Abstract
The 19-kD antigen is a cell wall-associated lipoprotein present in Mycobacterium tuberculosis and in bacille Calmette–Guérin (BCG) vaccine strains. Expression of the 19-kD antigen as a recombinant protein in two saprophytic mycobacteria—M. vaccae and M. smegmatis—resulted in abrogation of their ability to confer protection against M. tuberculosis in a murine challenge model, and in their ability to prime a DTH response to cross-reactive mycobacterial antigens. Induction of an immune response to the 19-kD antigen by an alternative approach of DNA vaccination had no effect on subsequent M. tuberculosis challenge. These results are consistent with a model in which the presence of the 19-kD protein has a detrimental effect on the efficacy of vaccination with live mycobacteria. Targeted inactivation of genes encoding selected antigens represents a potential route towards development of improved vaccine candidates.
Keywords: tuberculosis, mouse model, recombinant vaccines, immune response
INTRODUCTION
The 19-kD protein was originally identified as a major antigen of Mycobacterium tuberculosis on the basis of its recognition by murine MoAbs raised against crude bacterial extracts [1]. Subsequently, this antigen was shown to be the target of both antibody and T cell responses in the course of tuberculosis infection and M. bovis bacille Calmette–Guérin (BCG) vaccination in humans [2–4]. Related proteins are present in some slow-growing mycobacterial species [5], but database screening has failed to identify homologues in other organisms, and the biological function of the 19-kD protein remains to be determined. Rapid-growing non-pathogenic mycobacteria, such as M. smegmatis and M. vaccae, have no endogenous homologue of the 19-kD antigen, but readily express the protein following transformation with the appropriate gene from M. tuberculosis[6,7]. The recombinant protein expressed in mycobacterial systems resembles the native antigen in its modification by post-translational acylation and glycosylation [6,7].
In previous experiments to explore novel tuberculosis vaccines, we compared the effect of immunization of mice with M. vaccae alone with that of M. vaccae expressing the 19-kD antigen [7]. Despite induction of a strong type 1 immune response specific to the 19-kD protein, we observed an unexpected reduction in efficacy of vaccination with the 19-kD recombinant in comparison with control M. vaccae in low-dose aerosol and in high-dose i.v. challenge models [7]. This paradoxical result has, to our knowledge, no analogues amongst a variety of mycobacterial antigen vaccine candidates studied so far. There are two ways in which the anti-vaccine effect may have been generated. First, the existence of a type 1 response to the 19-kD antigen at the time of challenge might have the effect of exacerbating the M. tuberculosis infection. Alternatively, the presence of the 19-kD antigen in the vaccine preparation might interfere with the induction of events associated with protection.
To explore this phenomenon further, in the present study we compared the effect of the 19-kD antigen in two different mycobacterial delivery systems (live M. vaccae and M. smegmatis), and examined the consequences of induction of a type 1 response to the 19-kD antigen by an alternative procedure of DNA vaccination. The results are consistent with the hypothesis that the presence of the 19-kD antigen alters the response to vaccination with mycobacterial vectors.
MATERIALS AND METHODS
Recombinant mycobacterial strains
Mycobacterium vaccae NCTC11659 (supplied by Professor J. Stanford, University College London Medical School, London, UK) was grown at 30°C on Middlebrook 7H11 (Difco, Detroit, MI) agar plates or with shaking in 7H9 medium (Difco) supplemented with 2% glucose. Mycobacterium smegmatis mc2/1–2c [8] was grown under similar conditions at 37°C. Hygromycin B (Boehringer, Mannheim, Germany) was added at 50 μg/ml for cultures of strains transformed with shuttle plasmids; this was substituted by kanamycin the case of the 19-kD cosmid construct. Recombinant strains were generated by electroporation as described previously with a mycobacterium–Escherichia coli shuttle plasmid p16R1 [9] (vector control), or with p16R1-19, which has an additional 1·8-kb Sma I fragment including the M. tuberculosis 19-kD gene [7]. An M. smegmatis construct expressing the 19-kD antigen from a cosmid vector was prepared by transformation with cosmid T500 provided by Dr S. Cole (Institut Pasteur, Paris, France). Expression of the 19-kD antigen in recombinant constructs was confirmed by Western blot analysis using HYT6 antibodies as described previously [7]. For vaccination, exponential phase mycobacterial cultures were harvested, washed with PBS, and stored as aliquots at −20°C. Concentrations were determined by measurement of colony-forming units (CFU).
