Abstract
We studied the effects of the cytokines IL-1α, IL-6, tumour necrosis factor-alpha (TNF-α), IL-4, IL-10, IL-13 and transforming growth factor-beta (TGF-β) on fibronectin (FN) production by cultured-human monocytes. IL-1α, IL-6 and TNF-α all increased FN production, an indicator of monocyte activation. These cytokines increased FN production in a dose-dependent fashion, with a 4-h treatment being sufficient to measure FN production by radioimmunoassay. Conversely, IL-4, IL-10 and IL-13 strongly inhibited cytokine-induced FN production, while TGF-β only partially inhibited FN production. The combination of suboptimal doses of cytokines (IL-1α+IL-6, IL-1α+TNF-α, IL-6 +TNF-α), which could not singly induce substantial amounts of FN, were able to induce FN production by cultured monocytes. Northern blot analysis with a cDNA specific for FN confirmed the expression of FN mRNA in cultured monocytes stimulated with a single cytokine or a combination of cytokines. Our data demonstrate that monocytes may not always require high concentrations of cytokines for activation in vitro, and that the synergistic or additive action of low levels of cytokines on monocyte activation may be sufficient to promote immune or inflammatory reactions. Our data also suggest that certain T cell cytokines may regulate monocyte activation.
Keywords: fibronectin, monocyte, cytokine
INTRODUCTION
Fibronectin (FN) is a high molecular weight extracellular matrix glycoprotein that has been shown to be at sites of inflammation and tissue injury [1], where it may play an important role in immune response and the stimulation of wound repair [2–4]. At the cellular level, FN functions to regulate adhesion to other cells and substrates through its receptors, β1-integrins, which are also known as very late antigen-4 (VLA-4) or -5 (VLA-5). Furthermore, solid-phase FN has been shown to activate T cells [5,6], and results in increased tyrosine phosphorylation [7,8].
FN is also found in normal human plasma at a concentration of 300 μg/ml [9]; however, its physiological role in plasma and the effect of soluble FN in vitro in cultured cells remain unknown. In cultured human monocytes, FN-coated substrate enhanced both monocyte adhesion and cytokine production. We have previously shown that FN in culture medium activates monocytes through the VLA-5 receptor, inducing the production of proinflammatory cytokines such as IL-1, IL-6 and tumour necrosis factor-alpha (TNF-α) by these activated cultured human monocytes [10]. Many studies have shown that these same exogenous cytokines are also able to stimulate cultured human monocytes [1–3].
In this study we demonstrate that cultured human monocytes, stimulated with the cytokines IL-1α, IL-6 and TNF-α, produced FN as a result of their activation. We further demonstrate that cytokines such as IL-4, IL-10 and IL-13, and to a lesser extent transforming growth factor-beta (TGF-β), could negatively regulate FN production. We discuss the role of FN and β1-integrins in the activation of cultured human monocytes relating to cytokine production.
MATERIALS and METHODS
Isolation of monocytes
Heparinized peripheral blood was obtained from healthy donors. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque gradients, as previously described [11]. PBMC were washed three times in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan), and suspended at 1 ×106 cells/ml in RPMI 1640 medium, then incubated at 37°C in 5% CO2 for 2 h on a plastic plate (Becton Dickinson, Oxnard, CA; cat. no. 1005) coated with AB type human serum. Non-adherent cells were removed and culture wells are treated with cold medium containing 0·5 mm EDTA for 10 min, then agitated severely to detach the adherent cells as monocytes. The purity of the monocytes was assayed by immunoperoxidase staining with Leu-M3 MoAb (Becton Dickinson, Mountain View, CA), and was >95%.
Cell culture
The number of monocyte was adjusted to 2 ×106 cells/ml in RPMI 1640 medium containing 10% fetal calf serum (FCS; JRH Biosciences, Lemexa, KS), and cultured on a plastic plate (Corning, Corning, NY; cat. no. 25850) at 37°C in 5% CO2 for 4 h or 24 h with either a single cytokine or with a combination of cytokines (IL-1α+IL-6, IL-1α+TNF-α, IL-6 +TNF-α) (IL-1α, IL-6 and TNF-α were purchased from Genzyme, Cambridge, MA). Lipopolysaccharide (LPS; Sigma, St Louis, MO) was used as positive control for monocyte stimulation. To study the effects of IL-4 (provided by Ono Pharmacy, Osaka, Japan), IL-10 (Genzyme), IL-13 (Pepro Tech., London, UK) and TGF-β (Genzyme) on proinflammatory cytokine stimulation, monocytes were precultured with these cytokines for 2 h, the cells were then cultured with IL-1α, IL-6 or TNF-α. After culture the supernatants were collected and were assayed for FN. The viability of the cultured monocytes was confirmed by 0·2% trypan blue dye exclusion test, and was >90% in any culture plates.
