Abstract
Cytokines such as IL-1, tumour necrosis factor-alpha (TNF-α), IL-6 and IL-8 are increased in inflamed colonic mucosa after administration of mouse DSS. Nuclear factor κ B (NF-κ B) is a transcription factor which regulates the expression of these cytokine genes. The effect of intracolonically administered NF-κ B (p65) antisense phosphorothioate oligonucleotide was examined in mouse DSS-induced colitis using drinking water containing 5% DSS. When antisense oligonucleotide was given on day 0, the disease activity index (DAI) representing clinical symptoms improved and the histological score decreased; furthermore, IL-1, IL-6, and TNF-α concentrations in rectal mucosa were lower compared with the control group. Clinical and histological improvement was also observed when antisense oligonucleotide was begun on day 2 but not on day 7. In addition, the distribution of antisense oligonucleotides was investigated by confocal laser microscopy. In colonic mucosa, oligonucleotides were predominantly localized to cells in the lamina propria, but also in the epithelium. Western blot analysis using homogenized rectal mucosa showed the decreased expression of NF-κ B p65 in the antisense oligonucleotide-treated group, although it was increased in the colitis group. These results suggest that intracolonic administration of NF-κ B antisense oligonucleotide may be effective in ulcerative colitis.
Keywords: dextran sulphate sodium, inflammatory bowel disease, NF-κ B, antisense oligonucleotide
INTRODUCTION
Ulcerative colitis (UC) is an idiopathic, non-specific inflammatory disorder involving primarily the mucosa and submucosa of the colon. Since some UC patients have autoantibodies which react with colonic cells or have other autoimmune diseases, it is thought that an autoimmune mechanism is related to UC pathogenesis [1,2].
In the colonic mucosa of active UC patients, inflammatory cytokines such as IL-1, tumour necrosis factor-alpha (TNF-α), IL-6 and IL-8 are elevated; these play an important role in the development of colonic inflammation [3,4]. In addition, adhesion molecules such as endothelial leucocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) are up-regulated in inflamed colonic mucosa [5]. The expression of these cytokine and adhesion molecule genes is regulated by the transcription factor, nuclear factor κ B (NF-κ B). NF-κ B was originally identified as a heterodimeric complex of two subunits, p65 and p50 [6]. The NF-κ B family includes p65(RelA), RelB, c-Rel, p105(p50), and p100(p52) [7,8]. NF-κ B presents as a hetero- or homodimer in the cytosol bound to an inhibitory protein, inhibitor κ B (Iκ B). Phosphorylation of Iκ B by cytokine-activated Iκ B kinases (IKK) leads to degradation of Iκ B. This is followed by translocation of the activated NF-κ B into the nucleus and activation of target genes which contain the κ B motif (consensus: 5′-GGGRNNYYCC-3′) [9].
Rogler et al. showed that activated NF-κ B could be detected in inflamed colonic mucosa by in situ hybridization, while almost no activation of NF-κ B was observed in non-inflamed mucosa [10]. Schreiber et al. found increased activation of NF-κ B in nuclear extracts of colonic biopsy samples and lamina propria mononuclear cells (LPMC) obtained from inflammatory bowel disease (IBD) patients using Western blot and electrophoretic mobility shift assays [7]. Thus, NF-κ B plays a key role in the colonic inflammation of IBD. In the present study, DSS-induced colitis, which resembles human ulcerative colitis (UC), was produced in mice to examine the effect of intracolonically administered NF-κ B (p65) antisense oligonucleotide.
MATERIALS and METHODS
Animals
Nine-week-old female BALB/c mice (SLC Co., Shizuoka, Japan) weighing about 20 g were used in the study. Standard mouse chow pellets and water were supplied ad libitum.
Induction of experimental colitis
DSS, molecular weight 5000, was obtained from Meitou Sangyou (Osaka, Japan). Mice were given drinking water containing 5% DSS on the indicated days instead of tap water.
Preparation of antisense oligonucleotides
19-mer phosphorothioate antisense oligonucleotide against NF-κ B p65, containing its transcription initiation site, was synthesized; 19-mer sense or nonsense oligonucleotide were used as a control.
