Abstract
The up-regulated B cell responses detectable in cerebrospinal fluid (CSF) and the augmented myelin antigen-specific T cell responses observed in the CSF as well as systematically in patients with multiple sclerosis (MS) suggest the involvement of cytokines in disease development and perpetuation. Here we report on the parallel involvement of TNF-α, IL-6, IFN-γ and IL-10 in MS and controls, using enzyme-linked immunospot (ELISPOT) assays to detect and enumerate cytokine-secreting mononuclear cells (MNC) prepared from blood and, for IL-6 and IL-10, from CSF without in vitro stimulation. MS is associated with elevated levels of TNF-α-secreting blood MNC when compared with levels in groups of control patients with myasthenia gravis (MG) and other neurological diseases (OND) or healthy subjects. This elevation was confined to patients with untreated MS and not present in those examined during ongoing treatment with IFN-β. Untreated patients with MS had lower numbers of IL-10-secreting blood MNC compared with the three control groups. In patients undergoing treatment with IFN-β, numbers of IL-10-secreting cells were in the same range as in controls. Normalization of TNF-α from elevated, and of IL-10 from decreased levels could be one reason for the beneficial effects of IFN-β in MS, although it remains to be shown whether these changes reflect phenomena primarily involved in MS pathogenesis or secondary changes. In CSF, levels of IL-10-secreting cells were higher than in blood in both MS and OND, with no difference between these groups. Systemic aberrations of IL-6 and IFN-γ and of IL-6 in CSF in MS versus controls were only minor, irrespective of treatment with IFN-β.
Keywords: multiple sclerosis, cytokines, ELISPOT assay
INTRODUCTION
Multiple sclerosis (MS) is assumed to be an autoimmune disease of unknown aetiology affecting the central nervous system (CNS). MS is the second most common cause of disability after trauma among young and middle-aged individuals of Caucasian origin. Abnormal immune responses to CNS myelin antigens are considered pivotal in MS pathogenesis [1].
Cytokines are regulatory proteins secreted by a variety of cells. The pleiotropic actions of cytokines include numerous effects on cells of the immune system, resulting in modulation of immune responses. In experimental allergic encephalomyelitis (EAE) that is commonly used as an animal model of MS, T helper type 1 (Th1) cells that produce the proinflammatory cytokines TNF-α, IL-1 and IL-12 are involved in the initiation of disease, whereas Th2 cells producing IL-4 and IL-10 and the Th3 cell-related cytokine transforming growth factor-beta (TGF-β) appear in the CNS during the recovery phase [2]. Several studies have addressed the question of cytokine levels in MS, with partly conflicting results [3]. Thus, the role of individual cytokines like TNF-α[4,5], IL-6 [6,7], IFN-γ[8,9] and IL-10 [10,11] is still controversial.
The contrasting results on cytokine levels in MS most probably reflect methodological dilemmas as well as the complex biology of the cytokines. Cytokines are secreted locally and detected in unbound state at very low concentrations, have a short half-life and are rapidly taken up by surrounding cells and metabolized [12]. Most previous studies focused on examining one single cytokine. Although it is important to know the levels of individual cytokines, such studies do not allow analysis of any relationships between levels of different cytokines in the same subject. Since one cytokine can modulate the secretion of other cytokines, it seems more useful to examine several related cytokines in parallel in order to understand any involvement of the cytokine network in health and disease.
IFN-β-1b is beneficial in the treatment of relapsing remitting (RR) MS [13] and secondary progressive (SP) MS [14]. Even though it is assumed that the beneficial effect of IFN-β-1b is related to immunomodulation [15], the exact mechanisms of action of IFN-β-1b in MS are unsettled.
In the present study we investigated the numbers of IL-6, IL-10, TNF-α and IFN-γ-secreting blood mononuclear cells (MNC) in patients with MS and groups of controls by enzyme-linked immunospot (ELISPOT) assays, enabling the enumeration of individual cells secreting the mentioned cytokines per standardized (105) numbers of MNC prepared from peripheral blood. Levels of IL-6- and IL-10-secreting cells in cerebrospinal fluid (CSF) were examined in parallel.
