Abstract
Inflammatory bowel diseases (IBD) are characterized by a sustained inflammatory cascade that gives rise to the release of mediators capable of degrading and modifying bowel wall structure. Our aims were (i) to measure the production of matrix metalloproteinase-3 (MMP-3), and its tissue inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1), by inflamed and uninflamed colonic mucosa in IBD, and (ii) to correlate their production with that of proinflammatory cytokines and the anti-inflammatory cytokine, IL-10. Thirty-eight patients with IBD, including 25 with Crohn's disease and 13 with ulcerative colitis, were included. Ten controls were also studied. Biopsies were taken from inflamed and uninflamed regions and inflammation was graded both macroscopically and histologically. Organ cultures were performed for 18 h. Tumour necrosis factor-alpha (TNF-α), IL-6, IL-1β, IL-10, MMP-3 and TIMP-1 concentrations were measured using specific immunoassays. The production of both MMP-3 and the TIMP-1 were either undetectable or below the sensitivity of our immunoassay in the vast majority of uninflamed samples either from controls or from those with Crohn's disease or ulcerative colitis. In inflamed mucosa, the production of these mediators increased significantly both in Crohn's disease (P < 0·01 and 0·001, respectively) and ulcerative colitis (P < 0·001 and 0·001, respectively). Mediator production in both cases was significantly correlated with the production of proinflammatory cytokines and IL-10, as well as with the degree of macroscopic and microscopic inflammation. Inflamed mucosa of both Crohn's disease and ulcerative colitis show increased production of both MMP-3 and its tissue inhibitor, which correlates very well with production of IL-1β, IL-6, TNF-α and IL-10.
Keywords: inflammatory bowel disease, proinflammatory cytokines, IL-10, matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1
INTRODUCTION
Crohn's disease (CD) and ulcerative colitis (UC) are characterized by chronic inflammatory lesions of the digestive tract. A complex inflammatory cascade is responsible for the development and chronicity of the lesions. In both UC and CD, an increased production of proinflammatory cytokines, including tumour necrosis factor-alpha (TNF-α), IL-1β and IL-6 has been consistently described in chronic lesions [3–5]. Data on the production of anti-inflammatory cytokine IL-10 is more controversial [6–9]. The balance between these pro- and anti-inflammatory cytokines may be important in the regulation of the inflammatory cascade and tissue degradation. In particular, the production of various metalloproteinases and tissue inhibitors of metalloproteinase (TIMPs), which play a key role in tissue degradation and remodelling, is influenced by these cytokines. Proinflammatory cytokines stimulate several metalloproteinases and TIMPs in various experimental conditions [10–13], while IL-10 may have either an inhibitory [14,15] or a stimulatory effect [16]. Matrix metalloproteinase-3 (MMP-3) is a metalloproteinase capable of degrading a broad range of extracellular components including proteoglycans, type II, type IV, type IX and type XI collagens, laminin and fibronectin [17]. Using immunofluorescence and in situ hybridization techniques, MMP-3 was shown to be present both in regions of smooth muscle cell proliferation and mucosal degradation in CD, but only in regions of mucosal degradation in UC [18,19]. Furthermore, in an experimental model using explants of fetal small intestine, MMP-3 has been shown to play a prominent role in T cell and TNF-α-mediated gut injury [20,21]. The TIMPs regulate metalloproteinase activity, including MMP-3 [22,23]. Tissue inhibitor of metalloproteinase-1 (TIMP-1) has been shown to be expressed in the ulcers of CD and UC lesions [19]. To our knowledge, in inflammatory bowel disease (IBD) quantitative assessment of MMP-3 and TIMP-1 production has not been performed to date, nor has the correlation with production of pro- and anti-inflammatory cytokines been studied.
The aim of our work was to evaluate the spontaneous release of MMP-3 and TIMP-1 by the colonic mucosa of CD and UC patients in a model of organ culture and to assess their correlation with the release of proinflammatory cytokines and IL-10.