The sequences were as follows: antisense 5′-GAAACAGATCGTCCATGGT-3′; sense 5′-ACCATGGACGATCTGTTTC-3′; nonsense 5′-ATGGAGAATATGAAAGTG-3′.
Administration of p65 antisense oligonucleotide in the pre-inflammatory stage
Mice were randomized into four groups. The first group, designated as N (n = 5), received tap water for 7 days. The second group, designated C (n = 5), received 5% DSS for 7 days and 0·3 ml of distilled water given intracolonically on day 0. The third group, designated AS (n = 5), received 5% DSS for 7 days and 20 nmol of antisense phosphorothioate oligonucleotide in 0·3 ml of distilled water intracolonically on day 0. The fourth group, designated S (n = 5), received 20 nmol of sense or nonsense phosphorothioate oligonucleotide instead. Intracolonic administration was done by slow infusion through a polyethylene catheter (Clea Co., Tokyo, Japan) inserted 3 cm into the anus. Mice were killed under anaesthesia on day 7.
Administration of p65 antisense oligonucleotide in the early phase of colonic inflammation
Antisense oligonucleotide (20 nmol) was administered intracolonically on day 2 in the same fashion as described above. Mice were killed on day 7.
Administration of p65 antisense oligonucleotide for established colonic inflammation
Mice were given 5% DSS for 10 days, and 20 nmol of antisense oligonucleotide were administered intracolonically on day 7.
Evaluation of colitis
A disease activity index (DAI) was determined by an investigator blinded to the protocol by scoring the extent of body weight loss, stool haemoccult positivity or gross bleeding, and stool consistency in accordance with the method described by Murthy et al. [11,12]at sacrifice (Table 1).
Table 1.
Disease activity index
| Score | Weight loss (%) | Stool* consistency | Occult/gross bleeding |
|---|---|---|---|
| 0 | (–) | Normal | Normal |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose | Guiac (+) |
| 3 | 11–15 | ||
| 4 | >15 | Diarrhoea | Gross bleeding |
The disease activity index = (combined score of weight loss, stool consistency and bleeding)/3.
Normal stools = well formed pellets; loose = pasty stools which do not stick to the anus; diarrhoea = liquid stools that stick to the anus.
Blood samples were collected from the heart immediately before sacrifice for haemoglobin concentration. The rectum was removed and opened longitudinally; half of the sample was subjected to histological study and the rest was used to measure mucosal cytokine concentrations. For histology, the rectum was fixed in 10% neutral buffered formalin; 4-μm specimens were subjected to haematoxylin and eosin (H–E) staining. Randomly selected fields (n = 15) magnified 100 times in each section were inspected and graded by a pathologist blinded to the treatment protocol (Table 2)[13]. The mean score in each section was calculated.
Table 2.
Histological disease score
| Grade 0 = Normal colonic mucosa |
| Grade 1 =Loss of one‐third of the crypts |
| Grade 2 = Loss of two‐thirds of the crypts |
| Grade 3 = The lamina propria is covered with a single layer of |
| epithelium and mild inflammatory cell infiltration is present |
| Grade 4 = Erosions and marked inflammatory cell infiltration are present |
Randomly selected 15 fields (magnified 100 times) in each section were inspected and graded as below by a pathologist in our hospital who was blinded to the treatment protocol. By scoring the grades in 15 fields, the mean in each section was calculated.
IL-1β, IL-6 and TNF-α concentrations in homogenized rectal tissue were measured by ELISA according to the manufacturer’s protocol (R&D Systems, Abingdon, UK). Briefly, after biotinylated antibody reagent was added in 96-well plates, supernatants of homogenized rectal tissue were incubated at 37°C in 5% CO2 for 2 h. After washing with PBS, streptavidin-peroxidase (HRP) solution was added and the plate was incubated for 30 min at room temperature. The absorbance was measured at 590 nm using a microplate reader.
The total protein concentration was determined by the Lowry method, and the absorbance per milligram of protein was calculated.