PATIENTS and METHODS
Patients with MS and controls
Eighty-six patients (61 females) had clinically definite MS [16]. Their age range was 23–65 years (mean 45 years). The duration of MS was 2–38 years (mean 13 years). Six patients were examined during exacerbation, which was defined as a sudden appearance of new symptoms and signs or the sudden reappearance or worsening of earlier present symptoms lasting more than 24 h and occurring within 1 month before examination. Thirty-five patients were in remission. Thirty-eight patients were examined during the SP phase of MS. Seven patients had primary progressive (PP) MS. Twenty-five patients had no or slight disability, defined as an expanded disability status scale (EDSS) score <3, while 61 patients had moderate or severe disability defined as an EDSS score ≥3 [17]. The duration of MS was <10 years in 35 patients and ≥10 years in 51 patients. Twenty of the patients with MS were treated with IFN-β-1b (Betaferon; Schering AG, Berlin, Germany) at a dosage of 8 MU subcutaneously every other day at the time of inclusion in the study. None of the 66 untreated patients with MS had received corticosteroids or any other immunomodulatory therapy during the last 6 months before inclusion in the study.
In parallel, three control groups were examined: (i) 62 patients (26 females) had other neurological diseases (OND). Their age range was 20–79 years (mean 48 years). Twenty-two of the OND patients had tension headache, 13 had cerebrovascular diseases, 12 had epilepsia, six had dementia, four had Parkinson’s disease, two had vertigo, two had back pain and one had chronic pain syndrome; (ii) 18 patients had myasthenia gravis (MG). Their ages varied between 12 and 72 years (mean 52 years). The diagnosis of MG was based on characteristic clinical signs and symptoms, the results from laboratory tests that included determinations of serum antibody concentrations to acetylcholine receptor (AChR) and single fibre EMG, and response to treatment with choline esterase inhibitors. The onset of MG before the age of 40 was documented in 10 patients. The duration of MG varied between 2 and 52 years (mean 32 years). One patient had undergone thymectomy and eight patients were treated with immunosuppressive agents at the time of sampling. Eleven patients had mild generalized MG (IIa) according to the classification of Osserman & Genkins [18], three patients had ocular MG (I) and four patients were examined in remission; (iii) blood specimens were also obtained from 36 healthy subjects (25 females; staff of the Department) with an age range of 23–65 years (mean 35 years).
The study protocol was approved by the Ethical Committee of Karolinska Institutet at Huddinge University Hospital (Dnr 334/98) and informed consent was obtained from all patients.
Preparation of MNC from blood and CSF
Peripheral blood was obtained by venous puncture between 8 and 12 am. Sampling from the IFN-β-1b-treated patients took place 36–40 h after the last injection of IFN-β-1b. MNC were separated by density gradient centrifugation on Lymphoprep (Nycomed, Oslo, Norway) within 2 h after blood sampling. The cells from the interphase were collected, washed three times with Dulbecco’s modification of Eagle’s medium (Gibco, Paisley, UK) supplemented with antibiotics, 10% fetal calf serum (Gibco), 1% minimal essential medium (Gibco), and 1% of l-glutamine (Gibco), and adjusted to a concentration of 1 × 106 cells/ml. As per testing by the manufacturers, all solutions, medium, and chemicals used in the ELISPOT assays were either endotoxin-free or contained very low levels of endotoxin.
CSF was centrifuged for 10 min at 200 g. After discarding the supernatant, cells were counted and adjusted to a concentration of 1 × 105 cells/ml in medium (see above).
Cell viability as measured by trypan blue exclusion always exceeded 95%.
ELISPOT assays for detection of cytokine-secreting cells
To detect and enumerate IL-10- and IFN-γ-secreting MNC, an ELISPOT assay described by Czerkinsky et al. [19] was adopted [20]. Microtitre plates with nitrocellulose bottoms (Multiscreen-HA plates; Millipore, Mulsheim, France) were coated overnight at 4°C with 100 μl/well of MoAb to human IL-10 or IFN-γ (both from Mabtech, Stockholm, Sweden) diluted in filtered PBS (pH 7·4) to a concentration of 10 μg/ml. After removal of coating solutions by washings with PBS, 200-μl aliquots containing 2 × 105 blood MNC were applied to individual wells in duplicates. For CSF, 200-μl aliquots containing 1–2 × 104 MNC were applied to individual wells, if possible in duplicates. As a negative control, complete medium was also added to wells in the absence of MNC. Plates were incubated at 37°C for 48 h in humidified air containing 5% CO2, then emptied and washed. A biotinylated MoAb (100 μl) to human IL-10 or IFN-γ (12G8 and 7-B6-1, respectively; both from Mabtech) diluted in filtered PBS to a concentration of 1 μg/ml was added overnight at 4°C. Wells were washed, and 100 μl of streptavidin-alkaline phosphatase (Mabtech) diluted 1:1000 were added for 1·5 h at room temperature. After incubation, plates were emptied, washed and stained with BCIP-NBT (Gibco) diluted in Tris-buffer. Blue immunospots, each considered to represent a cell secreting IL-10 or IFN-γ, were counted with a dissection microscope under ×25 magnification.