PATIENTS AND METHODS
Intestinal biopsies
Thirty-eight patients with IBD (25 with CD and 13 with UC) were included. Diagnosis of CD and UC was made on the basis of clinical, radiological and histological data, according to standard criteria. Table 1 shows the clinical data and treatment at the time of the study. CD activity was assessed by the Crohn's disease activity index (CDAI) [24] and UC was classified as active or inactive on the basis of clinical criteria. Colonic biopsies were obtained from macroscopically and microscopically non-affected (n = 23 in CD; n = 9 in UC) or affected (n = 13 in CD; n = 12 in UC) areas during colonoscopy. All patients required colonoscopy for medical reasons. The control group included 10 patients without IBD who underwent endoscopy for polyp or colon cancer surveillance (n = 6), irritable bowel syndrome (n = 2) or lower gastrointestinal tract bleeding (n = 2). All controls were free of intestinal disease. In each patient a set of five biopsies, in both affected and non-affected regions, was taken. Two were fixed in formalin for histological assessment and three were placed at 4°C in a medium consisting of Ca2+- and Mg2+-free (CMF) Hanks' solution (Gibco, Merelbeke, Belgium) supplemented by 100 U/ml penicillin and 100 μg/ml streptomycin.
Table 1.
Clinical characteristics and treatment of the Crohn's disease (CD) and ulcerative colitis (UC) patients at the time of inclusion
| Patients with CD (n = 25) | |
|---|---|
| Age, years (mean (range)) | 33 (22–51) |
| Men/women | 8/17 |
| CDAI (mean (range)) | 222 (142–310) |
| Current treatment | |
| 5-ASA | 9 |
| Steroids | 3 |
| Azathioprine | 3 |
| No medication | 10 |
| Patients with UC (n = 13) | |
| Age, years (mean (range)) | 41 (19–61) |
| Men/women | 8/5 |
| Disease activity | |
| Active disease | 11 |
| Inactive disease | 2 |
| Current treatment | |
| 5-ASA | 6 |
| Steroids | 4 |
| No medication | 3 |
Endoscopic grading
Inflammation severity was endoscopically graded according to Wardle et al. [25]. The four-point scale corresponds to: (i) macroscopically normal; (ii) granular mucosa, contact bleeding; (iii) erythematous and oedematous mucosa, aphtoid or superficial ulceration; (iv) deep ulceration with slough, and inflammatory pseudo-polyp formation. Biopsy specimens were taken from both inflamed (grades 3 and 4) and non-inflamed (grade 1) areas. Grade 2 mucosa was not considered for this study to avoid an overlap in biopsy groups.
Tissue culture
After collection, biopsy specimens were transferred to the laboratory. Within a maximal lag of 3 h after biopsy, tissues were gently washed three times in CMF–Hank's medium supplemented with antibiotics, blotted carefully, weighed and individually placed in 24-well tissue culture plates in triplicate (1 ml culture medium/well). The culture medium consisted of RPMI 1640 (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco), 2 mm l-glutamine (Gibco), penicillin (100 U/ml), and streptomycin (100 μg/ml). According to Reimund et al. [3], after 18 h culture at 37°C in a humidified 95% air/5% CO2 atmosphere, medium was removed, centrifuged and stored at −80°C until required for cytokine assays. Structural integrity was assessed both by standard histology and measurement of lactate dehydrogenase release according to Wardle et al. [25]. After 18 h of culture no histological changes were noted compared with precultured tissues. Furthermore, the release of lactate dehydrogenase in cultured tissues was significantly lower than in uncultured tissues, i.e. tissues processed the same way as cultured tissues, but put in saline instead of culture medium (data not shown).