Macrophage staining
Mice were killed on days 0, 3 or 7, and rectal specimens were stained with anti-mouse macrophage MoAb, F4/80 (Cedarlane, Hornby, Ontario, Canada). F4/80 antibody reacts with the mouse macrophage F4/80 antigen, which is a 160-kD plasma membrane component on mouse mononuclear phagocytes [14]. Briefly, the specimen was fixed in periodate–lysine–paraformaldehyde (PLP) at 4°C overnight and embedded in OCT compound (Tissue Tek). Embedded samples were quickly frozen in liquid nitrogen, and 4-μm serial sections were prepared. Air-dried samples were fixed with acetone for 15 min, and endogenous peroxidase was blocked with 0·3% H2O2 in 50% methanol for 15 min at room temperature. Non-specific binding was blocked with 2% bovine serum albumin and the tissue was incubated with 20 μg/ml of biotinylated anti-mouse macrophage MoAb overnight at 4°C, followed by incubation with avidin–biotin peroxidase complex at room temperature for 20 min. After washing with PBS, the tissue was incubated with 0·03% 3-3-diaminobenzidine tetrahydrochloride (DAB; Sigma, St Louis, MO) containing 0·003% hydrogen peroxide. All sections were counterstained with Mayer’s haematoxylin [15].
Western blotting of NF-κ B p65 in the homogenized rectal tissue
Expression of NF-κ B p65 in the rectal mucosa was compared among three groups (groups N, C, and AS). Tissue lysates were prepared as described earlier [16]. Ten microlitres of the lysate (40–45 μg total protein) were separated on a 10% denaturing polyacrylamide gel at 40 ml for 1 h. Separated proteins were blotted to a 0·45-μm pore polyvinylidene difluoride membrane (PVDF; NEM Life Science, Boston, MA) using the Trans-Blot SD cell (BioRad, Tokyo, Japan) (140 mA; 45 min; transfer buffer 25 mm Tris, 192 mm glycine, 20% methanol, 0·5% SDS). Non-specific binding was blocked by incubating the membrane in blocking buffer (Block Ace; Dainippon Pharmaceutical Co., Osaka, Japan) overnight at 4°C. Subsequently the membrane was incubated with 0·5 μg/ml of a rabbit anti-human NF-κ B (p65) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. After washing with 20 mm Tris–HCl containing pH 7·5 500 mm NaCl and 0·1% Tween 20, the membrane was incubated with 1 μg/ml of an HRP-conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology), for 1 h at room temperature. The bands were visualized by placing the membrane in 20 mm Tris–HCl pH 7·5 containing 500 mm NaCl, 17% methyl alcohol, 0·015% H2O2, and 0·5 mg/ml of 4-chloro-1-naphthol (Nacalai Tesque, Inc., Kyoto, Japan).
Distribution of antisense oligonucleotides in the colonic mucosa
Twenty nmoles of 5′-end FITC-labelled antisense oligonucleotides in 0·3 ml of sterilized distilled water were administered intracolonically; 24 h later, mice were killed by perfusion with 4% paraformaldehyde plus 0·2% glutaraldehyde in PBS. The rectum was removed and fixed for 3 h in ice-cold perfusion buffer. After washing with PBS, samples were serially incubated in 12%, 15%, and 18% sucrose in PBS for 4 h at 4°C. The samples were embedded in OCT compound and frozen in liquid nitrogen. Serial sections (4 μm) were prepared and inspected using scanning confocal microscopy.
Statistical analysis
All numerical values are expressed as mean ±s.e.m. Data sets were analysed by Fischer’s protected least significant difference (PLSD) comparison tests for post hoc t-test. Differences of P < 0·01 were considered statistically significant.
RESULTS
The effect of p65 antisense oligonucleotide on clinical indices and histological disease score
In the control and p65 sense oligonucleotide-treated groups, pasty to liquid gross bloody stool, weight loss, and severe anaemia were observed in all mice. Their colons were shortened, but gross ulcerations were not evident. When p65 antisense oligonucleotide was administered on day 0 or 2, bloody stools were not evident, stools were better formed, and weight loss was lessened. Furthermore, DAIs were significantly lower in the mice treated with p65 antisense oligonucleotide on day 0 or 2 than in the control group (P < 0·01). Histologically, specimens obtained from the control and the p65 sense oligonucleotide-treated groups showed sporadic erosions with marked inflammatory cell infiltration in the lamina propria. In contrast, treatment with antisense oligonucleotide resulted in less erosions, and inflammatory cell infiltration was nearly absent in the rectum. Histological scores of the p65 antisense oligonucleotide-treated mice were significantly lower than in the control group (P < 0·01). However, no improvements in DAI or histological score were observed when antisense oligonucleotides were administered on day 7 (Table 3). The administration of nonsense oligonucleotides had no effects on the improvement of clinical parameters and histological scores (data not shown).