Intra-assay and interassay variations of the assays were <10% for numbers of IL-10- and IFN-γ-secreting cells [21].
To detect and enumerate IL-6- and TNF-α-secreting MNC, the ELISPOT assay of Czerkinsky et al. [19] as adopted by Rönnelid et al. [22] was employed. Ninety-six-well plastic immunoplates (Nunc, Roskilde, Denmark) were used. The plates were coated for 3 h at room temperature with 50 μl/well of MoAb to human IL-6 (PharMingen, San Diego, CA) or TNF-α (Genzyme, Cambridge, MA) diluted in PBS to a concentration of 15 μg/ml. This concentration was found to yield high numbers of cytokine-secreting cells in kinetic studies. After removal of coating solutions by washings with PBS, 200-μl aliquots containing 2 × 103 MNC/well for IL-6 and 1 × 103 MNC/well for TNF-α were applied to individual wells in duplicates. As a negative control, complete medium was also added to wells in the absence of MNC. Plates were incubated at 37°C for 20 h in humidified air containing 5% CO2, then emptied and washed. Biotinylated MoAbs (50 μl) to human IL-6 (PharMingen) or TNF-α (Genzyme) diluted in filtered PBS to a concentration of 1 μg/ml were added overnight at 4°C followed by enzyme-conjugated ABC (Dako, Glostrup, Denmark) and BCIP phosphatase substrate (Sigma, St Louis, MO). Blue immunospots, each considered to represent a cell secreting IL-6 or TNF-α, were counted with a dissection microscope under ×25 magnification.
The mean values of duplicates were calculated. Results were presented as numbers of IL-6, TNF-α, IL-10 and IFN-γ-secreting cells per 105 blood MNC. Variation of duplicates was regularly <10%.
To test the day to day variability of the assay, MNC were prepared from blood specimens on two consecutive days from five healthy subjects and examined for numbers of IL-6- and TNF-α-secreting cells. The intertest variation in numbers of IL-6- and TNF-α-secreting cells was <10% (data not shown).
Statistical analysis
The non-parametric Kruskal–Wallis anova and Mann–Whitney’s U-tests were used for group comparison. Wilcoxon signed rank test was used when comparing paired samples from the same individual. Spearman’s rank test was used for correlation.
RESULTS
The patients with MS had higher numbers of TNF-α-secreting cells in blood compared with patients with OND (P < 0·05) or MG (P < 0·05), or the healthy subjects (P < 0·01) (Fig. 1b). Numbers of IL-6-secreting blood MNC were also higher (P < 0·05) in MS compared with healthy subjects (Fig. 1a). No differences were observed in levels of IL-6-secreting cells when comparing MS with OND and MG. There were no differences for levels of IFN-γ- or IL-10-secreting blood MNC in MS versus OND, MG or healthy subjects (Fig. 1c,d).
Fig. 1.
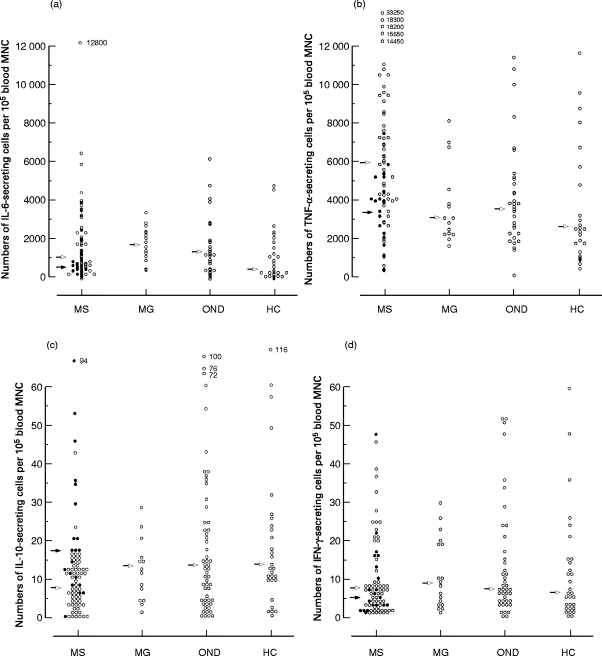
Numbers of (a) IL-6, (b) TNF-α, (c) IL-10 and (d) IFN-γ-secreting cells per 105 blood mononuclear cells (MNC) from patients with multiple sclerosis (MS), myasthenia gravis (MG), other neurological diseases (OND) and healthy subjects (HC). Arrows refer to median values. Closed circles and arrows refer to patients with MS treated with IFN-β-1b. Observe different scales used in (a,b) versus (c,d).