Histology and histological inflammation scoring
Biopsy specimens from each patient were fixed in 10% buffered formalin and embedded in paraffin wax after dehydration, clearing and impregnation. Subsequent 4-μ m sections were stained with haematoxylin and eosin. All biopsy specimens for histology were assigned an overall inflammation score adapted from Riley et al. [26]. Tissue examination was performed blindly according to the diagnosis and the endoscopic inflammation score. Sections were graded using five histological features: polymorphonuclear cell infiltrate in the lamina propria, crypt abscesses, surface epithelium integrity, chronic inflammatory cell infiltrate (mononuclear cells in the lamina propria) and crypt architectural integrity. The criterion of mucin depletion was not taken into account in patients with CD. Each histological feature was graded on a four-point intensity scale: none (1 point), mild (2 points), moderate (3 points) or severe (4 points). Thus, the overall inflammation score ranged from 5 to 24 for UC, and from 5 to 20 for CD. Only biopsy specimens with a histological score of 5 were used in both the control group and in the group of normal-appearing biopsies taken from patients with UC and CD. In UC and CD, overall (inflamed and uninflamed mucosa) endoscopic and histological grades were positively correlated (Spearman, r = 0·73; P < 0·001).
Immunoassays for cytokines
TNF-α, IL-1β, IL-6, IL-10, MMP-3 and TIMP-1 production were measured with specific immunoassays (EASIA from Biosources Europe, Fleurus, Belgium, except TIMP-1 (ELISA from Amersham Life Science, Aylesbury, UK). Immunoassays were performed according to the manufacturer's instructions. The detection limit was 3 pg/ml for TNF-α, 2 pg/ml for IL-6, 2 pg/ml for IL-1β, 1 pg/ml for IL-10, 100 pg/ml for MMP-3 and 1250 pg/ml for TMP-1. Cytokine, MMP-3 and TIMP-1 production was expressed by its concentration per ml of culture medium and per mg of tissue in culture. The final result for each patient corresponds to the mean of the three individual biopsy cultures. The mean variation coefficient between the three individual biopsies was 35·7 ± 13·2% for ΤΝF-α, 46·5 ± 12·4% for IL-6, 25·4 ± 15·3% for IL-1β, 37·3 ± 16·4% for IL-10, 35·4 ± 11·5% for MMP-3 and 39·5 ± 20·3% for TIMP-1.
We also calculated ratios between IL-1β or TNF-α and IL-10, representative of the proinflammatory/anti-inflammatory cytokine balance, as well as between MMP-3 and TIMP-1.
Data are expressed as a median (range). Results were compared using the Mann–Whitney U-test or Kruskall–Wallis, when multiple comparisons were made. A linear regression or a Spearman r-test was used to assess correlation. The level of significance was taken as P < 0·05.
RESULTS
Release of TNF-α, IL-6, IL-1β, IL-10, MMP-3 and TIMP-1 by organ cultures
Concentrations of TNF-α, IL-6, IL-1β, IL-10, MMP-3 and TIMP-1 measured in the supernatants of biopsy cultures were not different in controls, non-affected CD or non-affected UC mucosa, but were significantly higher in affected CD and UC mucosa (Table 2, Figs 1 and 2). In controls, as well as in uninflamed CD and UC, there was no significant amount of MMP-3 and TIMP-1 in the vast majority of the culture supernatants (Table 2, Figs 1 and 2). TNF-α/IL-10 and IL-1β/IL-10 ratios were not significantly different between controls, affected or non-affected UC and affected or non-affected CD (7·9 (1·93–35); 7·26 (1·55–39·09); 30 (6·21–163·63); 7·28 (1·17–27·69); 5·33 (4·29–12·37) and 9·33 (4·07–27·5); 21·19 (7·16–55·77); 23·49 (4·5–36·36); 13·75 (3–133·1); 22·1 (13·33–41·6), respectively).
Table 2.