DNA vaccines
DNA encoding the 19-kD antigen of M. tuberculosis was amplified by polymerase chain reaction (PCR) with primers containing a Bgl II site and the DNA fragment was digested with Bgl II and cloned into the Bgl II site of V1Jns.tPA (kindly provided by Dr J. B. Ulmer, Merck & Co., Inc., Gibbstown, NJ)[10]. Sequencing of plasmids with a primer derived from the tpa signal sequence confirmed in-frame ligation. The plasmids were also sequenced across the gene insert. The expression of the protein was confirmed by transfection of RD cells.
Animals
C57Bl/6JCit (B6) mice were bred in the Animal Facilities of the Central Institute for Tuberculosis (Moscow, Russia) under conventional conditions with water and food provided ad libitum. Female mice 8–10 weeks of age were used for vaccination and subsequent infection.
Vaccination
Mice in groups of 25 were immunized subcutaneously with 5 × 107 CFU/mouse of M. vaccae::p16R1 (vector control), M. vaccae::p16R1–19, M. smegmatis::p16R1 (vector control), M. smegmatis::p16R1-19, M. smegmatis::T500 or M. bovis BCG-Prague. All of the mycobacterial vaccines were delivered as a single injection of live organisms in 0·5 ml of PBS. Control mice were injected with PBS alone.
For DNA vaccination, mice were injected intramuscularly three times at 3-week intervals in both quadriceps with VIJNS-Tpa (vector control) or VIJNS-Tpa19 in saline. Each mouse received 100 μg of DNA at each injection.
Immune responses to vaccination
Four weeks after mycobacterial vaccination, DTH was estimated by injection of 40 μl saline containing 10 μg of tuberculin (Staten Serum Institut, Copenhagen, Denmark) into left footpads of 10 mice from each group. Right footpads were injected with 40 μl of saline control. Local swelling was measured with an engineer micrometer (Fowler, Osaka, Japan) after 24 h.
Mice receiving DNA vaccines were bled to obtain sera 17 days after the last immunization. Antibody responses were measured in an ELISA format using microtitre plates coated with 19-kD protein purified from recombinant E. coli as described previously [7]. To test the T cell response to DNA vaccination, splenocyte suspensions were prepared for cytokine assay as described below. Interferon-gamma (IFN-γ) production was measured in response to addition of the purified 19-kD antigen. The antigen was expressed in its native form in M. vaccae, or as a histidine-tagged recombinant in E. coli, with or without post-translational acylation as previously described [7].
Challenge with M. tuberculosis
Five weeks after mycobacterial vaccination, or 4 weeks after the final DNA immunization, mice were subjected to i.v. challenge with M. tuberculosis H37Rv. The challenge strain (from the collection of the Central Institute for Tuberculosis, Moscow, Russia) was grown initially on Loewenstein–Jensen medium at 37°C for 3 weeks. Bacterial mass was removed, weighed, rubbed with a glass stick against the walls of a thick glass tube and vigorously resuspended in gradually increasing volumes of sterile saline containing 0·05% Tween 20 and 0·1% bovine serum albumin (BSA; Sigma, St Louis, MO) to a final concentration of 10 mg (wet weight)/ml. To obtain a culture of exclusively live mid-log phase bacilli, 50 ml of this suspension were diluted in 5 ml of Dubos broth (Difco), supplemented with 0·5% BSA (Sigma). The culture was incubated without shaking for 1 week at 37°C in a 15-ml tube (first passage), and 0·5 ml was again diluted in 20 ml of fresh warm Dubos broth plus BSA and further cultured for 1 week in a 50-ml tube (second passage). After the second passage in liquid medium, the mycobacterial suspension was centrifuged at 3000 g for 20 min at 4°C; mycobacteria were resuspended in sterile saline, aliquoted and stored at −80°C until use. To estimate CFU, a 1-ml aliquot was thawed, diluted in 30 ml of saline, and 1:10 serial dilutions were plated onto Dubos oleic agar (Difco). Microcolonies were counted 3 days later using an inverted microscope. While microcolonies were growing, the bulk culture was stored for 3 days at 4°C; it was shown in separate experiments that no change in CFU content occurs during this period. After estimation of CFU content, the bacterial suspension was adjusted to a concentration of either 5 × 105 or 2 × 105 CFU in 0·5 ml of saline and immediately injected into mice.