In MS, a positive correlation was observed between numbers of TNF-α and IL-6 (r = 0·65; P < 0·0001), TNF-α and IFN-γ (r = 0·34; P < 0·01) and IL-6 and IFN-γ (r = 0·29; P < 0·05) secreting blood MNC. A positive correlation between TNF-α and IL-6 was also observed in patients with OND (r = 0·62; P < 0·0001), MG (r = 0·8; P < 0·0001) and healthy subjects (r = 0·56; P < 0·01).
Subgrouping of the patients with MS regarding treatment with IFN-β revealed that untreated patients had higher (P < 0·001) levels of TNF-α- and lower (P < 0·05) levels of IL-10-secreting cells in blood compared with the patients with MS examined during ongoing treatment with IFN-β (Fig. 1b,c). There were no differences for levels of IL-6- or IFN-γ-secreting cells between these two subgroups.
Subgrouping of the patients with MS regarding treatment with IFN-β revealed that the differences between MS and controls for TNF-α- and IL-6-secreting blood MNC were confined to the patients with untreated MS. These patients had thus higher levels of TNF-α-secreting blood MNC compared with patients with OND (P < 0·0001) or MG (P < 0·01), or with healthy subjects (P < 0·0001), and also higher levels of IL-6-secreting blood MNC compared with healthy subjects (P < 0·05). In contrast, no differences were observed for TNF-α- or IL-6-secreting blood MNC between patients with MS examined during ongoing treatment with IFN-β and any of the three control groups.
The patients with untreated MS had lower levels of IL-10-secreting blood MNC compared with the patients with OND (P < 0·01) or MG (P < 0·05), or the healthy subjects (P < 0·05). No such differences in levels of IL-10-secreting blood MNC were, on the other hand, encountered when considering the patients with MS treated with IFN-β.
There were no differences in IFN-γ-secreting blood MNC between patients with MS subgrouped regarding treatment with IFN-β and the three control groups.
In the group of patients with untreated MS, a positive correlation was observed between numbers of TNF-α and IL-6 (r = 0·71; P < 0·0001), TNF-α and IFN-γ (r = 0·35; P < 0·05) and IL-6 and IFN-γ-secreting blood MNC (r = 0·42; P < 0·01). In contrast, no correlations were observed between the levels of cells secreting cytokines in patients treated with IFN-β.
After subgrouping the untreated patients with MS regarding clinical variables, there were no significant differences in numbers of TNF-α, IL-6, IFN-γ and IL-10-secreting cells when comparing RR with SP course, high (EDSS ≥3) with low (EDSS <3) disability, or short (< 10 years) with long duration (≥ 10 years) of MS (Table 1).
Table 1.
