena JR, Rodriguez-Segade R. Increased concentrations of serum pentosidine in rheumatoid arthritis. Clin Chem. 1998;44:250–5. [PubMed] [Google Scholar]
- 8.Takahashi M, Suzuki M, Kushida K, Miyamoto S, Inoue T. Relationship between pentosidine levels in serum and urine and activity in rheumatoid arthritis. Br J Rheumatol. 1997;36:637–42. doi: 10.1093/rheumatology/36.6.637. [DOI] [PubMed] [Google Scholar]
- 9.Bucala R, Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 10.Vlassara H. Recent progress in advanced glycation end products and diabetic complications. Diabetes. 1997;46(Suppl. 2):S19–25. doi: 10.2337/diab.46.2.s19. [DOI] [PubMed] [Google Scholar]
- 11.Ligier S, Fortin PR, Newkirk MM. A new antibody in rheumatoid arthritis targeting glycated IgG: IgM anti-IgG-AGE. Br J Rheumatol. 1998;37:1307–14. doi: 10.1093/rheumatology/37.12.1307. [DOI] [PubMed] [Google Scholar]
- 12.Newkirk MM, Lepage K, Niwa T, Rubin L. Advanced glycation endproducts (AGE) on IgG, a target for circulating antibodies in North American Indians with rheumatoid arthritis (RA) Cell Mol Biol. 1998;44:1129–38. [PubMed] [Google Scholar]
- 13.Lucey M, Newkirk MM, Neville C, et al. Association between the IgM response to IgG damaged by glyoxidation and disease activity in rheumatoid arthritis. J Rheumatol. 2000;27 [PubMed] [Google Scholar]
- 14.Del Puente A, Knowler WC, Pettitt DJ, Bennett PH. High incidence and prevalence of rheumatoid arthritis in Pima Indians. Am J Epidemiol. 1989;129:1170–8. doi: 10.1093/oxfordjournals.aje.a115238. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsson LTH, Knowler WC, Pillemer S, et al. Rheumatoid arthritis and mortality. A longitudinal study in Pima Indians. Arthritis Rheum. 1993;36:1045–53. doi: 10.1002/art.1780360804. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SJ, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Zubler RH, Perrin LH, Creighton WD, et al. Use of polyethylene glycol (PEG) to concentrate immune complexes from serum or plasma samples. Ann Rheum Dis. 1977;36:23–25. [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Wray W, Boulikas T, Wray VP, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 20.Svenson KLG, Pollare T, Lithell H, Hallgren R. Impaired glucose handling in active rheumatoid arthritis: relationship to peripheral insulin resistance. Metabolism. 1988;37:125–30. doi: 10.1016/s0026-0495(98)90005-1. [DOI] [PubMed] [Google Scholar]
- 21.Sakul H, Pratley R, Cardon L, et al. Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. Am J Hum Genet. 1997;60:651–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Lunec J, Halloran SP, White AG, Dormandy TL. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981;8:233–45. [PubMed] [Google Scholar]
- 23.Kaur H, Edmonds SE, Blake DR, Halliwell B. Hydroxyl radical generation by rheumatoid blood and knee joint synovial fluid. Ann Rheum Dis. 1996;55:915–20. doi: 10.1136/ard.55.12.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch R, Lin JP, Scott WW, et al. Rheumatoid arthritis in the Pima Indians. Arthritis Rheum. 1998;41:1464–9. doi: 10.1002/1529-0131(199808)41:8<1464::AID-ART17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Engel A, Roberts J, Burch TA. Rheumatoid arthritis in adults United States 1960–62. Public Health Service Publication, No 1000, Series 11, no. 17. 1966 [PubMed] [Google Scholar]
- 26.Jacono J, Jacono B, Cano P, Segami M, Rubin L. An epidemiological study of rheumatoid arthritis in a northern Ontario clinical practice: the role of ethnicity. J Adv Nursing. 1996;24:31–35. doi: 10.1046/j.1365-2648.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 27.Kabat EA, Wu TT, Perry HM, et al. NIH Publication. 5. 1991. Sequences of proteins of immunological interest; pp. 91–3242. [Google Scholar]
- 28.Barlow AK, He X, Janeway C. Exogenously provided peptides of a self-antigen can be processed into forms that are recognized by self-T cells. J Exp Med. 1998;187:1403–15. doi: 10.1084/jem.187.9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]