Mortality in infected mice was monitored daily, and results expressed as mean survival time (MST) following challenge. To determine CFU levels in infected organs, serial dilutions of 0·2-ml samples of digested lung tissue or spleen cell suspension in sterile saline were plated on Dubos agar medium (Difco) 1, 2 and 3 or 5 weeks following infection. Colonies were counted 18–20 days later.
Immune responses following challenge
Vaccinated and infected mice were killed, and their lungs and spleens removed aseptically. Suspensions of interstitial lung cells were obtained and purified using the method introduced by Holt et al. [11] with our previously described modifications [12]. Similarly, splenocytes were enriched for T cells by separation on nylon wool columns, as described for lung cell suspensions [12]. Splenic or lung T cell-enriched suspensions pooled from three mice represented an experimental group.
To measure proliferative responses, 105 T cell-enriched lung or spleen cells were co-cultured with 3 × 105 irradiated syngeneic splenocytes (as antigen-presenting cells (APC)) in a well of a 96-well flat-bottomed plate (Costar, Badhoevedorp, The Netherlands), at 37°C, 5% CO2 in the presence of 10 μg/ml of PPD (Statum Serum Institut, Copenhagen, Denmark) [13]. All cultures were performed in triplicate, and non-stimulated wells served as controls. Cultures were pulsed with 0·5 μ Ci of 3H-thymidine for the last 18 h of a 72-h incubation. Uptake of label was measured in a liquid scintillation counter (Wallac, Turku, Finland) after harvesting the contents of each well onto fibreglass filters using a semiautomatic cell harvester (Scatron, Oslo, Norway).
To measure cytokine production, 1 × 106 cells/well were co-cultured in 24-well plates (Costar) with 5 × 105 APC and 10 μg/ml PPD. Cells were incubated at 37°C in a humidified 5% CO2 atmosphere. After 48 h, supernatants were harvested and stored at −30°C until use.
ELISAs were used to assay IL-4, IL-5, and IFN-γ in 48-h culture supernatants. Capture and detecting (biotinylated) MoAbs specific for mouse cytokines were purchased from PharMingen (San Diego, CA): IFN-γ, clones R4-6A2 and XMG1.2; IL-4, clones 11B11 and BVD6-24G2; IL-5, clones TRFK5 and TRFK4. ELISAs were performed following the manufacturers' instructions. A standard curve for each assay was generated with known concentrations of mouse rIL-4 (PharMingen), rIL-5 (PharMingen), and rIFN-γ (Genzyme, Boston, MA). The results for IFN-γ are expressed as mean ng/ml of a triplicate assessment; the standard deviation was <10%.
Statistical analysis
The statistical significance of the differences was estimated by Student's t-test. P < 0·05 was considered statistically significant.