Table 3.
The effect of p65 antisense oligonucleotide on clinical indices and histological disease score
| Group | Administration day | Haemoglobin (g/dl) | DAI | Histological disease score |
|---|---|---|---|---|
| N | 14·9 ± 0·46 | 0 | 0 | |
| C | 8·1 ± 0·44 | 3·883 ± 0·117 | 2·878 ± 0·279 | |
| S | 9·9 ± 0·65 | 3·777 ± 0·141 | 3·350 ± 0·076 | |
| AS | 0 | 13·8 ± 1·13* | 1·665 ± 0·273* | 1·625 ± 0·238* |
| 2 | 1·944 ± 0·389* | 1·480 ± 0·110* | ||
| 7 | 2·733 ± 0·125 | 2·616 ± 0·239 |
Results are expressed as the mean ± S.E.M. (n = 5).
Significantly different from colitis group at P<0·01.
DAI, Disease activity index; N, normal; C, control; S, p65 sense oligonucleotide; AS, p65 antisense oligonucleotide.
The effect of p65 antisense oligonucleotide on local cytokine concentrations
As shown in Table 4, concentrations of mucosal IL-1β, IL-6, and TNF-α in the DSS group were significantly higher than in the normal group. Treatment with antisense oligonucleotide on day 0 reduced cytokine concentrations significantly (P < 0·01).
Table 4.
The effect of p65 antisense oligonucleotide on local cytokine concentrations
| Group | IL‐1β (pg/mg) | IL‐6 (pg/mg) | TNF‐α (pg/mg) |
|---|---|---|---|
| N | 46·1 ± 10·8* | 35·2 ± 6·3* | 95·9 ± 26·6* |
| C | 282·6 ± 43·4 | 220·3 ± 53·3 | 749·6 ± 183·3 |
| S | 145·9 ± 44·3 | 150·5 ± 53·5 | 484·1 ± 63·8 |
| AS | 92·8 ± 5·1* | 17·3 ± 3·4* | 183·0 ± 30·7* |
Results are expressed as the mean ± S.E.M. (n = 5).
Significantly different from colitis group at P<0·01.
DAI, Disease activity index; N, normal; C, control; S, p65 sense oligonucleotide; AS, p65 antisense oligonucleotide.
Macrophage staining
Before induction of colitis, macrophages with polymorphic shapes were observed in the lamina propria around the basement membrane of crypts. However, macrophage infiltration was not obvious (Fig. 1a). On day 3, in the early phase of colonic inflammation, macrophages were infiltrated at the base of crypts (Fig. 1b), and on day 7, in established colonic inflammation, considerable numbers of macrophages were observed in the whole field of the lamina propria (Fig. 1c). In each section, some spindle-shaped cells, presumably fibroblasts, were stained. However, on day 7 the numbers of spindle-shaped cells stained by F4/80 MoAb were not increased compared with those of macrophages, polymorphic-shaped cells stained by its MoAb.
Fig. 1.
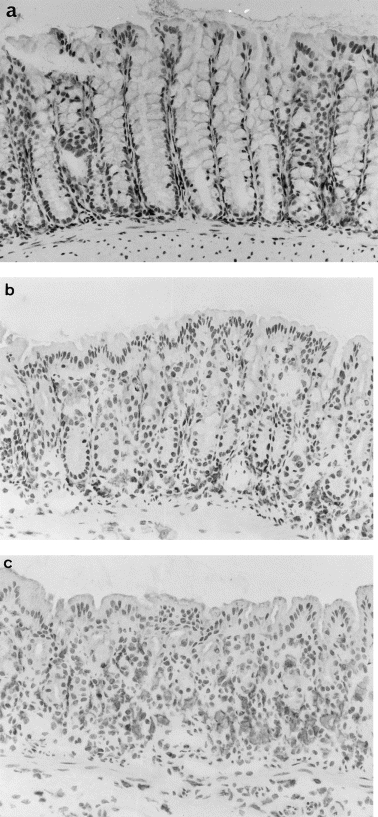
Immunohistochemical study of the rectal tissue in mice with DSS-induced colitis. Rectal tissue was stained with mouse MoAb F4/80 on (a) day 0, (b) day 3, and (c) day 7 and inspected with a microscope (× 400). F4/80+ macrophages were observed at the base of crypts on day 3. On day 7, considerable numbers of macrophages were observed in the entire field of the lamina propria. In each section, some spindle-shaped cells, presumably fibroblasts, were stained. However, on day 7 the numbers of spindle-shaped cells stained by F4/80 MoAb were not increased compared with those of macrophages, polymorphic shaped cells stained its MoAb.