Production of tumour necrosis factor-alpha (TNF-α), IL-6, IL-1β, IL-10, matrix metalloproteinase-3 (MMP-3) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in controls and affected or non-affected Crohn's disease (CD) and ulcerative colitis (UC) (pg/ml per mg)
| Controls | Non-affected CD | Affected CD | Non-affected UC | Affected UC | |
|---|---|---|---|---|---|
| TNF-α | 4·25 (1·4–17·8) | 4 (0·3–21·4) | 39·7 (4·2–85)** | 6 (1·4–21) | 24 (9·6–129)*** |
| IL-6 | 60·7 (15–860) | 43·1 (6·8–162) | 473 (127–5729)** | 74·3 (12–390) | 1243 (532–8848)*** |
| IL-1β | 7·5 (0·9–54·8) | 3·9 (0–46·6) | 29·5 (0·6–201·6)* | 6·8 (0·4–59·9) | 40·9 (9·9–204·7)** |
| IL-10 | 0·8 (0·04–2·8) | 0·4 (0–1·1) | 2·9 (0·2–33·8)* | 0·2 (0·01–1·9) | 2·9 (0·4–24·8)* |
| MMP-3 | 80 (0–1400) | 0 (0–300) | 620 (0–15 400)** | 0 (0–16) | 1440 (610–5400)*** |
| TIMP-1 | 650 (200–4700) | 400 (200–1600) | 2600 (600–10 600)*** | 300 (50–600) | 5500 (1600–11 500)*** |
P < 0·05
P < 0·01
P < 0·0001 compared with controls.
Fig. 1.
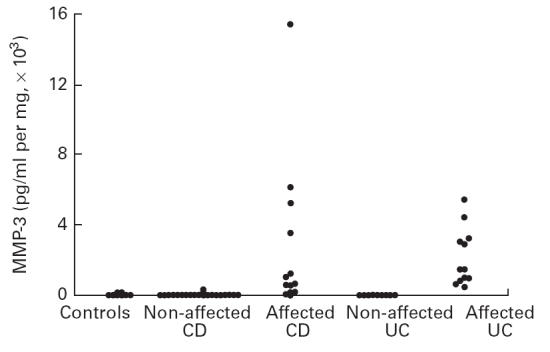
Production of matrix metalloproteinase-3 (MMP-3) by colonic mucosa in organ culture. Cultures were performed for 18 h without stimulus. MMP-3 concentrations were measured in the culture medium using a specific immunoassay. The detection limit of the test was 100 pg/ml. Each point corresponds to an individual patient and represents the mean of three biopsy cultures. There was an increased production of MMP-3 in inflamed Crohn's disease (CD) and ulcerative colitis (UC) (P < 0·01 and 0·001, respectively).
Fig. 2.
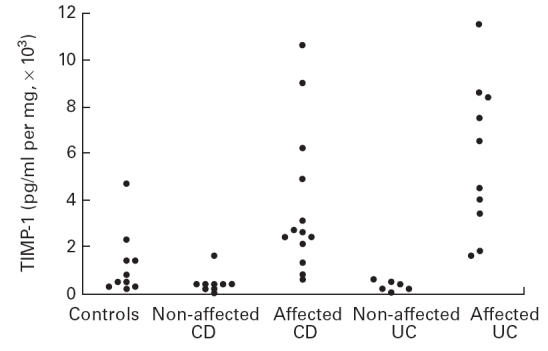
Production of tissue inhibitor of metalloproteinase-1 (TIMP-1) by colonic mucosa in organ culture. Cultures were performed for 18 h without stimulus. TIMP-1 concentrations were measured in the culture medium using a specific immunoassay. The detection limit of the test was 1250 pg/ml. Each point corresponds to an individual patient and represents the mean of three biopsy cultures. There was an increased production of TIMP-1 in inflamed Crohn's disease (CD) and ulcerative colitis (UC) (P < 0·001 and 0·001, respectively).
The MMP-3/TIMP-1 ratio was significantly higher in affected UC and CD compared with controls and non-affected UC or CD (0·39 (0·11–0·68; P < 0·001); 0·19 (0–2; P = 0·02); 0·005 (0–0·09); 0 (0–0·02); 0·1 (0–0·43), respectively).