Assay for FN
Microtitre plates (Sanco Junyaku, Tokyo, Japan; cat. no. 220-24K) were coated with polyclonal rabbit anti-FN antibody. The wells were washed three times, and unreactive sites were blocked with 1% bovine serum albumin (BSA)–PBS. Twenty microlitres of each sample and 80 μl of 125I-FN were added into these wells and incubated for 2 h at room temperature. After incubation the wells were washed three times, and the radioactivity was measured with a γ-scintillation counter.
Northern blotting
Total RNA was isolated from cultured human monocytes by the acid guanidinium thiocyanate-phenol-chloroform extraction method, as previously described [12]. The final RNA precipitate was pelleted by microcentrifugation and re-dissolved in 100 μl of diethlpyrocarbonate (DEPC)-treated sterile water. After quantification by spectrophotometry, equivalent amounts of RNA (10 μg/lane) were size-fractionated by electrophoresis in 1% agarose gels containing 2·2 m formaldehyde for 2·5 h. At the end of electrophoresis, gels were stained by ethidium bromide for assessment of equal loading. The RNA was then blotted by overnight capillary transfer onto nytron membrane (Schleicher and Schuell, Keene, NH). After prehybridization for 1 h, RNA blots were hybridized with radiolabelled FN cDNA probe for 2·5 h at 65°C using rapid hybridization buffer (Amersham, Aylesbury, UK). After hybridization, the blots were washed with 0·5–2 ×SSC, 0·2% SDS several times, and then exposed to film at −80°C.
Statistical analysis
The data for control and experimental groups are shown as mean ± s.d. Statistical analysis was variance test for multiple group comparisons. P <0·05 was used determine significance.
RESULTS
FN production by cultured human monocytes
Spontaneous FN production by cultured human monocytes after 4-h or 24-h cultures was 26 ± 755·6 ng/ml and 444·8 ± 50·1 ng/ml, respectively (Table 1). FN concentration in culture supernatants from monocytes stimulated with IL-1α, IL-6 or TNF-α increased in a dose-dependent fashion after both 4 h and 24 h of culture (P <0·01). FN production after 24-h culture was greater than that after 4-h culture in any cytokine stimulation. There were no differences in FN production between 24 h and 48 h of culture, nor were there any differences between the different cytokines used for stimulation (data not shown).
Table 1.
Fibronectin (FN) production by cultured human monocytes stimulated with lipopolysaccharide (LPS) or cytokines
| FN concentration (ng/ml) at | |||
|---|---|---|---|
| Stimulation with | 4 h | 24 h | |
| None | 267·3 ± 55·6 | 444·8 ± 50·1 | |
| IL-1α | 2 U/ml | 378·3 ± 43·3 | 578·4 ± 98·7 |
| 20 U/ml | 595·0 ± 143·5 | 818·4 ± 103·6* | |
| 200 U/ml | 802·2 ± 160·3* | 1079·4 ± 157·2* | |
| IL-6 | 2 U/ml | 390·3 ± 51·9 | 601·0 ± 38·6 |
| 20 U/ml | 589·0 ± 63·3 | 766·8 ± 169·5* | |
| 200 U/ml | 791·3 ± 120·1* | 1159·2 ± 202·5* | |
| TNF-α | 2 U/ml | 378·0 ± 30·8 | 533·2 ± 71·8 |
| 20 U/ml | 749·0 ± 217·5 | 948·0 ± 207·9* | |
| 200 U/ml | 988·6 ± 176·1* | 1248·6 ± 143·6* | |
| LPS | 2 μg/ml | 393·0 ± 33·6 | 582·7 ± 63·2 |
| 20 μg/ml | 624·1 ± 72·0 | 869·2 ± 182·7* | |
| 200 μg/ml | 808·7 ± 23·6* | 1168·2 ± 204·3* | |
Monocytes were cultured with medium, IL-1α, IL-6, tumour necrosis factor-alpha (TNF-α) or LPS (2 mg/ml, 20 mg/ml, 200 mg/ml) for 4 h or 24 h. FN in culture supernatants was measured by radioimmunoassay. Mean ± s.d., n = 16
P <0·01.