Distribution of antisense oligonucleotides in colonic mucosa
Twenty-four hours after administration, antisense oligonucleotides were distributed predominantly in cells of the lamina propria, although some oligonucleotides were detected in colonic epithelium (Fig. 2a,b).
Fig. 2.
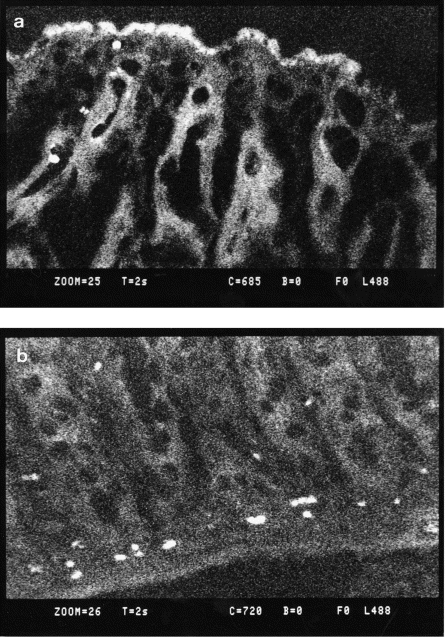
FITC-labelled oligonucleotides were administered intracolonically in DSS colitis, and the time course of distribution of the antisense oligonucleotides was examined with confocal laser microscopy. (a) Three hours after administration, FITC signals were detected mainly in the colonic epithelium, although some signals were detected in the lamina propria; (b) 24 h after administration, FITC signals were observed predominantly in the lamina propria.
Western blotting of NF-κ B p65 in the homogenized rectal tissue
As shown in Fig. 3, an intense band at 65 kD was observed in the control group, although a faint band was detected in the normal group. However, treatment with antisense oligonucleotides reduced the expression of NF-κ B p65 (Fig. 3).
Fig. 3.
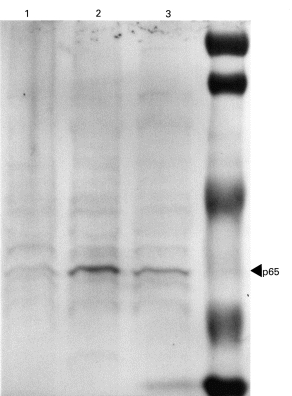
Expression of NF-κ B p65 in the rectal tissue was examined by Western blot analysis. Tissue lysates obtained from normal group (lane 1), control group (lane 2), and antisense oligonucleotide-treated group (lane 3) were subjected to SDS–PAGE. Subsequently, Western blot analysis was carried out using rabbit anti-human NF-κ B antibody. Intense band at 65 kD was observed in the control group, although only a faint band was detected in the normal group. The treatment with antisense oligonucleotide reduced the expression of NF-κ B p65.
DISCUSSION
Sulfasalazine (SASP) and 5-aminosalicylate (5-ASA) are used to treat mild to moderate UC; in more severe UC, corticosteroids are used in addition to SASP or 5-ASA. Corticosteroids inhibit NF-κ B activation by induction of Iκ Bα in target cells [17,18]. Ingested sulfasalazine is cleaved by colonic bacteria to yield sulfapyridine and 5-ASA. 5-ASA is thought to inhibit leukotriene B4 production through blocking the lipoxygenase pathway and as a free radical scavenger [19,20]. Sulfasalazine also inhibits the lipoxygenase pathway and recently was shown to inhibit NF-κ B activation by interfering with Iκ Bα phosphorylation [21].