Using linear regression, MMP-3 and TIMP-1 correlated very significantly with each other and with IL-6, IL-1β and IL-10, in both CD and UC (Table 3, Figs 3 and 4). The correlation obtained from a linear regression between MMP-3 or TIMP-1 and TNF-α was significant in UC but not CD. However, in CD these correlations were significant when a Spearman test was done (P < 0·01).
Table 3.
Correlation coefficients (linear regression) between matrix metalloproteinase-3 (MMP-3) or tissue inhibitor of metalloproteinase-1 (TIMP-1) and various pro- and anti-inflammatory cytokines in Crohn's disease (CD) and ulcerative colitis (UC)
| CD | IL-6 | IL-1β | IL-10 | TNF-α | TIMP-1 |
|---|---|---|---|---|---|
| MMP-3 | 0·95*** | 0·97*** | 0·7*** | 0·35 | 0·59** |
| TIMP-1 | 0·85** | 0·71** | 0·92*** | 0·36 | |
| UC | IL-6 | IL-1β | IL-10 | TNF-α | TIMP-1 |
| MMP-3 | 0·72** | 0·53* | 0·55** | 0·61** | 0·93*** |
| TIMP-1 | 0·68** | 0·58* | 0·61* | 0·71** | |
P < 0·05
P < 0·01
P < 0·0001
Fig. 3.
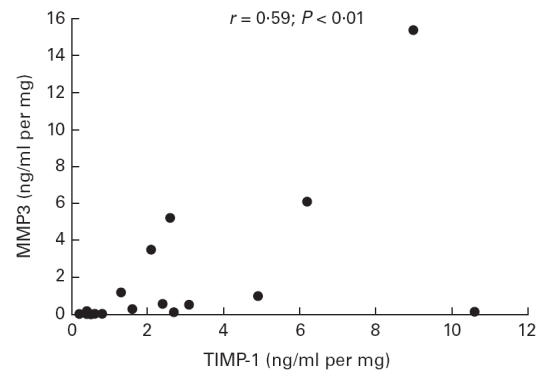
Correlation between production of matrix metalloproteinase-3 (MMP-3) and tissue inhibitor of metalloproteinase-1 (TIMP-1) by colonic mucosa in organ culture in Crohn's disease. Cultures were performed for 18 h without stimulus. MMP-3 and TIMP-1 concentrations were measured in the culture medium using a specific immunoassay. Each point corresponds to an individual patient and represents the mean of three biopsy cultures. Using linear regression, the two mediators were significantly correlated (r = 0·59; P < 0·01).
Fig. 4.
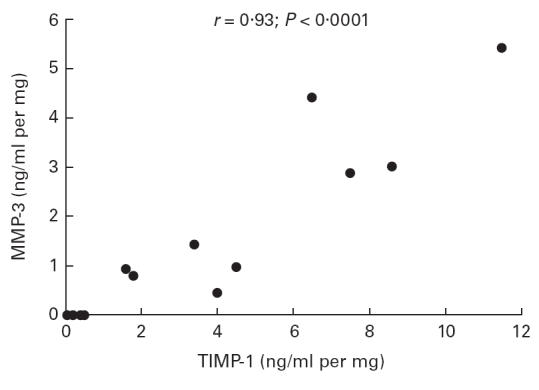
Correlation between production of matrix metalloproteinase-3 (MMP-3) and tissue inhibitor of metalloproteinase-1 (TIMP-1) by colonic mucosa in organ culture in ulcerative colitis. Cultures were performed for 18 h without stimulus. MMP-3 and TIMP-1 concentrations were measured in the culture medium using a specific immunoassay. Each point corresponds to an individual patient and represents the mean of three biopsy cultures. Using linear regression, the two mediators were significantly correlated (r = 0·93; P < 0·0001).