Combination effects of suboptimal doses of cytokines on FN production
Combinations of suboptimal doses (2 U/ml) of cytokines (IL-1α+IL-6, IL-1α+TNF-α, IL-6 +TNF-α), which could not singly induce substantial amounts of FN, were added to cultures of monocytes. These combinations of cytokines induced FN additively. Combination effects on FN production appeared to be greater after 24 h of culture than after 4 h of culture (Table 2).
Table 2.
The combination effects of suboptimal dose of cytokines on fibronectin (FN) production by cultured human monocytes
| FN concentration (ng/ml) at | ||
|---|---|---|
| Stimulation with | 4 h | 24 h |
| None | 267·3 ± 55·6 | 444·8 ± 50·1 |
| IL-1α 2 U/ml | 378·3 ± 43·3 | 578·4 ± 98·7 |
| IL-6 2 U/ml | 390·3 ± 51·9 | 601·0 ± 38·6 |
| TNF-α 2 U/ml | 388·0 ± 30·8 | 533·2 ± 71·8 |
| IL-1α 2 U/ml +IL-6 2 U/ml | 808·6 ± 46·9* | 1168·2 ± 125·9* |
| IL-1α 2 U/ml +TNF-α 2 U/ml | 867·1 ± 64·6* | 1123·4 ± 155·1* |
| IL-6 2 U/ml +TNF-α 2 U/ml | 752·7 ± 76·3* | 1015·6 ± 106·2* |
Monocytes were incubated with medium, suboptimal dose of IL-1α (2 U/ml), IL-6 (2 U/ml), tumour necrosis factor-alpha (TNF-α; 2 U/ml) or a combination of suboptimal dose of cytokines (IL-1α+IL-6, IL-1α+TNF-α, IL-6 +TNF-α) for 4 h or 24 h. The level of FN in the culture supernatants was measured by radioimmunoassay. (Mean ± s.d., n = 16
P <0·01.)
FN mRNA expression in cultured human monocytes
Using Northern blot analysis with a cDNA specific for FN, we examined the expression of FN mRNA in human monocytes. Human monocytes were cultured with optimal doses (200 U/ml) of IL-1α, IL-6 or TNF-α, or combinations of suboptimal doses (2 U/ml) of cytokines (IL-1α+IL-6, IL-1α+TNF-α, IL-6 +TNF-α) for 4 h. FN mRNA expression in cultured human monocytes significantly increased with 200 U/ml of IL-1α, IL-6 or TNF-α (Fig. 1), and with the combinations of suboptimal doses of cytokines (IL-1α+IL-6, IL-1α+TNF-α, IL-6 +TNF-α). However, FN mRNA expression did not increase significantly with suboptimal doses (2 U/ml) of single cytokines (IL-1α, IL-6 and TNF-α) (Fig. 2). In the time course studies of FN mRNA expression, significant expression was observed at 4 h to 24 h but not at 2 h stimulation (data not shown).
Fig. 1.
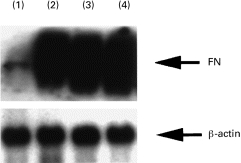
Fibronectin (FN) mRNA expression in cultured human monocytes stimulated with each cytokine for 4 h. 1, non-stimulated; 2, IL-1α 200 U/ml; 3, IL-6 200 U/ml; 4, tumour necrosis factor-alpha 200 U/ml.
Fig. 2.
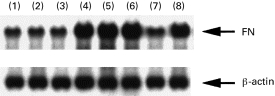
Fibronectin (FN) mRNA expression in cultured human monocytes stimulated with a combination of cytokines for 4 h. 1, IL-1α 2 U/ml; 2, IL-6 2 U/ml; 3, tumour necrosis factor-alpha (TNF-α) 2 U/ml; 4, IL-1α 2 U/ml +IL-6 2 U/ml; 5, IL-1α 2 U/ml +TNF-α 2 U/ml; 6, IL-6 2 U/ml +TNF-α 2 U/ml; 7, non-stimulation; 8, lipopolysaccharide 100 μg/ml.