NF-κ B p65 protein is increased in nuclear extracts of mucosal biopsy samples of IBD patients [7]. Since NF-κ B regulates the expression of various cytokines, chemokines, and adhesion molecules, sulfasalazine and corticosteroids appear to be effective in the treatment of IBD through inhibition of NF-κ B activation. However, corticosteroids have many undesirable side effects, such as impaired glucose tolerance, adrenal suppression, and increased risk of infection; furthermore, patients do not tolerate sulfasalazine. To overcome these problems, new therapeutic approaches directed at inhibition of NF-κ B itself have been tested in experimental colitis. Recently, antisense oligonucleotide against NF-κ B has been shown to be effective in trinitrobenzene sulphonic acid (TNBS) colitis, which resembles Crohn’s disease [22]. In the present study we examined the effect of p65 antisense oligonucleotide on mouse DSS-induced colitis, which is an experimental model analogous to human UC.
DSS-induced colitis is produced in hamsters [23], mice [24], and rats [25,26] and is characterized by ulceration, epithelial damage, mucosal or transmural inflammatory infiltrates, and lymphoid hyperplasia [24]. DSS-induced colitis is thought to be caused by (i) direct cytotoxicity, (ii) interference with the normal interaction between intestinal lymphocytes, epithelial cells, and extracellular matrix, (iii) aberrant modulation of the expression of integrin β7 receptors, other cell receptors or their functions [27], and (iv) changes in the intestinal microflora population [24].
The present study showed the effectiveness of NF-κ B (p65) antisense oligonucleotides on rectal inflammation when administered on day 0 or 2. However, no effect was observed when antisense oligonucleotides were administered on day 7. These results suggest that NF-κ B antisense oligonucleotides have inhibitory effects on DSS-induced colitis when administered in the early phase of inflammation. In the development of DSS colitis, activated macrophages play an important role by producing inflammatory cytokines such as IL-1β, TNF-α and IL-6. Administration of NF-κ B antisense oligonucleotides effectively down-regulated the production of these cytokines in the colonic mucosa. Western blotting analysis showed the decreased expression of NF-κ B p65 by the administration of p65 antisense oligonucleotides. Although we could not specify the exact target cells by immunohistochemistry, the uptake of antisense oligonucleotides was observed mainly in the inflammatory cells in the lamina propria. These data suggest that the p65 antisense oligonucleotide works on the inflammatory cells, mainly macrophages. In this study, we used F4/80 MoAb to examine the extent of macrophage infiltration. F4/80 antibody is reported to be a marker for mononuclear phagocytes in the mouse and not to cross-react significantly with any other cell type [14]. However, some spindle-shaped cells were stained and we assumed them to be fibroblasts with their typical shapes, although no previous reports indicate the cross-reactivity with fibroblasts.
Antisense oligonucleotides are single-stranded molecules (15–25 bases long) designed to hybridize to the mRNA and block the expression of the targeted protein. Recent reports have shown the effectiveness of antisense therapy in a variety of diseases, including IBD and an experimental animal model of this condition [5, 22 28 29]. Bennett et al. [5] reported that an ICAM-1 antisense oligonucleotide prevented or reversed DSS-induced colitis. Recently, Yacyshyn [30] reported that intravenous infusion of ICAM-1 antisense oligonucleotide (ISIS 2302) was effective in Crohn’s disease, and as a result corticosteroid dosage was reduced. In the present study we demonstrated that intracolonic administration of NF-κ B antisense oligonucleotide improved the inflammation by reducing the expression of NF-κ B p65 in the rectum, when antisense oligonucleotides were administrated in the early phase of colonic inflammation in DSS-induced colitis. Since rectal involvement is commonly observed in UC, intracolonic administration of p65 antisense oligonucleotides may be effective in UC, especially if more absorptive and stable antisense oligonucleotides can be developed.