Correlation between cytokine MMP-3 and TIMP-1 release and endoscopic grade
The Kruskall–Wallis test showed a significant difference between mediator levels in the culture supernatants of biopsies with endoscopic grades 1, 3 or 4, for all parameters tested (P < 0·001).
The Spearman correlation test also showed a significant correlation between mediator levels in the culture supernatant and endoscopic grades. The best correlation was obtained for MMP-3 (r = 0·86; P < 0·0001). The other correlations were all significant with a P level <0·001. The Spearman r values were 0·83, 0·64, 0·62, 0·58, 0·80 for IL-6, TNF-α, IL-10, IL-1β, and TIMP-1, respectively.
The MMP-3/TIMP-1 ratio was significantly higher in endoscopic grades 3 and 4 compared with grade 1 (0·39 (0–2) versus 0 (0–0·43); P < 0·01).
Correlation between cytokines, MMP-3 and TIMP-1 release and histological grade
Using the Spearman test, all mediators except TIMP-1 tested positively and significantly correlated with histological grades (P < 0·01 for each). The Spearman r values were 0·65, 0·72, 0·75, 0·68, 0·65 for IL-6, TNF-α, IL-10, IL-1β and MMP-3, respectively.
There was no significant correlation between MMP-3/TIMP-1 ratios and histological grades.
Relation with other variables
Cytokines, MMP-3 and TIMP-1 production by cultured colonic biopsies were not correlated to disease activity or type of medical treatment (data not shown).
DISCUSSION
We found increased production of both MMP-3 and TIMP-1 by inflamed colonic mucosa in both CD and UC. Increased production of both strongly correlated to each other and to the production of proinflammatory cytokines and the anti-inflammatory cytokine, IL-10.
In IBD an uncontrolled and sustained inflammatory cascade gives rise to the production of proteases, free radicals and metalloproteinases which are responsible for tissue degradation and lesion development. Our study provides some new data on the characteristics of the inflammatory cascade at the mucosal level.
We confirm an increased production of TNF-α, IL-6 and IL-1β, previously described in IBD, using various techniques, including organ culture of intestinal biopsies [3–5]. Data on IL-10 production have been more controversial [6–9]. The most recent study, using in situ hybridization and immunohistochemistry, showed the absence of general deficiency in IL-10 production in IBD [27]. However, there was a possible relative defect of IL-10 production in the lamina propria. In the present study we did not find any prominent defect in IL-10 production in the mucosa of patients with IBD. IL-10 was significantly higher in the supernatants of inflamed biopsies compared with uninflamed biopsies or biopsies from normal subjects. This increase correlated very well with increased production of proinflammatory cytokines. Furthermore, no significant difference was found in ratios between IL-10 and TNF-α, IL-1β or IL-6 in various experimental conditions.
MMP-3, which is produced by smooth muscle cells and fibroblasts, is capable of degrading a wide range of extracellular matrix components [17]. In an ex vivo model using explant cultures of fetal small intestine, MMP-3 has been shown to play a prominent role in T cell and TNF-mediated gut injury [20,21]. In a previous study using immunofluorescence, MMP-3 was shown to be present extracellularly in mucosal damaged regions in both UC and CD [18]. However, in macroscopically healthy regions, very little MMP-3 was detected. A more recent study using in situ hybridization showed the presence of MMP-3 in granulation tissue of CD ulcers [19]. TIMP-1, a tissue inhibitor of metalloproteinase [22,23], was also present in the intestinal ulcers [19]. TNF-α, IL-1β and, to a lesser extent, IL-6 have been shown to stimulate metalloproteinase production, including MMP-3, in various experimental conditions [9,28]. In contrast, IL-10 may decrease production of several metalloproteinases [14]. However, in some conditions, such as keratinocyte cultures, it may also increase MMP-3 production [16]. Note that a recent study has shown a decreased production of MMP-3 in explants of fetal small intestine after stimulation by IL-10 [15]. We did not detect a significant concentration of MMP-3 in supernatants of control biopsies or uninflamed CD and UC. In inflamed mucosa both in UC and CD, our data confirm an increased production of MMP-3. This increase was similar in UC and CD and strongly correlated with an increase in proinflammatory cytokine production. A significant difference between CD and UC is worth mentioning here. The correlation between MMP-3 and TNF-α was stronger in UC than in CD. This may indicate a difference in the regulation of MMP-3 production between these two diseases. It is important to note that in both illnesses, there was no parallel defect in IL-10 production, which was also very significantly correlated with MMP-3. This suggests that despite a significant inhibitory effect when added to a fetal small intestine culture model [15], the concentrations of IL-10 reached in our colonic biopsy cultures were not sufficient to suppress MMP-3 production significantly.