Numbers of TNF-α, IL-6, IFN-γ and IL-10-secreting cells per 105 blood mononuclear cells (MNC) from patients with untreated multiple sclerosis (MS) upon subgrouping regarding clinical variables
| MS patients groups | TNF-α | IL-6 | IFN-γ | IL-10 | |
|---|---|---|---|---|---|
| All untreated patients | Range | 650–33 250 | 25–12 800 | 1–46 | 1–43 |
| Mean (s.d.) | 7014 (5086) | 1812 (2075) | 10 (11) | 9 (7) | |
| Median | 6000 | 1013 | 7 | 8 | |
| No. exam. | 58 | 55 | 55 | 56 | |
| PP | Range | 2850–33 250 | 110–6600 | 2–21 | 5–13 |
| Mean (s.d.) | 11 390 (12 621) | 1827 (2704) | 9 (11) | 8 (4) | |
| Median | 5900 | 700 | 3 | 6 | |
| No. exam. | 6 | 6 | 6 | 6 | |
| MS exacerbation | Range | 650–10 500 | 250–4100 | 4–20 | 8–17 |
| Mean (s.d.) | 5470 (3681) | 1820 (1559) | 9 (7) | 12 (5) | |
| Median | 4800 | 1350 | 7 | 12 | |
| No. exam. | 6 | 6 | 5 | 4 | |
| MS remission | Range | 1100–18 200 | 25–12 800 | 1–46 | 1–24 |
| Mean (s.d.) | 6528 (4723) | 2222 (3052) | 10 (11) | 10 (6) | |
| Median | 4425 | 1075 | 7 | 12 | |
| No. exam. | 16 | 18 | 21 | 21 | |
| SP | Range | 1900–14 450 | 150–6025 | 1–39 | 1–43 |
| Mean (s.d.) | 6710 (2920) | 1599 (1402) | 9 (11) | 9 (9) | |
| Median | 6325 | 1000 | 5 | 6 | |
| No. exam. | 27 | 28 | 24 | 24 | |
| EDSS <3 | Range | 650–18 200 | 6600–12 800 | 1–46 | 1–16 |
| Mean (s.d.) | 6781 (5365) | 2385 (3222) | 11 (12) | 11 (5) | |
| Median | 4350 | 1075 | 7 | 12 | |
| No. exam. | 16 | 18 | 19 | 18 | |
| EDSS ≥3 | Range | 1900–33 250 | 110–6600 | 1–39 | 1–43 |
| Mean (s.d.) | 7308 (5495) | 1704 (1200) | 8 (10) | 10 (8) | |
| Median | 6000 | 1200 | 5 | 9 | |
| No. exam. | 39 | 40 | 37 | 37 |
PP, Primary progressive phase of MS; SP, secondary progressive phase of MS; EDSS, expanded disability status scale. There were no differences between patients examined during exacerbation versus remission, versus SP, or between patients with EDSS <3 versus EDSS ≥3.
MNC were prepared from CSF and blood obtained in parallel from some of the untreated patients with MS as well as with OND, and examined for IL-6- and IL-10-secreting cells (Fig. 2). In both MS and OND, levels of IL-10-secreting cells were higher in CSF than in blood (P < 0·0001 for both comparisons). This was not seen for IL-6-secreting cell levels.
Fig. 2.
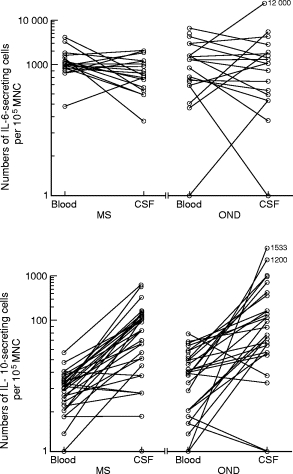
Numbers of IL-6- and IL-10-secreting cells per 105 cerebrospinal fluid (CSF) and blood mononuclear cells (MNC) from patients with multiple sclerosis (MS) and other neurological diseases (OND).
In MG, numbers of IL-6-secreting cells were higher compared with healthy subjects.
DISCUSSION
Analysis of cytokine production patterns is becoming increasingly important in understanding physiological responses and pathological mechanisms during immune stimulation and to improve therapies. ELISPOT assays are among the most sensitive and specific assays available for cytokine research. They permit the ex vivo identification of cells actively secreting cytokines. ELISPOT assays are 10–200 times more sensitive than ELISAs [23], efficient (when using cytokine gene transfectants, 100% of cells can be detected) and fast. ELISPOT assays can detect a single cell out of a million, and they can detect cells secreting <100 molecules of a certain cytokine per second [24]. Drawbacks of ELISPOT assays are that they do not allow detection of the phenotype of cell secreting cytokine, are difficult to establish, and are expensive.
The discrepancies between present data obtained with ELISPOT assays and previous data using other methods such as in situ hybridization (ISH) and ELISA are most probably related to the methodological differences used and the different variables measured by these methods. Furthermore, the levels of cytokines change not only with the health status of the individual but also with age [25], physical activity [26], diet [27] and psychological stress [28]. It is difficult to exclude the influence of such factors on the present as well as most previously published cytokine data.
Using ELISPOT assays, Sun et al. [8] reported similar levels of IFN-γ-secreting cells in MS compared with controls. Elevated levels of IL-2-secreting blood MNC were reported in patients with MS compared with tension headache [29]. Interestingly, lower levels of IL-10-secreting blood MNC in patients with MS were also reported [21]. This is the first study where ELISPOT assays have also been used to detect TNF-α- and IL-6-secreting cells. As both TNF-α and IL-6 might play a role in MS, detection of their levels could give new evidence about their importance.