RESULTS
Effect of mycobacterial vaccines on MST
Intravenous challenge of mice with a lethal dose of 5 × 105 or 2 × 105 virulent M. tuberculosis resulted in an MST of 25 and 32 days, respectively (Table 1). Prior s.c. vaccination with live BCG conferred significant protection, more than doubling the MST. Vaccination with an equivalent number of live M. smegmatis or M. vaccae conferred a much lower degree of protection, although a significant increase in MST (P < 0·01) was observed during challenge with the lower dose of M. tuberculosis (Table 1). The weak protection associated with immunization with saprophytic mycobacteria in this model is consistent with the results of previous experiments involving multiple immunization with heat-killed M. vaccae[7], and our previous observation of rapid clearance of live M. smegmatis following s.c. injection [14]. There was no evidence of a significant difference in the degree of protection conferred by M. vaccae compared with M. smegmatis. In both cases however the presence of the recombinant 19-kD antigen in the vaccine constructs had a significant deleterious effect (P < 0·05) on protective efficacy as judged by MST (Table 1). The effect of the 19-kD antigen was less in the M. smegmatis construct transformed with a cosmid vector (group 5 in Table 1). This could be due to a reduced level of expression of the 19-kD antigen in this construct (5–10-fold lower as assessed by Western blot assay), or to expression of some additional protective antigen encoded by other genes carried on the approx. 40-kb cosmid insert. These results confirm earlier findings and demonstrate that the effect of the 19-kD antigen in the lethal challenge model is not limited to a single delivery system.
Table 1.
Mortality of B6 mice following vaccination and Mycobacterium tuberculosis H37Rv challenge
| MST ± s.e.m. (days) | ||
|---|---|---|
| Group | A. 5 × 105 CFU/mouse | B. 2 × 105 CFU/mouse |
| 1. Non-vaccinated control | 24·8 ± 1·3 | 31·6 ± 2·9 |
| 2. M. bovis BCG-Prague | > 70 | > 90 |
| 3. M. smegmatis::p16R1 | 25·1 ± 0·7 | 66·3 ± 4·7 |
| 4. M. smegmatis::p16R1-19 | 24·1 ± 0·7 | 42·9 ± 5·9* |
| 5. M. smegmatis::cosmid 19-kD | 29·5 ± 1·7 | 52·5 ± 3·2* |
| 6. M. vaccae::p16R1 | ND | 57·8 ± 2·0 |
| 7. M. vaccae::p16R1-19 | ND | 46·6 ± 2·5* |
| 8. VIJNS-Tpa | 20·0 ± 0·72 | ND |
| 9. VIJNS-Tpa19 | 28·9 ± 2·61** | ND |
Significant decrease in mean survival time (MST) compared with the corresponding vector control group.
Significantly different from the plasmid control group (P < 0·01 by Student's t-test).
Effect of mycobacterial vaccines on bacterial load
Increased MST in BCG-vaccinated mice was associated with a significantly lower bacterial load in lungs and spleens following low-dose challenge (P < 0·001) (Fig. 1). Interestingly, CFU counts either stabilized (lungs) or dropped (spleens) between weeks 2 and 5 post-infection in the non-vaccinated control group. A drop in numbers of mycobacteria populating the spleen is often characteristic of the terminal phase of tuberculosis infection in mice (our unpublished observation). In mice immunized with M. vaccae vector control however, the bacterial load continued to rise up to 5 weeks, reaching a level higher than that seen in the non-vaccinated animals, in spite of their postponed mortality. The dynamics of the bacterial load in the group receiving M. vaccae expressing the 19-kD antigen resembled that seen in the non-vaccinated controls (Fig. 1).
Fig. 1.
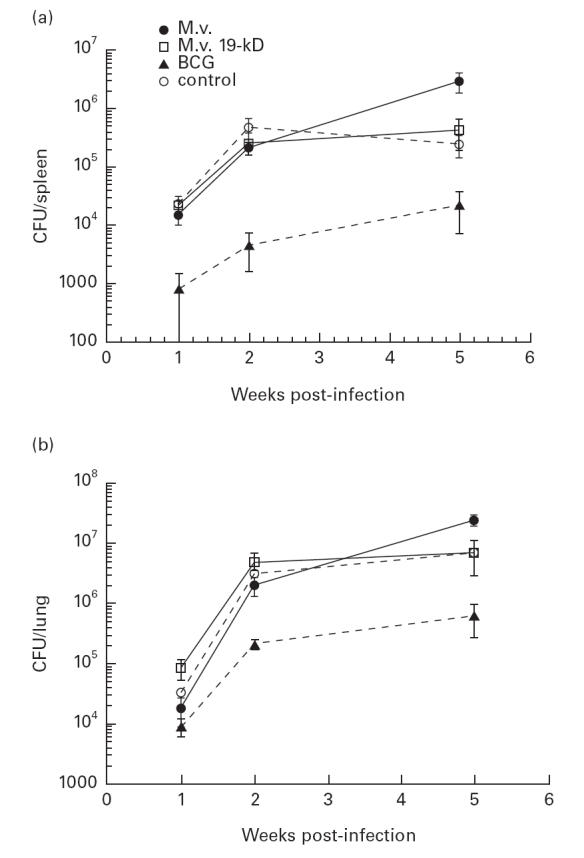
Bacterial load in organs of the low-dose infected mice. Bacterial load was measured by counting colony-forming units (CFU) in tissue homogenates prepared from spleens (a) and lungs (b) of mice challenged with 2 × 105Mycobacterium tuberculosis H37Rv. A reduction of approximately 10-fold was observed in bacille Calmette–Guérin (BCG)-immunized mice. In mice immunized with M. vaccae, the bacterial load after 5 weeks was higher than in control mice, in spite of a decreased mortality.