Effect of anti-inflammatory cytokines on FN production by cultured human monocytes
The effects of anti-inflammatory cytokines, such as IL-4, IL-10, IL-13 and TGF-β, on FN production by cultured human monocytes stimulated with cytokines were studied. Each anti-inflammatory cytokine was preincubated with the monocyte culture, prior to stimulation with proinflammatory cytokines. IL-4, IL-10 and IL-13 strongly inhibited FN production (% inhibition was 93·6 ± 4% by IL-4, 98·8 ± 2·6% by IL-10 and 91·1 ± 4·8% by IL-13) (Fig. 3). TGF-β, however, showed only a weak inhibition of FN production following stimulation with IL-6 and TNF-α, and failed to inhibit IL-1α-mediated stimulation. Toxic effects of these anti-inflammatory cytokines were dissimilar, because the cell viability of recovered monocytes was >90%.
Fig. 3.
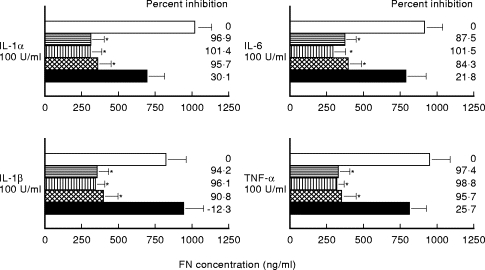
Effect of anti-inflammatory cytokines (IL-4, IL-10, IL-13 and transforming growth factor-beta (TGF-β)) on fibronectin production by cultured human monocytes stimulated for 24 h with IL-1α, IL-6, tumour necrosis factor-alpha and lipopolysaccharide. □, None; horizontal hatching, IL-4 100 U/ml; vertical hatching, IL-10 100 U/ml; cross-hatched, IL-13 100 U/ml; ▪, TGF-β 100 U/ml (mean ± s.d., n =8; *P <0·01).
Effects of anti-inflammatory cytokines on FN mRNA expression
Effects of anti-inflammatory cytokines (IL-4, IL-10, IL-13 and TGF-β) on FN mRNA expression were studied. Monocytes were cultured with both anti- and proinflammatory cytokines for 4 h. FN production by IL-1α was inhibited strongly by IL-4, IL-10 and IL-13 (Fig. 4). However, TGF-β could not inhibit FN mRNA expression in cultured human monocytes stimulated with IL-1α. The similar inhibitory effects with IL-4, IL-10 and IL-13, but not TGF-β, were observed with IL-1β, IL-6 and TNF-α stimulation (data not shown).
Fig. 4.
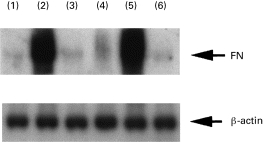
Fibronectin mRNA expression in cultured human monocytes stimulated with IL-1α for 24 h in the presence of anti-inflammatory cytokines (IL-4, IL-10, IL-13 and transforming growth factor-beta (TGF-β)). 1, non-stimulated; 2, IL-1α 100 U/ml; 3, IL-1α 100 U/ml +IL-4 100 U/ml; 4, IL-1α 100 U/ml +IL-10 100 U/ml; 5, IL-1α 100 U/ml +TGF-β 100 U/ml; 6, IL-1α 100 U/ml +IL-13 100 U/ml.
DISCUSSION
In the present study we demonstrated that the cytokines IL-1α, IL-6 and TNF-α stimulated cultured human monocytes and led to the production of FN. We further demonstrated that cultured human monocytes could also be stimulated to produce FN by combinations of suboptimal doses of cytokines. The increased expression of FN mRNA was demonstrated, not only in cultured monocytes after stimulation with these cytokines, but also after stimulation with combinations of suboptimal doses of cytokines. These findings suggest that if small amounts of multiple cytokines were produced simultaneously, high concentrations of inflammatory cytokines might not always be required to activate monocytes towards an inflammatory response. In fact, in chronic inflammatory diseases such as systemic lupus erythematosus, the elevation of plasma cytokine levels has not been demonstrated, despite the spontaneous high production of cytokines by monocytes in vitro[12].