TNF-α is a proinflammatory cytokine with functions ranging from induction of cell adhesion molecules and MHC class II expression to differentiation of many cell types [30]. A TNF-α-dependent cytokine network was shown to favour development of Th1 immune responses through induction of IL-12 production [31].
Accumulating evidence supports an essential role of IL-6 in the development, differentiation, regeneration and degeneration of neurones in the peripheral and central nervous system [32]. IL-6 was found in MS brain lesions [33]. In rats with protracted relapsing EAE, peak IL-6 mRNA expression in CNS sections and CSF corresponded to the maximum severity of clinical disease [2], and IL-6-deficient mice are resistant to myelin oligodendrocyte glycoprotein (MOG)-induced EAE [34]. However, recent reports suggest that IL-6 has also many anti-inflammatory and immunosuppressive effects [35]. The role of IL-6 in MS is unknown. The elevated numbers of IL-6-secreting blood MNC observed in MS compared with healthy subjects, presented here, were not specific for MS but also observed in OND and MG.
The immunoregulatory properties of IL-10 such as suppression of antigen-specific proliferation of Th1 clones and inhibition of the synthesis of many Th1 cell-related cytokines (IFN-γ, TNF-α, TNF-β, IL-1, IL-2, IL-6) [36] could be beneficial in MS. The low levels of IL-10-secreting cells observed in untreated MS might result from an augmentation of proinflammatory cytokines that could be harmful in MS. Nasal administration of IL-10 suppressed acute and protracted-relapsing EAE [37]. IL-10 is a potential candidate to treat MS.
In the present study patients with MS, regardless of IFN-β-1b treatment, had similar levels of IFN-γ-secreting cells to controls.
These are the first data on IL-6 and IL-10 employing ELISPOT assays on CSF cells. In contrast to blood levels, the numbers of IL-10-secreting MNC in CSF were similar in untreated MS patients compared with patients with OND. The reason for this discrepancy could be the possible different nature of cytokine regulation in the CSF compared with blood. No difference was observed for IL-6-secreting cell levels in CSF compared with blood either in MS or in OND. Patients with MS and OND had similar levels of IL-6 in CSF as well, a finding that was recently reported using bioassays [38].
IFN-β-1b benefits patients with MS. Whether IFN-β affects the course of MS through the induction or suppression of cytokines or by other mechanisms is not known. In vitro tests revealed that IFN-β-1b increased IL-10 production by T cells [39] and monocytes [40]. In the present study, IFN-β-1b-treated patients with MS had higher numbers of IL-10- and lower numbers of TNF-α-secreting cells compared with untreated patients when blood MNC were studied without any stimulation. The present findings support the role of IFN-β-1b in up-regulating IL-10. This might reflect a direct effect of IFN-β-1b on cytokine production, as was shown in vitro[41], but it is also possible that this is just a reflection of immune responses regulated by other mechanisms. Recently, Rudick et al. [42] reported higher plasma levels of IL-10 in MS after IFN-β-1a treatment.
Numbers of IL-6-secreting blood MNC were elevated in patients with MG compared with healthy subjects. IL-6 might be important for antibody production against AChR in MG pathogenesis.
In conclusion, untreated MS is associated with high levels of TNF-α- and low levels of IL-10-secreting blood MNC compared with controls. The finding indicates an imbalance in MS between Th1 and Th2 cytokines. This imbalance was not observed in patients with MS undergoing treatment with IFN-β-1b. Thus treated patients had numbers of IL-10- and TNF-α-secreting blood MNC similar to those observed in controls. The molecular mechanisms and clinical relevance of this cytokine dysregulation are not known.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council, the Swedish Medical Association and funds from the Karolinska Institute.