19-kD DNA vaccination
We have previously reported that immunization with the purified 19-kD antigen in Freund's incomplete adjuvant (FIA) had no effect on MST in the lethal challenge model [7]. However, we also noted that this protocol was less effective than immunization with the M. vaccae recombinant in induction of a type 1 immune response. To investigate the effect of a strong type 1 immune response on M. tuberculosis challenge, we adopted the approach of DNA vaccination. As reported for M. tuberculosis antigen 85 [10], immunization of mice with plasmid DNA carrying the 19-kD gene resulted in induction of strong type 1 immune response as judged by antigen-specific release of IFN-γ by splenocytes and by a relatively high level of IgG2a antibodies (Fig. 2). However, DNA vaccination had no effect on MST in the lethal challenge model (Table 1), suggesting that the presence of a type 1 response to the 19-kD antigen does not in itself have a deleterious effect on the course of infection.
Fig. 2.
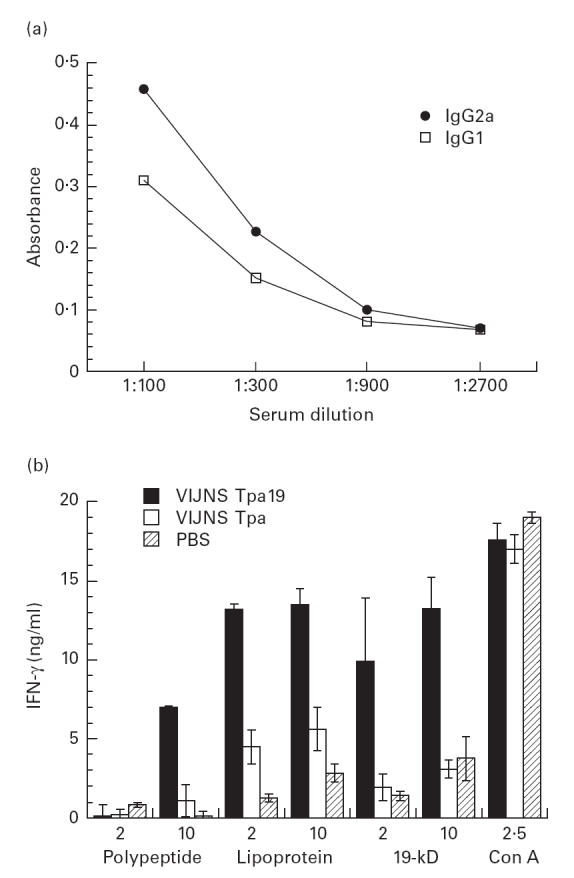
Induction of a type 1 immune response to the 19-kD antigen by DNA vaccination. (a) Antibody responses to the 19-kD antigen were measured by ELISA assay in sera from mice immunized by DNA vaccination. A strong IgG2a response was consistent with induction of a type 1 immune response. There was no detectable antibody response to the 19-kD antigen in mice immunized with vector control. (b) Splenocytes from vaccinated and control mice were incubated with 19-kD antigen purified from recombinant mycobacteria (‘19 kD’), or from Escherichia coli after expression as an acylated (‘lipoprotein’) or non-acylated (‘polypeptide’) histidine-tagged recombinant. In each case, a strong IFN-γ response was observed using splenocytes from mice immunized with the 19-kD construct.