The activation of immunocompetent cells by cytokines is regulated by another group of regulatory cytokines that include IL-4, IL-10, IL-13, and TGF-β[13]. Our data showed that all of these exogenous cytokines except for TGF-β strongly inhibited FN production by inflammatory cytokine-activated monocytes. It has been established that interferon-gamma (IFN-γ) is produced mainly by CD8+ T cells and also by CD4+ T helper 1 (Th1) cells, and IL-4, IL-10, and IL-13 are produced mainly by CD4+ T helper 2 (Th2) cells [14–17]. In general, Th1 cytokines and Th2 cytokines have been reported to act antagonistically by suppressing each other. In our study, exogenous Th2 cytokines suppressed FN production by cultured monocytes, while IFN-γ, a Th1 cytokine, did not. Although the cellular mechanism of the suppression of FN production by cultured monocytes remains unknown, our data suggest that monocyte activation is also regulated by certain T cell cytokines.
FN is a high molecular weight glycoprotein that is produced by liver cells [18], endothelial cells [19,20] and monocytes [21] at the early phase of inflammation. In general, plasma FN levels increase in the inflammatory state [22–24]. However, the physiological role of plasma FN is still unknown. We have previously investigated the effect of the soluble form of FN on the production of IL-1α, IL-1β, IL-6 and TNF-α by cultured human monocytes from normal individuals [10]. FN markedly stimulated the secretion of IL-1α, IL-1β, IL-6 and TNF-α. A marked increase of mRNA for each cytokine was also observed [10]. MoAbs to VLA-5 integrin and GRGDSP, an analogue oligopeptide of the cellular binding domain of FN, inhibited (>50%) FN-induced cytokine production [10]. These data demonstrate that the soluble form of FN stimulates monocyte via the integrin, VLA-5, and RGD residues on the FN molecule. Taken together, our previous and present studies demonstrate that cultured human monocytes can be stimulated both by the soluble form of FN and by exogenous cytokines, and can consequently produce both materials. It is not clear, however, whether the FN, produced by cultured human monocytes after stimulation with inflammatory cytokines, is still active and functional.
Exogenous IL-4, IL-10 and IL-13 inhibited not only IL-1, IL-6 and TNF-α production by cultured human monocytes in previous studies [25–28], but also inhibited FN production in the present study. Our results suggest that β1-integrins and their natural ligand, FN, play an important role in the complex cytokine network that regulates the inflammatory and immune responses.
Acknowledgments
We would like to thank all subjects who volunteered to participate in this study. We are grateful to Ono Pharmacy Co. Ltd. for providing material.
REFERENCES
- 1.Hayashi M, Yamada KM. Domain structure of the carboxyl half of human plasma fibronectin. J Biol Chem. 1983;258:3332–40. [PubMed] [Google Scholar]
- 2.Rennard SI, Chen YF, Robbins RA, Gadek JE, Crystal GG. Fibronectin mediates cell attachment to C1q: a mechanism for the localization of fibrosis in inflammatory disease. Clin Exp Immunol. 1983;54:239–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Tada K, Tuan T-L, Brown PJ, Brown PJ, Grinnell F. Fibronectin receptors of human keratinocytes and their expression during cell culture. J Cell Biol. 1987;105:3097–104. doi: 10.1083/jcb.105.6.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albelda SM, Buck CA. Integrin and other cell adhesion molecules. FASEB J. 1990;4:2868–79. [PubMed] [Google Scholar]
- 5.Shimizu Y, Seventer GAV, Horgan KJ, Horgan KJ, Shaw S. Costimulation of proliferative responses of resting CD4+T cell by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990;145:59–67. [PubMed] [Google Scholar]
- 6.Nojima Y, Humphries MJ, Mould AP, Komoriya A, Yamada KM. VLA-4 mediates CD3-dependent CD4+T cell activation via the CS1 alternatively spliced domain of fibronectin. J Exp Med. 1990;172:1185–92. doi: 10.1084/jem.172.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis LS, Oppenheimer-Marks N, Bednarczyk JL, Mcintyre BW, Lipsky PE. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990;145:785–93. [PubMed] [Google Scholar]