REFERENCES
- 1.Stanley R, Hamilton Basil C. Gastroenterology. 5. Bockus: Morison; 1991. pp. 1326–37. [Google Scholar]
- 2.Das KM, Dasgupta A, Madal A, Geng X. Autoimmunity to cytoskeletal protein tropomyosin. J Immunol. 1993;150:2487–93. [PubMed] [Google Scholar]
- 3.Reinacker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumor necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Scholmerich J, Gross V, Falk W. Neutralization of tumor necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulfate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353–8. doi: 10.1111/j.1365-2249.1997.291-ce1184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett CF, Kornbrust D, Henry S, et al. An ICAM-1 antisense oligonucleotide prevents and reverses dextran sulfate sodium-induced colitis in mice. J Pharmacol Exp Ther. 1997;280:988–1000. [PubMed] [Google Scholar]
- 6.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor κΒ in inflammatory bowel disease. Gut. 1998;42:477–84. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neurath MF. Pathogenesis of inflammatory bowel disease: transcription factors in the spotlight. Gut. 1998;42:458–9. doi: 10.1136/gut.42.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:149–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 10.Rogler G, Brand K, Vogl D, et al. Nuclear factor B is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–69. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 11.Murthy SNS, Cooper HS, Shim H, Shah RS, Ibarahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 12.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through a NF-κB half-site. Mol Cell Biol. 1995;15:5258–67. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 14.Hume DA, Robinson AP, Macpherson GG, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of the antigen F4/80; relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983;158:1522–36. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteland JL, Nicholls SM, Shimeld C, Easty DL, William NA, Hill TJ. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. J Histochem Cytochem. 1995;43:313–20. doi: 10.1177/43.3.7868861. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning; a laboratory manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1989. pp. 18.60–18.63. [Google Scholar]
- 17.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin As., Jr Role of transcriptional activation by IκB in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–6. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 18.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NFκB activity through induction of IκB synthesis. Science. 1995;270:286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 19.Aruoma OI, Wasil H, Halliwell B, Hoey BM, Butler J. The scavenging of oxidants by sulphasalazine and its metabolites. Biochem Pharmacol. 1987;36:3739–42. doi: 10.1016/0006-2952(87)90028-1. [DOI] [PubMed] [Google Scholar]
- 20.Gionchetti P, Guarnieri C, Campieri M, Belluzzi A, Brignola C, Iannone P, Miglioli M, Barbara L. Scavenger effect of salfasalazine, 5-aminosalicylic acid, and olsalazine on superoxide radical generation. Dig Dis Sci. 1991;36:174–8. doi: 10.1007/BF01300752. [DOI] [PubMed] [Google Scholar]
- 21.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–74. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath MF, Pettersson S, Hermann K, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates established experimental colitis in mice. Nature Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 23.Ohkusa T. Production of experimental ulcerative colitis in hamsters by dextran sulfate sodium and changes in intestinal microflora. Jpn J Gastroenterol. 1985;82:1327–36. [PubMed] [Google Scholar]
- 24.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 25.Takizawa H, Sasakawa T, Nakazawa T, Tsuchiya M, Nagura H, Hibi T. Frontiers of mucosal immunology. Amsterdam: Excerpta Medica; 1991. pp. 853–4. [Google Scholar]
- 26.Shintani N, Nakajima T, Nakakubo H, Nagai H, Kagitani Y, Takizawa H, Asakura H. Intravenous immunoglobulin (IVIG) treatment of experimental colitis induced by dextran sulfate sodium in rats. Clin Exp Immunol. 1997;108:340–5. doi: 10.1046/j.1365-2249.1997.d01-1021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni J, Chen SF, Hollander D. Effects of dextran sulfate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut. 1996;39:234–41. doi: 10.1136/gut.39.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitajima I, Sinohara T, Bilakovics J, Brown DA, Xu X, Nerenberg M. Ablation of transplanted HTLV-I tax transformed tumors in mice by antisense inhibition of NF-κB. Science. 1992;258:1792–5. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- 29.Funatomi H, Itakura J, Ishiwata T, Pastan I, Thompson SA, Johnson GR, Korc M. Amphiregulin antisense oligonucleotide inhibits the growth of T3M4 human pancreatic cancer cells and sensitizes the cells to EGF receptor-targeted therapy. Int J Cancer. 1997;72:512–7. doi: 10.1002/(sici)1097-0215(19970729)72:3<512::aid-ijc21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Yacyshyn BR, Yacyshyn MB, Jewell L, Tami JA, Bennett CF, Kisner DL, Shanahan Wr., Jr A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn’s disease. Gastroenterology. 1998;114:1133–42. doi: 10.1016/s0016-5085(98)70418-4. [DOI] [PubMed] [Google Scholar]