Immune responses to vaccination
To investigate the possibility that the presence of the 19-kD alters the immunogenicity of the mycobacterial vaccines, the DTH response to PPD was measured in vaccinated and control groups prior to challenge. The question concerning correlation of DTH response to mycobacterial antigens with protection against experimental tuberculosis infection in mice remains controversial [15,16]. However, in our hands the highest level of DTH response to PPD in BCG-vaccinated and M. tuberculosis-infected mice was always observed in genetically more resistant or better protected mouse strains [13,17]. In this study, the level of DTH response to PPD assessed in vaccinated mice 1 week before challenge again correlated well with the degree of protection reflected by the MST data. As shown in Table 2, the most prominent reaction was registered in the BCG-vaccinated group; weak but positive reactions were observed in groups receiving the saprophytic mycobacteria, and mice vaccinated with 19-kD-recombinant mycobacteria were unresponsive. The level of DTH response in mice vaccinated with M. smegmatis transformed with 19-kD on a cosmid vector did not differ from that in the M. smegmatis vector control group; again in parallel with a lower decrease in the MST of these mice compared with those receiving the 19-kD plasmid construct (Table 1). Reduction in the DTH response observed following vaccination with the saprophytic mycobacteria is consistent with an effect of the 19-kD antigen on the process of immunization.
Table 2.
DTH response to PPD in vaccinated mice prior to challenge
| Group | DTH* |
|---|---|
| 1. Non-vaccinated control | 0·08 ± 0·03 |
| 2. M. bovis BCG-Prague | 0·24 ± 0·03 |
| 3. M. smegmatis::p16R1 | 0·18 ± 0·03 |
| 4. M. smegmatis::p16R1-19 | 0·07 ± 0·01** |
| 5. M. smegmatis::cosmid 19-kD | 0·15 ± 0·03 |
| 6. M. vaccae::p16R1 | 0·16 ± 0·02 |
| 7. M. vaccae::p16R1-19 | 0·04 ± 0·02** |
Footpad swelling (mm) ± s.e.m.
Significant (P = 0·01) decrease compared with corresponding vector control.
Immune responses following challenge
The capacity to proliferate and to produce IFN-γ in response to mycobacterial antigens was assessed in T cell-enriched lung and splenocyte preparations from vaccinated and control groups 1 and 2 weeks after challenge with M. tuberculosis (2 × 105 CFU/mouse). No significant difference was observed between groups immunized with M. vaccae and M. vaccae::19-kD. In each case a proliferative response with a low level of IFN-γ production was observed after 2 weeks in the lung preparations, and a strong IFN-γ response in splenocytes at the 1-week time point (Table 3). Interestingly, IFN-γ production peaked 1 week post-infection and decreased during week 2; thus the IFN-γ response reached its maximum before T cells acquired capacity to specifically proliferate, and dropped as mycobacteria increased in numbers. In contrast to the control and M. vaccae-immunized groups, in mice receiving BCG, a more rapid induction of proliferation and IFN-γ production was observed after 1 week in the lungs, in the absence of any detectable splenocyte response. The early response in the lung is consistent with the presence of appropriately primed T cells following BCG vaccination. The absence of a splenocyte response is consistent with previous observations of relatively weak T cell priming in the spleen of mice receiving s.c. BCG vaccination (A. Apt, unpublished observations), and is also a reflection of the fact that the number of M. tuberculosis challenge organisms reaching the spleen in these animals may be below the threshold for T cell activation (Fig. 1). IL-4 and IL-5 were not detectable in any of the groups.
Table 3.