- 8.Yamada A, Nikaido T, Nojima Y, Schlossman SF, Morimoto C. Activation of human CD4+T lymphocyte. Interaction of fibronectin with VLA-5 receptor on CD4 cells induces the AP-1 transcription factor. J Immunol. 1991;146:53–56. [PubMed] [Google Scholar]
- 9.Nishinarita S, Yamamoto T, Takizawa T, Hayakawa J, Karasaki M, Sawada S. Increased plasma fibronectin in patients with systemic lupus erythematosus. Clin Rheumatol. 1990;9:214–9. doi: 10.1007/BF02031971. [DOI] [PubMed] [Google Scholar]
- 10.Takizawa T, Nishinarita S, Kitamamura N, et al. Interaction of the cell-binding domain of fibronectin with VLA-5 integrin induces monokine production in cultured human monocytes. Clin Exp Immunol. 1995;101:376–82. doi: 10.1111/j.1365-2249.1995.tb08367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyum A. Isolation of lymphocytes, granulocytes macrophages. Scand J Immunol. 1976;5(Suppl.):9–15. [PubMed] [Google Scholar]
- 12.Riet VI, Greef DC, Favero DH, Demanet C, Camp BV. Production of fibronectin and adherence to fibronectin by human melanoma cell line. Br J Haematol. 1994;87:258–65. doi: 10.1111/j.1365-2141.1994.tb04907.x. [DOI] [PubMed] [Google Scholar]
- 13.De Waal Malefyt R, Fidgor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-γ or IL-10. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 14.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clones: definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 15.Mosmann TR, Coffmann RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 16.Abbas KA, Williams ME, Burstein HJ, Chang TL, Lichtman AH. Activation and functions of CD4+T-cell subsets. Immunol Rev. 1991;123:5–22. doi: 10.1111/j.1600-065x.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann TR, Schumacher JH, Street NF, et al. Diversity of cytokine synthesis and function of mouse CD4+T cells. Immunol Rev. 1991;123:209–29. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 18.Owen MR, Cimino CD. Synthesis of fibronectin by the isolated perfused rat liver. Blood. 1980;59:1305–9. [PubMed] [Google Scholar]
- 19.Schmidt ME, Dougias SD. Disappearance and recovery of human monocyte IgG receptor activity after phagocytosis. J Immunol. 1972;109:914–7. [PubMed] [Google Scholar]
- 20.Haakenstad AO, Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immumol. 1974;112:1939–48. [PubMed] [Google Scholar]
- 21.Alitalo K, Hovi T, Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980;151:602–13. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carsons S, Parenti D, Lavietes BB, Diamond HS, Singer A, Boxer M. Plasma fibronectin in systemic lupus erythematosus relationship to clinical activity, DNA binding and acute phase proteins. J Rheumatol. 1985;12:1088–92. [PubMed] [Google Scholar]
- 23.Scott DL, Wainwright AC, Walton KW, Williamson N. Significance of fibronectin in rheumatoid arthritis and osteoarthritis. Ann Rheum. 1981;40:142–53. doi: 10.1136/ard.40.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott DL, Walton KW. The significance of fibronectin in rheumatoid arthritis. Semin Arthritis Rheum. 1984;13:244–54. doi: 10.1016/0049-0172(84)90028-3. [DOI] [PubMed] [Google Scholar]
- 25.Hart PH, Ahern MJ, Smith MD, Finlay-Jones JJ. Regulatory effects of IL-13 on synovial fluid macrophages and blood monocytes from patients with inflammatory arthritis. Clin Exp Immunol. 1995;99:331–9. doi: 10.1111/j.1365-2249.1995.tb05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano S, Sone S, Nishioka Y, Mukaida N, Matsushima K, Ogura T. Differential effects of anti-inflammatory cytokines (IL-4, IL-10 and IL-13) on tumoricidal and chemotactic properties of human monocytes induced by monocyte chemotactic and activating factor. J Leuk Biol. 1995;57:303–9. doi: 10.1002/jlb.57.2.303. [DOI] [PubMed] [Google Scholar]
- 27.Kucharzik T, Lgering N, Adolf M, Domschke W, Stoll R. Synergistic effect of immunoregulatory cytokine on peripheral blood monocytes from patients with inflammatory bowel disease. Dig Dis Sci. 1997;42:805–12. doi: 10.1023/a:1018872332387. [DOI] [PubMed] [Google Scholar]
- 28.Hart PH, Ahern MJ, Jones CA, Jones KL, Jones KL, Smith MD, Finlay-Jones JJ. Synovial fluid macrophages and blood monocytes differ in their responses to IL-4. J Immunol. 1993;151:3370–80. [PubMed] [Google Scholar]