REFERENCES
- 1.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 2.Diab A, Zhu J, Xiao BG, Mustafa M, Link H. High IL-6 and low IL-10 in the central nervous system are associated with protracted relapsing EAE in DA rats. J Neuropathol Exp Neurol. 1997;56:641–50. [PubMed] [Google Scholar]
- 3.Navikas V, Link H. Cytokines and the pathogenesis of MS. J Neurosci Res. 1996;45:322–33. doi: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Franciota DM, Grimaldi LME, Martino GV, Piccolo G, Bergamaschi R, Cittero A, Melzi d’Eril GV. Tumor necrosis factor in serum and cerebrospinal fluid of patients with multiple sclerosis. Ann Neurol. 1989;26:787–9. doi: 10.1002/ana.410260618. [DOI] [PubMed] [Google Scholar]
- 5.Navikas V, Be B, Link J, et al. Augmented expression of tumor necrosis factor-α and lymphotoxin mRNA in mononuclear cells in multiple sclerosis and optic neuritis. Brain. 1996;119:213–23. doi: 10.1093/brain/119.1.213. [DOI] [PubMed] [Google Scholar]
- 6.Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991;32:67–74. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- 7.Navikas V, Matusevicius D, Söderström M, Fredrikson S, Kivisäkk P, Ljungdahl Å, Höjeberg B, Link H. Increased interleukin-6 mRNA expression in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis. J Neuroimmunol. 1996;64:63–69. doi: 10.1016/0165-5728(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 8.Sun JB, Olsson T, Wang WZ, Xiao BG, Kostulas V, Fredrikson S, Ekre HP, Link H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991;21:1461–8. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- 9.Link J, Söderström M, Olsson T, Höjeberg B, Ljundahl Å, Link H. Increased transforming growth factor-β, interleukin-4 and interferon-γ in multiple sclerosis. Ann Neurol. 1994;36:379–86. doi: 10.1002/ana.410360309. [DOI] [PubMed] [Google Scholar]
- 10.Navikas V, Link J, Palasik W, Söderström M, Fredrikson S, Olsson T, Link H. Increased mRNA expression of IL-10 in mononuclear cells in multiple sclerosis and optic neuritis. Scand J Immunol. 1995;41:171–8. doi: 10.1111/j.1365-3083.1995.tb03550.x. [DOI] [PubMed] [Google Scholar]
- 11.Salmaggi A, Dufour A, Eoli M, Corsini E, La Mantia L, Massa G, Nespolo A, Milanese C. Low serum interleukin levels in multiple sclerosis: further evidence for decreased systemic immunosuppression? J Neurol. 1996;243:13–17. doi: 10.1007/BF00878525. [DOI] [PubMed] [Google Scholar]
- 12.Barnes A. Measurements of serum cytokines. Lancet. 1998;352:324–5. doi: 10.1016/S0140-6736(05)60303-0. [DOI] [PubMed] [Google Scholar]
- 13.The IFNB Multiple Sclerosis Study Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45:1277–85. [PubMed] [Google Scholar]
- 14.European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicenter randomized trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491–7. [PubMed] [Google Scholar]
- 15.Arnason BW, Reder AT. Interferons and multiple sclerosis. Clin Neuropharm. 1994;154:495–547. [Google Scholar]
- 16.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Osserman KE, Genkins G. Studies in myasthenia gravis: review of twenty year experience in over 1200 patients. Mount Sinai J Med. 1971;38:497–537. [PubMed] [Google Scholar]
- 19.Czerkinsky CC, Andersson G, Ekre HP, Nilsson LÅ, Klareskog L, Ouchterlony Ö. Reverse ELISPOT assay for clinical analysis of cytokine production. I. Enumeration of gamma-interferon secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 20.Link H, Olsson O, Sun J, et al. Acetylcholine receptor-reactive T and B cells in myasthenia gravis and controls. J Clin Invest. 1991;87:2191–6. doi: 10.1172/JCI115253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özenci V, Kouwenhoven M, Huang YM, Xiao BG, Kivisäkk P, Fredrikson S, Link H. Multiple sclerosis: levels of IL-10 secreting blood mononuclear cells are low in untreated patients but augmented during IFN-β-1b treatment. Scand J Immunol. 1999;49:554–61. doi: 10.1046/j.1365-3083.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 22.Rönnelid J, Klareskog L. A comparison between ELISPOT methods for the detection of cytokine producing cells: greater sensitivity and specificity using ELISA plates as compared to nitrocellulose membranes. J Immunol Methods. 1997;15:17–26. doi: 10.1016/s0022-1759(96)00170-6. [DOI] [PubMed] [Google Scholar]