As for MMP-3, no significant amount of TIMP-1 was detected in supernatants of control biopsies or uninflamed mucosa of CD and UC. However, in inflamed mucosa our data confirm increased production, and thus show no sign of overall defect in this endogenous protective mechanism in IBD. Again, increased production in the inflamed area was similar in CD and UC. Like MMP-3, TIMP-1 was also very well correlated with proinflammatory cytokines as well as with IL-10. An exception can be noted here: TIMP-1 production was less strongly correlated with TNF-α production in CD than in UC. As for MMP-3, this may suggest a difference in the regulation of TIMP-1 production between these diseases. Despite the strong correlation between MMP-3 and TIMP-1, which suggests an adapted regulatory response of TIMP-1 to the increase of MMP-3, when one looks at ratios between MMP-3 and TIMP-1, there was a significant increase in inflamed mucosa in both CD and UC. This may be due to irrelevant variations in MMP-3 or TIMP-1 concentrations below the detection limit of the ELISA in uninflamed mucosa and, in our opinion, does not permit any functional conclusions. Any such conclusions would require specific functional studies. Furthermore, our ELISA for MMP-3 recognizes either free MMP-3, pro-MMP-3 or MMP-3 linked to TIMP-1. The same holds true for TIMP-1, rendering functional interpretations even more difficult. A recent paper on this point has shown a significant increase in metalloproteinase activity assessed by zymography in inflamed as well as uninflamed mucosa in CD and UC [29]. This suggests that the proportional increase of TIMP-1 we found may be insufficient to neutralize the increased MMP-3 activity. This could be due, for example, to a modification in ratios between active free MMP-3 and TIMP-1, which we were unable to assess precisely with our ELISA. The correlation we found between MMP-3 (as well as TNF-α, IL-1β, IL-6 and IL-10) and histological grade may also be an argument for the functional relevance of its increase, while the absence of correlation for TIMP-1 may be due to its complex protective role and to the lack of assessment of active TIMP-1, as stated earlier.
In conclusion, we found an increased production of both MMP-3 and TIMP-1, its tissue inhibitor, in inflamed mucosa of both CD and UC. This increased production was strongly correlated with increased production of proinflammatory cytokines and was not associated with a prominent defect in IL-10 production. The functional significance of these increased productions of MMP-3 and TIMP-1 remains to be clarified by functional studies.
Acknowledgments
E.L., C.R. and D.F. are supported by the National Fund for Scientific Research of Belgium. The authors are indebted to Mrs Y. Vrindts-Gevaert and S. Gaspard for their expert technical assistance.