PPD-stimulated proliferation (SI) and IFN-γ (ng/ml) production by lung and spleen cells at the early phases of disease
| Lung cells | Spleen cells | ||||
|---|---|---|---|---|---|
| Group | Week | SI | IFN-γ | SI | IFN-γ |
| 1. Non-vaccinated control | 1 | 1 | 8 | 7 | 201 |
| 2 | 8 | 6 | 9 | 94 | |
| 2. M. bovis BCG-Prague | 1 | 8 | 23 | 2 | < 0·3 |
| 2 | 12 | 10 | 2 | < 0·3 | |
| 3. M. vaccae::p16R1 | 1 | 1·4 | 11 | 1·5 | 159 |
| 2 | 23 | 9 | 9 | 81 | |
| 4. M. vaccae::p16R1-19 | 1 | 1·3 | 10 | 1·6 | 311 |
| 2 | 21 | 8 | 7 | 141 | |
DISCUSSION
With the elucidation of the sequence of the complete antigenic complement of M. tuberculosis as part of the mycobacterial genome initiative [18], efforts are underway to identify an appropriate combination of antigens to constitute a subunit vaccine capable of matching the protective efficacy conferred by live BCG. While considerable progress has been made towards this goal [19], there is no evidence to date of any vaccine candidates able to surpass BCG in protective efficacy. Previous results with the 19-kD antigen of M. tuberculosis indicated an unusual property of this protein in its ability to decrease the protection conferred by immunization with M. vaccae in two animal challenge models [7]. We were interested in exploring the possibility that this antigen may be detrimental to the process of protective immunity, either by inducing some pathogenic form of immune response, or by altering responses to other protective antigens present in the vaccine.
The results of the present study demonstrate that the initial observation of a detrimental effect of the 19-kD antigen is not limited to a single vaccination system, with an analogous response associated with its addition to live and to heat-killed vaccines, and using M. smegmatis as well as M. vaccae as delivery vehicle. Two lines of experimental evidence suggest that the detrimental activity of the 19-kD antigen is mediated by its effect on recognition of other antigens present in the vaccine rather than by a pathological immune response to the antigen itself. First, induction of a strong type 1 immune response to the 19-kD antigen delivered in the form of a DNA vaccine had no effect—either detrimental or beneficial—on the subsequent course of infection with virulent M. tuberculosis. This observation is consistent with the absence of any effect on intranasal infection with BCG following DNA vaccination with the 19-kD gene as reported recently by Erb et al. [20]. Several other M. tuberculosis antigens have been shown to confer significant protection when delivered as DNA vaccines, including antigen 85 [10,21], the 65-kD heat shock protein [22], and a 38-kD lipoprotein implicated in phosphate transport [23]. These protective antigens include proteins localized in the bacterial cytoplasm, proteins associated with the cell wall, and proteins secreted into the culture medium; there are no obvious criteria which would predict the anomalous vaccination effects of the cell wall-associated 19-kD antigen. Zhu et al. observed a protective effect of vaccination when the 19-kD antigen was delivered in the form of a recombinant vaccinia construct [24], further demonstration that an immune response to the 19-kD is not in itself directly detrimental.
A second observation suggesting an effect of the 19-kD on the response to other antigens in the vaccines is the reduced PPD reactivity assessed by DTH measurements in the different vaccine groups (Table 2). The response induced in mice receiving M. vaccae or M. smegmatis alone presumably reflects the presence of shared antigens between M. tuberculosis and the saprophytic mycobacteria. This response to cross-reactive antigens was lost when the saprophytic mycobacteria were transformed with the 19-kD plasmid. The mechanism of this apparent suppression is unknown. The 19-kD antigen could act as a competitive inhibitor of recognition of other antigens—by preferential binding to antigen-presenting molecules, for example—or it could have some other effect on APC function which causes a reduction in vaccine immunogenicity.
Expression of the 19-kD protein by BCG vaccine strains (and by M. tuberculosis itself) may have an analogous deleterious effect on induction of protective immune responses. If so, mutation of the appropriate gene to create a 19-kD negative strain may present a novel route towards generation of a BCG vaccine with improved protective efficacy.
Acknowledgments
This research was supported by the Wellcome Trust Collaborative Research Initiative Grant and the World Health Organization Global Programme for Vaccines and Immunization. We are grateful to Dr Stewart Cole for provision of cosmid T500 and to Dr Jeffrey Ulmer for providing the DNA vaccine vector VIJNS-Tpa. The assistance of Marie-Pierre Gares in preparation of recombinant constructs is gratefully acknowledged.