- 23.Tanguay S, Killion J. Direct comparison of ELISPOT and ELISA based assays for detection of individual cytokine secreting cells. Lymphokine Cytokine Res. 1994;12:903–11. [PubMed] [Google Scholar]
- 24.Shirai A, Holmes K, Klinman DM. Detection and quantitation of cells secreting IL-6 under physiological conditions in BALB/c mice. J Immunol. 1993;150:793–9. [PubMed] [Google Scholar]
- 25.O’Mahony L, Holland J, Jackson J, Feigherty C, Hennessy TPJ, Mealy K. Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clin Exp Immunol. 1998;113:213–9. doi: 10.1046/j.1365-2249.1998.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nehlsen-Cannarella SL. Cellular response to moderate and heavy exercise. Can J Physiol Pharmacol. 1998;76:485–9. doi: 10.1139/cjpp-76-5-485. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes G, Venkatraman JT, Turturro A, Attwood VG, Hart RW. Effect of food restriction on life span and immune functions in long-lived Fisher-344 x Brown Norway F1 rats. J Clin Immunol. 1997;17:85–95. doi: 10.1023/a:1027344730553. [DOI] [PubMed] [Google Scholar]
- 28.Ackerman KD, Martino M, Heyman R, Moyna RH, Rabin BS. Stressor-induced alternations of cytokine production in multiple sclerosis patients and controls. Psychosom Med. 1998;60:484–91. doi: 10.1097/00006842-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Lu CZ, Fredrikson S, Xiao BG, Link H. Interleukin-2 secreting cells in multiple sclerosis and controls. J Neurol Sci. 1993;120:99–106. doi: 10.1016/0022-510x(93)90032-t. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Tracey KJ. Tumor necrosis factor. In: Thomson A, editor. The cytokine handbook. 3. San Diego: Academic Press; 1999. pp. 517–48. [Google Scholar]
- 31.Becher B, Blain M, Giacomini PS, Antel JP. Inhibition of Th1 polarization by soluble TNF receptor is dependent on antigen presenting cell-derived IL-12. J Immunol. 1999;162:684–8. [PubMed] [Google Scholar]
- 32.Gadient RA, Otten UH. Interleukin-6 (IL-6)—a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52:379–90. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 33.Maimone D, Guazzi GC, Annunziata P. IL-6 detection in multiple sclerosis brain. J Neuro Sci. 1997;146:59–65. doi: 10.1016/s0022-510x(96)00283-3. [DOI] [PubMed] [Google Scholar]
- 34.Okuda Y, Sakoda S, Bernard CCA, Fujimara H, Saeki Y, Kishimato T, Yanagihara T. IL-6 deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10:703–8. doi: 10.1093/intimm/10.5.703. [DOI] [PubMed] [Google Scholar]
- 35.Tilg H, Dinarello CA, Mier JW. IL-6 and AAPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–32. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 36.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao BG, Bai XF, Zhang GX, Link H. Suppression of acute and protracted-relapsing experimental allergic encephalomyelitis by nasal administration of low-dose IL-10 in rats. J Neuroimmunol. 1998;84:230–7. doi: 10.1016/s0165-5728(97)00264-6. [DOI] [PubMed] [Google Scholar]
- 38.Sekizawa T, Openshaw H, Ohbo K, Sugamura K, Itoyama Y, Niland JC. Cerebrospinal fluid interleukin 6 in amyotropic parameter and comparison with inflammatory and non-inflammatory central nervous system diseases. J Neurol Sci. 1998;154:194–9. doi: 10.1016/s0022-510x(97)00228-1. [DOI] [PubMed] [Google Scholar]
- 39.Rep MHG, Hintzen RQ, Polman CH, van Lier RAW. Recombinant interferon-β blocks proliferation but enhances interleukin-10 secretion by activated human T cells. J Neuroimmunol. 1996;67:111–8. doi: 10.1016/0165-5728(96)00060-4. [DOI] [PubMed] [Google Scholar]
- 40.Porrini AM, Gambi D, Reder AT. Interferon effects on interleukin-10 secretion. Mononuclear cell response to interleukin-10 is normal in multiple sclerosis patients. J Neuroimmunol. 1995;61:27–34. doi: 10.1016/0165-5728(95)00070-i. [DOI] [PubMed] [Google Scholar]
- 41.Yong VW, Chabot S, Stuve O, Williams G. Interferon beta in the treatment of multiple sclerosis. Neurology. 1998;51:682–9. doi: 10.1212/wnl.51.3.682. [DOI] [PubMed] [Google Scholar]
- 42.Rudick RA, Ransohoff RM, Lee JC, Peppler R, Yu M, Mathisen PM, Tuohy VK. In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology. 1998;50:1294–300. doi: 10.1212/wnl.50.5.1294. [DOI] [PubMed] [Google Scholar]