REFERENCES
- 1.Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology. 1997;113:118–26. doi: 10.1016/s0016-5085(97)70116-1. [DOI] [PubMed] [Google Scholar]
- 2.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 3.Reimund JM, Wittersheim C, Dumont S, Muller CD, Kennet JS, Baumann R, Poindron P, Duclos B. Increased production of tumor necrosis factor-α, interleukin-1β, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut. 1996;39:684–9. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinecker H-C, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6 and IL-1β by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens C, Wlaz G, Singaram C, et al. Tumor necrosis factor-α, interleukin-1β, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992;37:818–26. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen OH, Koppen T, Rüdiger N, Horn T, Eriksen J, Kirman I. Involvement of interleukin-4 and -10 in inflammatory bowel disease. Dig Dis Sci. 1996;41:1786–93. doi: 10.1007/BF02088746. [DOI] [PubMed] [Google Scholar]
- 7.Schmit A, Van Gossum A, Carol M, Mascart-Lemone F. Different cytokine patterns within inflamed and non inflamed colonic mucosa in Crohn's disease. Gastroenterology. 1998;114:G4417. (Abstr.). [Google Scholar]
- 8.Kucharzik T, Stoll R, Lügering N, Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;100:452–6. doi: 10.1111/j.1365-2249.1995.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434–44. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 10.Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, Kamoi K, Suda T. Regulation of matrix metalloproteinases (MMP-2-3-9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998;139:1338–45. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 11.Saren P, Welgus HG, Kovanen PT. TNF-α and IL-1β selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–65. [PubMed] [Google Scholar]
- 12.Murphy G, Reynolds JJ, Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-o-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985;260:3079–83. [PubMed] [Google Scholar]
- 13.Lotz M, Guerne P-A. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA) J Biol Chem. 1991;266:2017–20. [PubMed] [Google Scholar]
- 14.Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer J-M. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–10. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pender SLF, Breese EJ, Günther U, Howie D, Wathen NC, Schuppan D, McDonald TT. Suppression of cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology. 1998;115:573–83. doi: 10.1016/s0016-5085(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 16.Reitamo S, Remitz A, Tamai K, Uitto J. Interleukin-10 modulates type 1 collagen and matrix metalloproteinase gene expression in cultured human skin fibroblasts. J Clin Invest. 1994;94:2489–92. doi: 10.1172/JCI117618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stetler-Stevenson W. Type IV collagenase in tumour invasion and metastasis. Cancer Metastasis Rev. 1990;9:289–303. doi: 10.1007/BF00049520. [DOI] [PubMed] [Google Scholar]
- 18.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994;47:113–6. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saarialho-Kere UK, Vaalmo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg M-L. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996;148:519–26. [PMC free article] [PubMed] [Google Scholar]
- 20.Pender SLF, Tickle SP, Docherty AJP, Howie D, Wathen NC, McDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582–90. [PubMed] [Google Scholar]
- 21.Pender SLF, Fell JME, Chamow SM, Ashkenazi A, McDonald TT. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol. 1998;160:4098–103. [PubMed] [Google Scholar]
- 22.Albin RJ, Senior RM, Welgus HG, Connoly NL, Campbell EJ. Human alveolar macrophages release an inhibitor of metalloproteinase elastase in vitro. Am Rev Respir Dis. 1987;135:1281–5. doi: 10.1164/arrd.1987.135.6.1281. [DOI] [PubMed] [Google Scholar]
- 23.Howard EW, Bullen EC, Banda MJ. Regulation of the autoactivation of human 72-kDa progelatinase by tissue inhibitor of metalloproteinase-2. J Biol Chem. 1991;266:13064–9. [PubMed] [Google Scholar]
- 24.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn's disease activity index (CDAI) Gastroenterology. 1979;77:843–6. [PubMed] [Google Scholar]
- 25.Wardle TD, Hall L, Turnberg LA. Use of coculture of colonic biopsies to investigate the release of eicosanoids by inflamed and uninflamed mucosa from patients with inflammatory bowel disease. Gut. 1992;33:1644–51. doi: 10.1136/gut.33.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean ? Gut. 1991;32:174–8. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Autsbach F, Braunstein J, Helmke B, et al. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121–30. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito A, Ito Y, Sasaguri Y, Norimatsu M, Mori Y. Effects of interleukin-6 on the metabolism of connective tissue components in rheumatoid synovial fibroblasts. Arthritis Rheum. 1992;35:1197–201. doi: 10.1002/art.1780351012. [DOI] [PubMed] [Google Scholar]
- 29.Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–22. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]