REFERENCES
- 1.Engers HD, Houba V, Bennedsen J, et al. Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986;51:718–20. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackett PS, Bothamely GH, Bathra HV, et al. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988;26:2313–8. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faith A, Moreno C, Lathigra R, et al. Analysis of human T-cell epitopes in the 19, 000 MW antigen of Mycobacterium tuberculosis: influence of HLA–DR. Immunology. 1991;74:1–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Harris DP, Vordermeier HM, Friscia G, et al. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T-cells. J Immunol. 1993;150:5041–50. [PubMed] [Google Scholar]
- 5.Young DB, Kaufmann SHE, Hermans PWM, Thole JER. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–45. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- 6.Garbe TR, Harris D, Vordermeier HM, et al. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect Immun. 1993;61:260–7. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Zeid C, Gares M-P, Inwald J, et al. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856–62. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Lathigra R, Garbe T, Catty D, Young DB. Genetic analysis of superoxide dismutase, the 23 kDa antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991;5:381–91. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 9.Garbe TR, Barathi J, Barnini S, et al. Transformation of mycobacterial species using hygromycin resistance as selectable marker. Microbiology. 1994;140:133–8. doi: 10.1099/13500872-140-1-133. [DOI] [PubMed] [Google Scholar]
- 10.Huygen K, Content J, Denis O, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature Med. 1996;2:893–8. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 11.Holt PG, Degebrodt AO, Leary C, Krska K, Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985;62:586–93. [PMC free article] [PubMed] [Google Scholar]
- 12.Lyadova IV, Yeremeev VV, Majorov KB, et al. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect Immun. 1998;66:4981–8. doi: 10.1128/iai.66.10.4981-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apt AS, Avdienko VG, Nikonenko BV, Moroz AM, Skamene E. Distinct H-2 complex control of mortality, and immune responses to tuberculosis infection in virgin and BCG-vaccinated mice. Clin Exp Immunol. 1993;94:322–9. doi: 10.1111/j.1365-2249.1993.tb03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeremeev VV, Majorov KB, Avdienko VG, Kondrashov SV, Apt AS. Experimental analysis of Mycobacterium smegmatis as a possible vector for the development of the new antituberculosis vaccines. Probl Tuberculeza. 1996;N1:49–52. (in Russian) [PubMed] [Google Scholar]
- 15.Orme IM, Anderson P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–97. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 16.Rook GAW, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–84. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 17.Nikonenko BV, Apt AS, Mezhlumova MB, et al. Influence of the mouse Bcg, Tbc-1 and xid genes on resistance and immune responses to tuberculosis infection and efficacy of BCG vaccination. Clin Exp Immunol. 1996;104:37–43. doi: 10.1046/j.1365-2249.1996.d01-643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 19.Copenhagen: Statens Seruminstitut; 1999. WHO Meeting on the GVP Task Force on Animal Models for Tuberculosis, 11 February. [Google Scholar]
- 20.Erb KJ, Kirman J, Woodfield L, et al. Identification of potential CD8+ T-cell epitopes of the 19 kDa and AphC proteins from Mycobacterium tuberculosis. No evidence for CD8+ T-cell priming against the identified peptides after DNA-vaccination of mice. Vaccine. 1998;16:692–7. doi: 10.1016/s0264-410x(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 21.Denis O, Tanghe A, Palfliet K, et al. Vaccination with plasmid DNA encoding mycobacterial antigen 8A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–33. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Venkataprasad N, Thangaraj HS, et al. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–6. [PubMed] [Google Scholar]
- 23.Tascon RE, Colston MJ, Ragno S, et al. Vaccination against tuberculosis by DNA injection. Nature Med. 1996;2:888–92. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 24.Zhu X, Venkataprasad N, Ivanyi J, Vordermeier HM. Vaccination with recombinant vaccinia viruses protects mice against Mycobacterium tuberculosis infection. Immunology. 1997;92:6–9. doi: 10.1046/j.1365-2567.1997.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


